Abstract
Interactions between the dual Bcr/Abl and aurora kinase inhibitor MK-0457 and the histone deacetylase inhibitor vorinostat were examined in Bcr/Abl+ leukemia cells, including those resistant to imatinib mesylate (IM), particularly those with the T315I mutation. Coadministration of vorinostat dramatically increased MK-0457 lethality in K562 and LAMA84 cells. Notably, the MK-0457/vorinostat regimen was highly active against primary CD34+ chronic myelogenous leukemia (CML) cells and Ba/F3 cells bearing various Bcr/Abl mutations (ie, T315I, E255K, and M351T), as well as IM-resistant K562 cells exhibiting Bcr/Abl-independent, Lyn-dependent resistance. These events were associated with inactivation and down-regulation of wild-type (wt) and mutated Bcr/Abl (particularly T315I). Moreover, treatment with MK-0457 resulted in accumulation of cells with 4N or more DNA content, whereas coadministration of vorinostat markedly enhanced aurora kinase inhibition by MK-0457, and preferentially killed polyploid cells. Furthermore, vorinostat also interacted with a selective inhibitor of aurora kinase A and B to potentiate apoptosis without modifying Bcr/Abl activity. Finally, vorinostat markedly induced Bim expression, while blockade of Bim induction by siRNA dramatically diminished the capacity of this agent to potentiate MK-0457 lethality. Together, these findings indicate that vorinostat strikingly increases MK-0457 activity against IM-sensitive and -resistant CML cells through inactivation of Bcr/Abl and aurora kinases, as well as by induction of Bim.
Introduction
Chronic myelogenous leukemia (CML) is characterized by the Philadelphia chromosome (Ph; 22q), which is responsible for the chimeric fusion oncoprotein Bcr/Abl. The Bcr/Abl kinase is constitutively active and signals downstream to multiple survival pathways,1 providing CML cells with a survival advantage over their normal counterparts and conferring resistance against cytotoxic agents.2 The treatment of CML has been revolutionized by the introduction of the kinase inhibitor imatinib mesylate (IM; Gleevec, Novartis, Basel, Switzerland), which is highly active in patients with chronic-phase CML3 but less active in patients with accelerated or blast-phase disease.4 However, almost all patients who initially respond eventually develop resistance to this agent. Mechanisms of resistance include bcr/abl gene amplification, increased expression of the Bcr/Abl protein, and most commonly, point mutations in various domains of the Bcr/Abl kinase, including the activation loop, the phosphorylation loop, or the gatekeeper region.5 This phenomenon stimulated the development of second-generation Bcr/Abl kinase inhibitors (eg, dasatinib and nilotinib), which are active against proteins bearing most mutations.6,7 However, these agents are inactive against cells with gatekeeper region mutations, most notably T315I,8 prompting the search for newer Bcr/Abl kinase inhibitors active against such mutants.
The aurora kinases (A, B, and C) represent a family of serine/threonine kinases involved in the control of mitosis.9 Deregulation of aurora kinase activity leads to disruption of cell-cycle progression, mitotic abnormalities, and genetic instability.10 Importantly, aurora kinases are overexpressed and/or activated in a variety of tumor cells, suggesting a role for this family in tumorigenesis.9,10 MK-0457 is a small-molecule, novel pan–aurora kinase inhibitor9 with demonstrated activity against wild-type (wt) and mutated Bcr/Abl,11-13 including the T315I mutation, as well as FLT3 and JAK2. MK-0457 delays entry into mitosis, leads to aberrant cytokinesis, induces apoptosis in several human tumor types, and is being evaluated in patients with a variety of malignant diseases.9 MK-0457 potently inhibits aurora kinases (particularly aurora A and B) in tumor cells, manifested by down-regulation of phosphorylated histone H3 at Ser10.9 This results in multiple events, including aberrant cell-cycle progression and accumulation of polyploid cells with DNA content of 4N or more, which collectively trigger cell death.14,15 Very recently, it was reported that MK-0457 also potently inhibits the Bcr/Abl T315I mutation,11-13 which confers resistance to first-generation (ie, IM) and second-generation (eg, dasatinib and nilotinib) kinase inhibitors.8,16 Moreover, MK-0457 is also highly effective against other commonly detected dasatinib-resistant mutations (eg, V299L).17 MK-0457 binds to the kinase domain of an IM-resistant mutant form of the Abl kinase, indicating this agent favors the active conformation of Bcr/Abl.11,12 Furthermore, a phase 1 clinical trial indicates that MK-0457 has significant activity in patients with T315I phenotype–refractory CML or Ph+ ALL.18 In accord with these findings, a phase 2 trial in the specific setting of T315I+ Ph+ leukemia is forthcoming.19
Vorinostat (Zolinza/NSC-701852, previously known as suberoylanilide hydroxamic acid [SAHA]; Merck Pharmaceuticals, Whitehouse Station, NJ) is a pan–histone deacetylase inhibitor (HDACI) displaying activity against both nuclear (class I) as well as cytoplasmic (class II) HDACs,20 and has recently been approved for the treatment of cutaneous T-cell lymphoma.21 In preclinical studies, vorinostat kills neoplastic cells through multiple mechanisms,22 including activation of the extrinsic and/or intrinsic apoptotic pathways23,24 and induction of oxidative damage,25 as well as induction of autophagic cell death26,27 and senescence.28 HDACIs like vorinostat, through inhibition of the class II HDAC6, results in acetylation and loss of function of the chaperone protein Hsp90.29 This prevents complex formation with client proteins, including Bcr/Abl, which subsequently undergo polyubiquitination and proteasomal degradation.30-32 In previous studies, vorinostat and other HDACIs have been shown to interact synergistically with other targeted agents, including Bcr/Abl kinase inhibitors such as IM33 and dasatinib.34 It is postulated that synergism between HDACIs and Bcr/Abl kinase inhibitors stems, at least in part, from disruption of Hsp90 function, leading to Bcr/Abl degradation.35
The ability of HDACIs like vorinostat to promote the lethality of IM and other Bcr/Abl kinase inhibitors,33,34 as well as the shared capacity of HDACIs and aurora kinase inhibitors to induce aberrant mitotic progression,15,28,36 gave rise to the possibility that vorinostat might increase the activity of MK-0457 in CML cells. To address this issue, we have examined interactions between MK-0457 and vorinostat in Bcr/Abl+ cells sensitive or resistant, through various mechanisms, to IM. Our results indicate that vorinostat dramatically potentiates MK-0457 lethality toward CML cells, including those bearing the T315I mutation, in association with Bcr/Abl inactivation/down-regulation, diminished aurora kinase function, and induction of the proapoptotic protein Bim.
Methods
Cells and reagents
LAMA84 and K562 cells are cell lines both derived from human CML in blast crisis, which exhibit either hyper- or hypotriploid karyotype and carry at least one Ph chromosome (a small percentage of cells may carry 3 or 4 chromosomes).37 These cells and their IM-resistant counterparts (K562-R) were obtained and cultured as described previously.38 K562 cells were stably transfected with pSR-Bim and pSR-control constructs as described previously.39 Parental Ba/F3 cells were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), and cultured in RPMI1640 medium containing 10% fetal bovine serum (FBS) and 10 ng/mL IL-3. Ba/F3 cells expressing wt and mutant forms of Bcr/Abl (eg, T315I, E255K, and M351T) were kindly provided by Dr Brian J. Druker (Oregon Health & Science University Cancer Institute, Portland, OR).40 Ba/F3 cells transfected with Bcr/Abl (wt or mutant) were cultured in 10% FBS/RPMI1640 medium without IL-3 because they are transformed by Bcr/Abl to growth factor independence.41 All experiments were performed using logarithmically growing cells (4-6 × 105 cells/mL).
Bone marrow was obtained with informed consent, in accordance with the Declaration of Helsinki, from 3 patients with CML undergoing routine diagnostic bone marrow aspiration, and from a patient with a nonmyeloid hematologic disorder (iron deficiency). Patients 1 and 2 had been treated with IM and displayed progressive disease, while patient 3 was newly diagnosed. All studies were sanctioned by the institutional review board of Virginia Commonwealth University. Mononuclear cells were isolated by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO). In some cases, CD34+ CML cells were isolated using a Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech, Auburn, CA) according to the manufacturer's protocol.42 In addition, normal CD34+ cells were purchased from StemCell Technologies (Vancouver, BC), thawed, and maintained as per the manufacturer's instructions. The cells were diluted to a density of 106 cells/mL for drug treatment.
The dual Bcr/Abl and aurora kinase inhibitor MK-0457 (formerly known as VX-680) and the HDACI vorinostat (suberoylanilide hydroxamic acid [SAHA]) were provided by Merck Pharmaceuticals, the latter in conjunction with the Cancer Treatment and Evaluation Program (National Cancer Institute, Bethesda, MD). Aurora kinase inhibitor II (4-[4′-benzamidoanilino]-6,7-dimethoxyquinazoline) was purchased from Calbiochem (San Diego, CA). They were dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C. In all experiments, the final concentration of DMSO did not exceed 0.1%.
Apoptosis and mitochondrial membrane potential (Δψm)
The extent of cell death was evaluated by staining with 0.5 μg/mL 7-amino actinomycin D (7AAD; Sigma-Aldrich) at 37°C for 20 minutes; the percentage of 7AAD+ cells was then determined using a Becton Dickinson FACScan (BD Biosciences, Franklin Lakes, NJ). In some cases, cells exhibiting apoptosis or loss of Δψm were assessed by annexin V–fluorescein isothiocyanate (FITC) staining (BD Pharmingen, San Diego, CA) or DiOC6 (Invitrogen, Carlsbad, CA) and flow cytometry as described previously.38
Western blot
Whole-cell lysates were prepared and subjected to Western blot analysis after the procedures described in detail previously.38 Where indicated, the blots were reprobed with antibodies against β-actin (Transduction Laboratories, Lexington, KY) or α-tubulin (Calbiochem) to ensure equal loading and transfer of proteins. The following antibodies were used as primary antibodies: phospho-Bcr (Tyr177), phospho-CrkL (Tyr207), CrkL (32H4, mouse monoclonal; for human CML cells), cleaved caspase-3 (Asp175), cleaved poly–(ADP-ribose) polymerase (PARP; Asp214), phospho-Lyn (Tyr507), Lyn, and aurora A and aurora B (Cell Signaling Technology, Danvers, MA); c-Abl, CrkL (C-20, rabbit polyclonal; for Ba/F3 cells), and histone H3 (Santa Cruz Biotechnology, Santa Cruz, CA); phosphohistone H3 (Ser10; Upstate Biotechnology, Lake Placid, NY); caspase-3 and caspase-9 (BD Pharmingen); PARP (Biomol, Plymouth Meeting, PA); and Bim (Calbiochem). In some cases, the density of blots was quantified using the FluoChem 8800 Imaging System (Alpha Innotech, San Leandro, CA) and VideoTesT-Master software (VideoTesT, St Petersburg, Russia).
Coimmunoprecipitation
The interaction between Bcr/Abl and Hsp90 was evaluated by coimmunoprecipitation analysis as previously reported.43 Briefly, cell lysates in radioimmunoprecipitation assay (RIPA) buffer were incubated with c-Abl antibody (Santa Cruz Biotechnology) overnight at 4°C. Dynabeads (Dynal, Oslo, Norway) were then added and incubated for an additional 4 hours. After washing, the bead-bound protein was eluted and subjected to Western blot analysis using Hsp90 antibody (Santa Cruz Biotechnology).
Cell-cycle analysis
Cell-cycle analysis was used to assess cell polyploidy.9 Briefly, cells were fixed in 67% ethanol/phosphate-buffered saline (PBS) on ice overnight, stained with 0.01 mg/mL propidium iodide (PI) containing 0.5 mg/mL RNase on ice for 3 hours, and subjected to flow cytometry using CellQuest software (BD Biosciences).44
Immunocytochemistry
Cytospin slides were fixed in 3.7% formaldehyde/PBS for 20 minutes at room temperature, and permeabilized in 0.5% Triton X-100 for 2 minutes. Cells were then incubated with FITC-conjugated phosphohistone H3 (Ser10) antibody (Upstate Biotechnology) for 1 hour, washed with PBS, and mounted with Vectashield Mounting Medium with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA).
Statistical analysis
For analysis of cell death, values represent the means plus or minus SD for at least 3 separate experiments performed in triplicate. The significance of differences between experimental variables was determined using the Student t test. Analysis of synergism was performed according to median dose effect analysis using the Calcusyn software program (Biosoft, Ferguson, MO).45
Results
MK-0457 induces apoptosis in CML cell lines in association with inhibition of both Bcr/Abl and aurora kinases
Earlier in vitro competition binding assays revealed that MK-0457 binds to wt ABL1 (Kd = 20 nM) as well as drug-resistant mutants, particularly T315I ABL1 (Kd = 5 nM).12,13 MK-0457 also inhibited autophosphorylation of T315I Bcr/Abl in Ba/F3 cells expressing this mutant protein (concentration that inhibits response by 50% [IC50] ≈ 5 μM).13 Moreover, a clear inhibitory effect of Bcr/Abl-mediated signaling (reflected by diminished CrkL phosphorylation, an adaptor protein phosphorylated primarily by Bcr/Abl), was observed with 10 μM MK-0457 in primary CML cells harboring the T315I mutation obtained from a patient who developed resistance to IM and subsequently had no response to dasatinib.12 To test the potency of MK-0457 with respect to inhibition of wt Bcr/Abl kinase in intact cells, K562 and LAMA84 cells were exposed to various concentrations (5 nM-10 μM) of MK-0457 for 24 to 48 hours, after which Bcr/Abl activity, manifested by expression of phospho-Bcr/Abl (Tyr177 of Bcr) and phospho-CrkL, as well as apoptosis, were monitored. As shown in Figure 1A, treatment with 1 μM MK-0457 resulted in 40% to 55% cells displaying loss of Δψm and 37% to 47% cell death (7AAD+), accompanied by caspase-3 cleavage and PARP degradation (Figure 1B). These events were associated with diminished phosphorylation of Bcr/Abl and CrkL, but no change in total protein levels (Figure 1B). Exposure to MK-0457 also diminished phosphorylation of the aurora kinase target histone H39 (Figure 1B). Collectively, these findings indicate that micromolar concentrations of MK-0457 induce apoptosis in Bcr/Abl+ leukemia cells, a phenomenon associated with inhibition of both wt Bcr/Abl and aurora kinases.
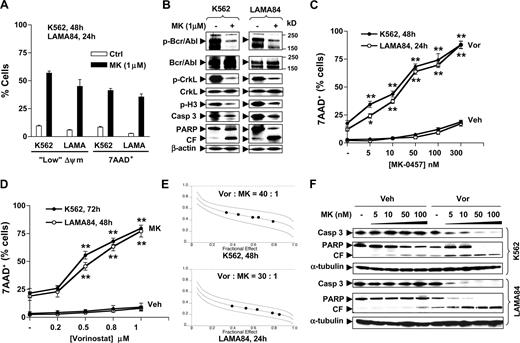
Vorinostat interacts synergistically with MK-0457 to induce apoptosis in CML cell lines. (A) K562 and LAMA84 cells were exposed to 1 μM MK-0457 (MK) for 24 hours (LAMA84) or 48 hours (K562), after which the percentage of cells exhibiting “low” Δψm or 7AAD positivity was determined by flow cytometry. (B) K562 and LAMA84 cells were treated as described in panel A, after which cells were lysed and subjected to Western blot using the indicated antibodies. (C) K562 and LAMA84 cells were incubated with increasing concentrations (5-300 nM) of MK in the absence or presence of vorinostat (Vor K562, 2 μm; LAM484, 1.5 μm) for 48 hours (K562) or 24 hours (LAMA84), after which the percentage of 7AAD+ cells was determined by flow cytometry. (D) K562 and LAMA84 cells were incubated with increasing concentrations (0.2-1 μM) of vorinostat in the absence or presence of 100 nM MK for 72 hours (K562) or 48 hours (LAMA84), after which the percentage of 7AAD+ cells was determined by flow cytometry. (E) K562 (top panel) and LAMA84 cells (bottom panel) were treated with a range of MK and Vor concentrations alone and in combination for 48 hours (K562) or 24 hours (LAMA84) at a fixed ratio as indicated. At the end of this period, the percentage of 7AAD+ cells was determined by flow cytometry; fractional effect values were determined by comparing results with those of untreated controls, and median dose effect analysis was used to characterize the nature of the interaction. Combination index (CI) values less than 1.0 denote a synergistic interaction. Two additional studies yielded equivalent results. (F) K562 and LAMA84 cells were treated with 5 to 100 nM MK with or without Vor (K562, 2 μM; LAMA84, 1.5 μM), after which Western blot analysis was performed to monitor cleavage of caspase-3 and PARP. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate (A,C,D). For each condition involving combined treatment, the net increase over treatment with Vor (C) or MK (D) alone was determined and evaluated for significance using the Student t test. Asterisks indicate significantly greater than values for treatment of cells with the single agent at the same concentrations (*P < .05; **P < .01). For panels B and F, each lane was loaded with 30 μg protein; blots were stripped and reprobed with β-actin or α-tubulin antibodies to ensure equal loading and transfer of protein. CF indicates cleavage fragment; Veh, vehicle. Two additional studies yielded equivalent results.
Vorinostat interacts synergistically with MK-0457 to induce apoptosis in CML cell lines. (A) K562 and LAMA84 cells were exposed to 1 μM MK-0457 (MK) for 24 hours (LAMA84) or 48 hours (K562), after which the percentage of cells exhibiting “low” Δψm or 7AAD positivity was determined by flow cytometry. (B) K562 and LAMA84 cells were treated as described in panel A, after which cells were lysed and subjected to Western blot using the indicated antibodies. (C) K562 and LAMA84 cells were incubated with increasing concentrations (5-300 nM) of MK in the absence or presence of vorinostat (Vor K562, 2 μm; LAM484, 1.5 μm) for 48 hours (K562) or 24 hours (LAMA84), after which the percentage of 7AAD+ cells was determined by flow cytometry. (D) K562 and LAMA84 cells were incubated with increasing concentrations (0.2-1 μM) of vorinostat in the absence or presence of 100 nM MK for 72 hours (K562) or 48 hours (LAMA84), after which the percentage of 7AAD+ cells was determined by flow cytometry. (E) K562 (top panel) and LAMA84 cells (bottom panel) were treated with a range of MK and Vor concentrations alone and in combination for 48 hours (K562) or 24 hours (LAMA84) at a fixed ratio as indicated. At the end of this period, the percentage of 7AAD+ cells was determined by flow cytometry; fractional effect values were determined by comparing results with those of untreated controls, and median dose effect analysis was used to characterize the nature of the interaction. Combination index (CI) values less than 1.0 denote a synergistic interaction. Two additional studies yielded equivalent results. (F) K562 and LAMA84 cells were treated with 5 to 100 nM MK with or without Vor (K562, 2 μM; LAMA84, 1.5 μM), after which Western blot analysis was performed to monitor cleavage of caspase-3 and PARP. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate (A,C,D). For each condition involving combined treatment, the net increase over treatment with Vor (C) or MK (D) alone was determined and evaluated for significance using the Student t test. Asterisks indicate significantly greater than values for treatment of cells with the single agent at the same concentrations (*P < .05; **P < .01). For panels B and F, each lane was loaded with 30 μg protein; blots were stripped and reprobed with β-actin or α-tubulin antibodies to ensure equal loading and transfer of protein. CF indicates cleavage fragment; Veh, vehicle. Two additional studies yielded equivalent results.
Vorinostat interacts with MK-0457 to induce apoptosis in Bcr/Abl+ leukemia cells in a highly synergistic manner
Attempts were then made to determine whether vorinostat could enhance MK-0457 lethality. Exposure of K562 (48 hours) or LAMA84 cells (24 hours) to low MK-0457 concentrations ranging from 5 to 300 nM minimally induced cell death (Figure 1C). However, coadministration of marginally toxic concentrations (eg, 1.5-2 μM) of vorinostat, which produced cell death in approximately 18% of K562 and 12% of LAMA84 cells, resulted in a highly significant increase in MK-0457 lethality in both cell lines, even at very low MK-0457 concentrations (eg, 5-10 nM; P < .05 or P < .01 compared with treatment with MK-0457 alone). Coadministration of vorinostat produced approximately 90% cell death as MK-0457 concentrations approached 300 nM. Essentially identical effects were observed when loss of Δψm was monitored (data not shown). In addition, cotreatment of K562 or LAMA84 cells with lower, nontoxic concentrations of vorinostat (ie, 0.5 μM to 1.0 μM) very significantly increased the lethality of minimally toxic concentrations of MK-0457 (100 nM) at later exposure intervals (72 or 48 hours, respectively; P < .01 in each case versus vorinostat alone; Figure 1D). Isobologram analysis revealed combination index values less than 0.6 (K562) or less than 0.4 (LAMA84), indicating a highly synergistic interaction (Figure 1E). Moreover, coadministration of vorinostat dramatically potentiated the ability of minimally toxic concentrations of MK-0457 (5-100 nM) to induce caspase-3 cleavage and PARP degradation in both cell lines (Figure 1F). Together, these findings indicate that minimally toxic concentrations of vorinostat strikingly increase the lethality of MK-0457, even at extremely low concentrations (ie, 5-10 nM), in Bcr/Abl+ leukemia cells.
Vorinostat potentiates MK-0457 lethality in primary CML cells while relatively sparing normal cells
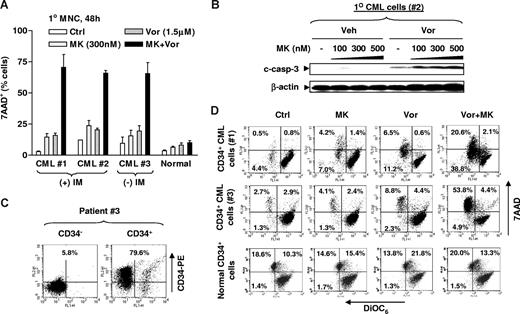
Parallel studies were performed in bone marrow mononuclear cells (MNCs) and isolated CD34+ cells from 3 patients with CML. Exposure (48 hours) of CML MNCs to 300 nM MK-0457 or 1.5 μM vorinostat individually resulted in minimal toxicity, whereas combined treatment resulted in a substantial increase in cell death in each patient sample but not normal bone marrow MNCs (Figure 2A). Dose-response studies with other minimally toxic concentrations of either MK-0457 or vorinostat yielded similar results (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Western blot analysis of cell lysates from patient 2 exhibited a clear increase in expression of cleaved forms of caspase-3 after combined treatment (Figure 2B). Studies were then performed on the CD34+ cells isolated from 2 of the CML MNC samples (nos. 1 and 2), for which a sufficient number of cells were available. A representative sample isolated by immunomagnetic microbeads yielded approximately 80% CD34+ cells (Figure 2C) with more than 90% viability determined by trypan blue exclusion. As shown for the representative flow cytometric data in Figure 2D, a major shift to the left (indicating loss of Δψm, reflected by “low” DiOC6 uptake) and upward (indicating 7AAD+ dead cells) was observed in CD34+ cells from both patients, consistent with the results in CML MNCs. Notably, the vorinostat/MK-0457 regimen exhibited only modest lethality toward normal CD34+ cells, compared with untreated controls (Figure 2D bottom panels). These findings indicate that vorinostat dramatically increases MK-0457 lethality in primary CML cells, and raise the possibility that this regimen may be relatively sparing toward normal hematopoietic cells.
Vorinostat potentiates the lethality of MK-0457 in primary CML cells, while largely sparing normal bone marrow MNCs. (A) MNCs were isolated from bone marrow samples obtained from 2 patients with CML in chronic phase (patients 1 and 2) who had progressed after IM treatment, and from an additional patient (patient 3) who had not yet been treated, as well as from a normal bone marrow specimen. MNCs were exposed to 300 nM MK with or without 1.5 μM Vor for 48 hours, after which the percentage of 7AAD+ cells was determined by flow cytometry. (B) MNCs from patient 2 were treated with 100 to 500 nM MK with or without 1.5 μM Vor for 48 hours, after which cells were lysed and subjected to Western blot analysis to monitor caspase-3 cleavage. (C,D) Primary CD34+ CML cells isolated from bone marrow MNCs were obtained from patients 1 and 3. Cells were then stained with PE-conjugated CD34 antibody and subjected to flow cytometry to determine the purity of CD34+ cells. A representative result is shown (C). Numbers reflect the percentage of cells in the 2 top quadrants. Isolated primary CD34+ CML cells as well as normal CD34+ cells were exposed to 300 nM MK with or without 1.5 μM Vor for 24 to 48 hours, after which cells were stained with both DiOC6 and 7AAD and subjected to flow cytometry. “Low” DiOC6 uptake/7AAD− (bottom left quadrant) corresponds to early apoptosis (mitochondrial damage, reflected by loss of Δψm) and “low” DiOC6/7AAD+ (top left quadrant) corresponds to late apoptosis. Values reflect the percentage of cells in the corresponding quadrants. Results represent the means plus or minus SD for experiments performed in triplicate (A). (B) Each lane was loaded with 100 μg protein. The results of a representative experiment are shown; an additional experiment yielded equivalent results. Veh indicates vehicle.
Vorinostat potentiates the lethality of MK-0457 in primary CML cells, while largely sparing normal bone marrow MNCs. (A) MNCs were isolated from bone marrow samples obtained from 2 patients with CML in chronic phase (patients 1 and 2) who had progressed after IM treatment, and from an additional patient (patient 3) who had not yet been treated, as well as from a normal bone marrow specimen. MNCs were exposed to 300 nM MK with or without 1.5 μM Vor for 48 hours, after which the percentage of 7AAD+ cells was determined by flow cytometry. (B) MNCs from patient 2 were treated with 100 to 500 nM MK with or without 1.5 μM Vor for 48 hours, after which cells were lysed and subjected to Western blot analysis to monitor caspase-3 cleavage. (C,D) Primary CD34+ CML cells isolated from bone marrow MNCs were obtained from patients 1 and 3. Cells were then stained with PE-conjugated CD34 antibody and subjected to flow cytometry to determine the purity of CD34+ cells. A representative result is shown (C). Numbers reflect the percentage of cells in the 2 top quadrants. Isolated primary CD34+ CML cells as well as normal CD34+ cells were exposed to 300 nM MK with or without 1.5 μM Vor for 24 to 48 hours, after which cells were stained with both DiOC6 and 7AAD and subjected to flow cytometry. “Low” DiOC6 uptake/7AAD− (bottom left quadrant) corresponds to early apoptosis (mitochondrial damage, reflected by loss of Δψm) and “low” DiOC6/7AAD+ (top left quadrant) corresponds to late apoptosis. Values reflect the percentage of cells in the corresponding quadrants. Results represent the means plus or minus SD for experiments performed in triplicate (A). (B) Each lane was loaded with 100 μg protein. The results of a representative experiment are shown; an additional experiment yielded equivalent results. Veh indicates vehicle.
Vorinostat increases MK-0457 lethality in CML cells exhibiting a Bcr/Abl-independent, Lyn-dependent form of IM resistance
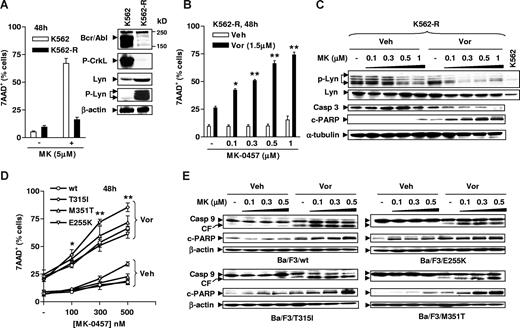
The effects of vorinostat on MK-0457 lethality were then examined in IM-resistant K562 cells (K562-R) exhibiting a Bcr/Abl-independent mechanism characterized by overexpression/activation of the Src kinase Lyn.38,46,47 As previously reported,38 these cells displayed a dramatic reduction in Bcr/Abl expression and activity, manifested by diminished CrkL phosphorylation, accompanied by a marked increase in expression of Lyn and phosphorylated Lyn (Figure 3A inset). Interestingly, these cells were also highly resistant to MK-0457 concentrations as high as 5 μM (48 hours; Figure 3A), presumably due to the loss of Bcr/Abl-dependence. However, coadministration (48 hours) of a modestly toxic concentration of vorinostat (1.5 μM) significantly increased the lethality of MK-0457 at concentrations of 100 nM or higher (Figure 3B). Notably, MK-0457, at concentrations of 500 nM or higher, clearly inhibited phosphorylation of Lyn, and this event was substantially enhanced by coadministration of vorinostat, which by itself had minimal effects (Figure 3C). Moreover, the vorinostat/MK-0457 regimen induced a marked increased in caspase-3 processing and PARP degradation in these cells. These results indicate that vorinostat significantly enhances MK-0457–mediated Lyn inhibition and lethality in CML cells displaying a Bcr/Abl-independent, Lyn-dependent form of resistance.
The MK-0457/vorinostat regimen induces apoptosis in cells resistant to IM through disparate mechanisms. (A) IM-resistant K562-R cells were established as described in “Cells and reagents,” and Western blot analysis was performed to monitor expression of Bcr/Abl and phospho-CrkL, as well as levels of total and phosphorylated Lyn (inset). Parental K562 and K562-R cells were exposed to 5 μM MK for 48 hours, after which the percentage of 7AAD+ cells was determined by flow cytometry. (B) K562-R cells were incubated with 0.1 to 1 μM MK with or without 1.5 μM Vor for 48 hours, after which flow cytometry was performed to monitor the percentage of 7AAD+ cells. (C) K562R cells were treated as described in panel B, after which Western blot analysis was performed to monitor expression of total and phosphorylated Lyn, as well as processing of caspase-3 and cleavage of PARP. (D) Ba/F3 cells bearing wt or various point mutations, including T351I, M351T, and E255K, were exposed to 100 to 500 nM MK-0457 with or without 1.5 μM vorinostat for 48 hours, after which the percentage of 7AAD+ cells was determined by flow cytometry. (E) Alternatively, cells were lysed and subjected to Western blot analysis to evaluate cleavage of caspase-9 and PARP. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate (A,B,D). For each condition involving combined treatment, the net increase compared with treatment with Vor alone was determined and the significance of differences evaluated using the Student t test. Asterisks indicate significantly greater than values obtained after treatment of cells with MK alone at the same concentrations (*P < .05; **P < .01). For panels A inset, C, and E, each lane was loaded with 30 μg protein; blots were stripped and reprobed with antibodies to β-actin or α-tubulin to ensure equal loading and transfer. CF indicates cleavage fragment; Veh, vehicle. Two additional studies yielded equivalent results.
The MK-0457/vorinostat regimen induces apoptosis in cells resistant to IM through disparate mechanisms. (A) IM-resistant K562-R cells were established as described in “Cells and reagents,” and Western blot analysis was performed to monitor expression of Bcr/Abl and phospho-CrkL, as well as levels of total and phosphorylated Lyn (inset). Parental K562 and K562-R cells were exposed to 5 μM MK for 48 hours, after which the percentage of 7AAD+ cells was determined by flow cytometry. (B) K562-R cells were incubated with 0.1 to 1 μM MK with or without 1.5 μM Vor for 48 hours, after which flow cytometry was performed to monitor the percentage of 7AAD+ cells. (C) K562R cells were treated as described in panel B, after which Western blot analysis was performed to monitor expression of total and phosphorylated Lyn, as well as processing of caspase-3 and cleavage of PARP. (D) Ba/F3 cells bearing wt or various point mutations, including T351I, M351T, and E255K, were exposed to 100 to 500 nM MK-0457 with or without 1.5 μM vorinostat for 48 hours, after which the percentage of 7AAD+ cells was determined by flow cytometry. (E) Alternatively, cells were lysed and subjected to Western blot analysis to evaluate cleavage of caspase-9 and PARP. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate (A,B,D). For each condition involving combined treatment, the net increase compared with treatment with Vor alone was determined and the significance of differences evaluated using the Student t test. Asterisks indicate significantly greater than values obtained after treatment of cells with MK alone at the same concentrations (*P < .05; **P < .01). For panels A inset, C, and E, each lane was loaded with 30 μg protein; blots were stripped and reprobed with antibodies to β-actin or α-tubulin to ensure equal loading and transfer. CF indicates cleavage fragment; Veh, vehicle. Two additional studies yielded equivalent results.
The vorinostat/MK-0457 regimen is highly active against Ba/F3 cells bearing various IM-resistant Bcr/Abl mutations, particularly T315I
Additional studies were then performed in Ba/F3 cells bearing various mutations (ie, M351T, E255K, and T315I) conferring resistance to IM and, in the case of T315I, to dasatinib and nilotinib.8 Exposure (48 hours) to 100 to 500 nM MK-0457 had only minor effects on the survival of Ba/F3 cells expressing wt or mutant Bcr/Abl, although T315I cells appeared slightly more sensitive to MK-0457 compared with the others (Figure 3D). However, coadministration of a minimally toxic concentration (eg, 1.5 μM) of vorinostat significantly potentiated MK-0457 lethality in Ba/F3 cells bearing either wt or mutant forms of Bcr/Abl, particularly at relatively high MK-0457 concentrations (ie, 500 nM) in T315I cells. These events were accompanied by pronounced cleavage of caspase-9 and PARP (Figure 3E). Such findings argue that a strategy combining vorinostat with MK-0457 is highly active toward leukemia cells bearing either wt Bcr/Abl or mutants conferring resistance to IM as well as second-generation Bcr/Abl kinase inhibitors. Parallel experiments were performed in parental Ba/F3 cells untransfected with either wt or mutant bcr/abl. Interestingly, while Bcr/Abl-expressing cells displayed resistance to both agents, potentiation of MK-0457 lethality by vorinostat was also observed in parental Ba/F3 cells (Figure S2A), even when marginally toxic concentrations of both agents were combined (Figure S2B).
Combination of vorinostat with low concentrations of MK-0457 results in inactivation and down-regulation of both wild-type and T315I Bcr/Abl
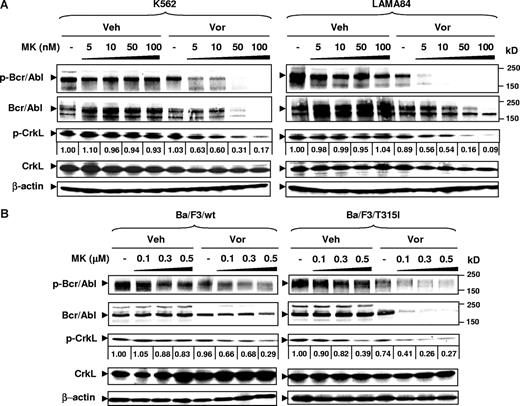
To evaluate these interactions further in Bcr/Abl+ cells, the effects of MK-0457 alone or combined with vorinostat were examined in relation to expression and activation of wt and mutant Bcr/Abl. Notably, in both K562 and LAMA84 cells, coadministration of vorinostat (eg, 1.5-2 μM), which by itself had minimal effects, dramatically potentiated the inhibitory activity of very low concentrations of MK-0457 (ie, 5-100 nM) on Bcr/Abl activity, manifested by the pronounced dephosphorylation of both Bcr/Abl on Tyr177, a site which is autophosphorylated in the Bcr-Abl fusion protein and which plays an important role in transforming activity of Bcr-Abl48 , and CrkL (Figure 4A). In addition, while total CrkL protein levels were not reduced, combined treatment with vorinostat and MK-0457 markedly diminished total Bcr/Abl protein expression in both K562 and LAMA84 cells (Figure 4A). Moreover, while MK-0457 (100-500 nM) modestly decreased Bcr/Abl activity in Ba/F3 cells bearing wt or T315I Bcr/Abl, this event was strikingly enhanced by coadministration of vorinostat (Figure 4B). Notably, down-regulation of phosphorylated and total Bcr/Abl by the vorinostat/MK-0457 regimen was if anything more pronounced in T315I Ba/F3 cells compared with their wt counterparts (Figure 4B). Results comparable with those in wt Bcr/Abl cells were observed in Ba/F3 cells bearing other IM-resistant mutants (eg, M351T and E255K; data not shown). Notably, exposure of LAMA84 cells to 100 nM MK-0457 with or without 1.5 μM vorinostat for only 16 hours resulted in a clear reduction in expression of phospho-Bcr/Abl, but only minimal changes in total Bcr/Abl expression levels at this interval (Figure S3). This suggests that inhibition of Bcr/Abl activity occurs before down-regulation of the Bcr/Abl protein. Moreover, vorinostat treatment with or without MK-0457 resulted in a marked decrease in Hsp90 coimmunoprecipitating with c-Abl (Figure S3). Together, these findings indicate that vorinostat interacts with MK-0457 to inhibit and subsequently down-regulate Bcr/Abl, and that these events are at least as pronounced in cells expressing mutant Bcr/Abl.
Coadministration of MK-0457 and vorinostat results in inactivation and down-regulation of wt and T315I Bcr/Abl kinases. (A) K562 and LAMA84 cells were incubated with 5 to 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours (K562) or 24 hours (LAMA84). (B) Ba/F3 cells with wt or T315I Bcr/Abl were treated with 0.1 to 0.5 μM MK with or without 1.5 μM Vor for 48 hours. (A,B) After drug treatment, cells were lysed, and Western blot analysis was performed to assess expression of total and phosphorylated forms of Bcr/Abl and CrkL. Each lane was loaded with 30 μg protein; blots were stripped and reprobed with antibodies to β-actin to ensure equal loading and transfer. Results are representative of 3 separate experiments. Protein bands of phospho-CrkL were quantified using an imaging system as described in “Western blot.” Values reflect the ratio of integrated densitometric determinations between untreated and drug-treated cells. Veh indicates vehicle. Two additional studies yielded equivalent results.
Coadministration of MK-0457 and vorinostat results in inactivation and down-regulation of wt and T315I Bcr/Abl kinases. (A) K562 and LAMA84 cells were incubated with 5 to 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours (K562) or 24 hours (LAMA84). (B) Ba/F3 cells with wt or T315I Bcr/Abl were treated with 0.1 to 0.5 μM MK with or without 1.5 μM Vor for 48 hours. (A,B) After drug treatment, cells were lysed, and Western blot analysis was performed to assess expression of total and phosphorylated forms of Bcr/Abl and CrkL. Each lane was loaded with 30 μg protein; blots were stripped and reprobed with antibodies to β-actin to ensure equal loading and transfer. Results are representative of 3 separate experiments. Protein bands of phospho-CrkL were quantified using an imaging system as described in “Western blot.” Values reflect the ratio of integrated densitometric determinations between untreated and drug-treated cells. Veh indicates vehicle. Two additional studies yielded equivalent results.
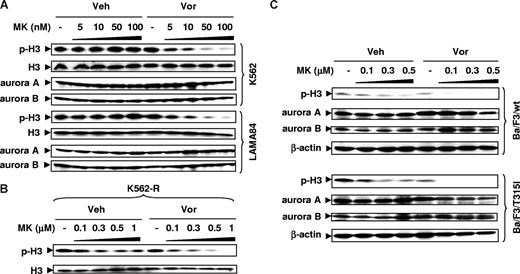
Vorinostat enhances MK-0457–mediated aurora kinase inhibition in IM-sensitive and -resistant cells
The possibility that vorinostat might enhance MK-0457–mediated inhibition of aurora kinases was then investigated. As shown in Figure 5A (top panels), low MK-0457 concentrations in K562 cells (ie, 5-100 nM) had minimal effects on phosphorylation of histone H3 at Ser10, an established marker of aurora kinase activity.9 However, coadministration of vorinostat, which by itself had little effect, strikingly reduced expression of phosphohistone H3. Similar results were observed in LAMA84 cells (Figure 5A bottom panels). Analogous results were also observed in IM-resistant K562-R cells (Figure 5B) as well as in Ba/F3 cells bearing wt or T315I Bcr/Abl, although in these lines, 100 nM MK-0457 by itself slightly reduced phosphohistone H3 expression (Figure 5C). Notably, MK-0457 with or without vorinostat did not diminish expression of aurora A and aurora B.
Vorinostat enhances inhibition of aurora kinases by MK-0457 in IM-sensitive and -resistant cells, including those bearing the T315I mutation. (A) K562 and LAMA84 cells were exposed to 5 to 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours (K562) or 24 hours (LAMA84). (B) IM-resistant K562-R cells were treated with 0.1 to 1 μM MK with or without 1.5 μM Vor for 48 hours. (C) Ba/F3 cells with wt or T315I Bcr/Abl were treated with 0.1 to 0.5 μM MK with or without 1.5 μM Vor for 48 hours. (A-C) After drug treatment, Western blot analysis was performed to monitor expression of total and phosphorylated (Ser 10) histone H3, as well as total aurora A and B. Each lane was loaded with 30 μg protein; blots were stripped and reprobed with antibodies to β-actin to ensure equal loading and transfer. Two additional studies yielded equivalent results.
Vorinostat enhances inhibition of aurora kinases by MK-0457 in IM-sensitive and -resistant cells, including those bearing the T315I mutation. (A) K562 and LAMA84 cells were exposed to 5 to 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours (K562) or 24 hours (LAMA84). (B) IM-resistant K562-R cells were treated with 0.1 to 1 μM MK with or without 1.5 μM Vor for 48 hours. (C) Ba/F3 cells with wt or T315I Bcr/Abl were treated with 0.1 to 0.5 μM MK with or without 1.5 μM Vor for 48 hours. (A-C) After drug treatment, Western blot analysis was performed to monitor expression of total and phosphorylated (Ser 10) histone H3, as well as total aurora A and B. Each lane was loaded with 30 μg protein; blots were stripped and reprobed with antibodies to β-actin to ensure equal loading and transfer. Two additional studies yielded equivalent results.
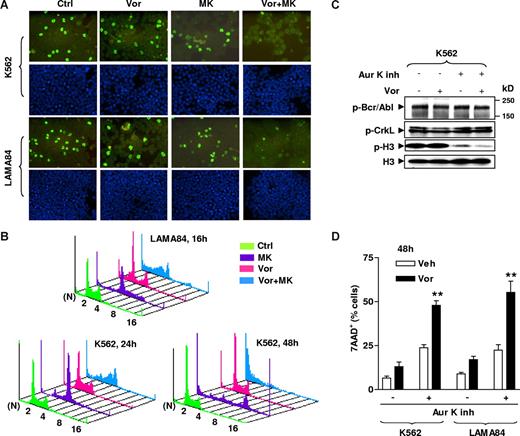
To confirm the results of Western blot analysis (Figure 5), immunocytochemical staining was performed using a FITC-conjugated antibody against phosphohistone H3 (Ser10). As shown in Figure 6A, cotreatment with both vorinostat and MK-0457 for 24 hours (K562; top panels) or 16 hours (LAMA84; bottom panels) led to a marked reduction in the number of cells exhibiting phosphohistone H3 positivity compared with treatment with MK-0457 alone. Cell-cycle analysis was then performed to determine whether such aurora kinase inhibition affected cell ploidy. Consistent with earlier findings,9,15 MK-0457 alone clearly induced an increase in polyploid cells (≥ 4N DNA content; Figure 6B), a characteristic consequence of aurora kinase inhibition.9 Vorinostat by itself had little effect on cell ploidy. Notably, coadministration of vorinostat with MK-0457 resulted in a striking increase in the sub-G1 compartment, accompanied by the marked disappearance of polyploid cells (Figure 6B). Together, these findings argue that vorinostat potentiates MK-0457–mediated aurora kinase inhibition in both IM-sensitive and -resistant CML cells, and raise the possibility that this phenomenon contributes to enhanced killing of polyploid cells by the combination regimen.
Aurora kinase inhibition contributes functionally to the lethality of MK-0457/vorinostat in CML cells. (A) K562 (top) and LAMA84 (bottom) cells were treated with 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 24 hours (K562) or 16 hours (LAMA84), after which cytospin slides were prepared and stained with FITC-conjugated antibodies to phosphorylated (Ser 10) histone H3 (top panels) as described in “Immunocytochemistry.” Bottom panels exhibit DAPI staining. Images were captured with an Olympus BX40 microscope at 20×/0.50 (Olympus America, Center Valley, PA) and a CE digital camera (Alpha Innotech, San Leandro, CA) with RS Image software version 1.7.3. (Roper Scientific Photometrics, Tucson, AZ). (B) LAMA84 and K562 cells were incubated with 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 16 hours (LAMA84) or 24 and 48 hours (K562), after which cells were stained with PI and DNA content was analyzed by flow cytometry. N indicates number of ploidy. (C) K562 cells were exposed to 10 μM of a selective aurora kinase A and B inhibitor (Aur K inh) in the absence or the presence of Vor (2 μM) for 48 hours, after which cells were lysed and subjected to Western blot to monitor expression of phospho-Bcr/Abl and phospho-CrkL, as well as levels of total and phosphorylated histone H3. Each lane was loaded with 30 μg protein. (D) K562 and LAMA84 cells were treated with 10 μM Aur K inh with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours, after which the percentage of 7AAD+ cells were determined by flow cytometry. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate. Asterisks indicate significantly greater than the value for treatment of cells with Aur K inh alone at same concentrations (**P < .01). The results of a representative experiment are shown (A-C); 2 additional studies yielded equivalent results.
Aurora kinase inhibition contributes functionally to the lethality of MK-0457/vorinostat in CML cells. (A) K562 (top) and LAMA84 (bottom) cells were treated with 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 24 hours (K562) or 16 hours (LAMA84), after which cytospin slides were prepared and stained with FITC-conjugated antibodies to phosphorylated (Ser 10) histone H3 (top panels) as described in “Immunocytochemistry.” Bottom panels exhibit DAPI staining. Images were captured with an Olympus BX40 microscope at 20×/0.50 (Olympus America, Center Valley, PA) and a CE digital camera (Alpha Innotech, San Leandro, CA) with RS Image software version 1.7.3. (Roper Scientific Photometrics, Tucson, AZ). (B) LAMA84 and K562 cells were incubated with 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 16 hours (LAMA84) or 24 and 48 hours (K562), after which cells were stained with PI and DNA content was analyzed by flow cytometry. N indicates number of ploidy. (C) K562 cells were exposed to 10 μM of a selective aurora kinase A and B inhibitor (Aur K inh) in the absence or the presence of Vor (2 μM) for 48 hours, after which cells were lysed and subjected to Western blot to monitor expression of phospho-Bcr/Abl and phospho-CrkL, as well as levels of total and phosphorylated histone H3. Each lane was loaded with 30 μg protein. (D) K562 and LAMA84 cells were treated with 10 μM Aur K inh with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours, after which the percentage of 7AAD+ cells were determined by flow cytometry. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate. Asterisks indicate significantly greater than the value for treatment of cells with Aur K inh alone at same concentrations (**P < .01). The results of a representative experiment are shown (A-C); 2 additional studies yielded equivalent results.
Additional studies were performed to determine whether vorinostat could enhance the lethality of other aurora kinase inhibitors. To this end, a selective inhibitor of aurora kinase A and B (aurora kinase inhibitor II)49 was used. Exposure to 10 μM of this agent resulted in a clear reduction in expression of phosphohistone H3 in K562 (Figure 6C) and LAMA84 cells (data not shown). However, coadministration of a minimally toxic concentration of vorinostat led to a further reduction in phosphohistone H3 expression without changes in phospho-Bcr/Abl or phospho-CrkL expression (Figure 6C). Vorinostat also significantly increased aurora kinase inhibitor II–induced cell death in K562 and LAMA84 cells (Figure 6D), consistent with results involving MK-0457. Because vorinostat also potentiated MK-0457 lethality in K562-R cells exhibiting loss of Bcr/Abl (Figure 5B) as well as Ba/F3 cells untransfected with bcr/abl (Figure S2), such findings suggest that interactions between these agents may also involve Bcr/Abl-independent mechanisms.
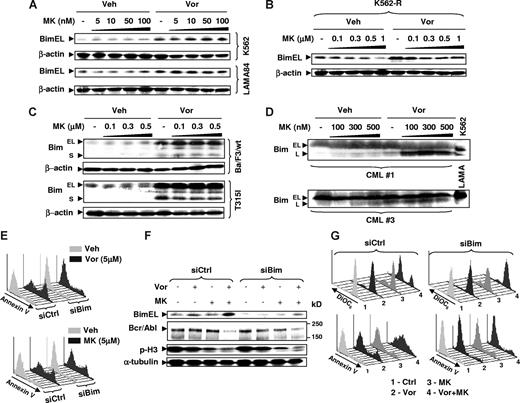
Up-regulation of Bim by vorinostat plays a significant role in potentiation of MK-0457 lethality
In view of accumulating evidence implicating induction of Bim in HDACI lethality,50 the role of this proapoptotic protein in vorinostat/MK-0457 interactions was investigated. While MK-0457 by itself had little effect on Bim expression, exposure to vorinostat, with or without MK-0457, resulted in a pronounced increase in expression of Bim (particularly the BimEL isoform) in K562 and LAMA84 cells (Figure 7A), as well as in IM-resistant K562-R cells (Figure 7B). Interestingly, in both wt and T315I Bcr/Abl Ba/F3 cells, vorinostat also robustly induced expression of the BimS isoform (Figure 7C), while basal or vorinostat-induced expression of this isoform was not detectible in human CML cells (data not shown). Notably, exposure of primary CML cells to vorinostat induced a clear increase in expression of the BimL isoform but not that of BimEL (Figure 7D). The results suggest that the specific Bim isoform induced by vorinostat is likely cell-type specific.
Induction of Bim by vorinostat plays a functional role in interactions between vorinostat and MK-0457. (A) K562 (top) and LAMA84 (bottom) cells were treated with 5 to 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours (K562) or 24 hours (LAMA84). (B) IM-resistant K562-R cells were treated with 0.1 to 1 μM MK with or without 1.5 μM Vor for 48 hours. (C) Ba/F3 cells with wt or T315I Bcr/Abl were treated with 0.1 to 0.5 μM MK with or without 1.5 μM Vor for 48 hours. (D) MNCs from bone marrow samples obtained from CML patients 1 and 3 were exposed to 100 to 500 nM MK with or without 1.5 μM Vor for 48 hours. (A-D) After drug treatment, cells were lysed and subjected to Western blot analysis to monitor expression of Bim. (E) K562 cells were stably transfected with constructs encoding siRNA against Bim (siBim) or nonspecific sequence (siCtrl). Cells were exposed to 5 μM of Vor (top panel) or MK (bottom panel) for 48 hours, after which cells were stained with annexin V–FITC and subjected to flow cytometry. (F) K562 cells transfected with siBim or siCtrl were treated with 100 nM MK with or without 2 μM Vor for 48 hours, after which cells were lysed and Western blot analysis was performed to monitor expression of Bim, Bcr/Abl, and phosphohistone H3. (G) Alternatively, cells were stained with DiOC6 (top panels) or annexin V–FITC (bottom panels), respectively, and subjected to flow cytometry. Each lane was loaded with 30 μg (A-C,F) or 100 μg (D) of protein; blots were stripped and reprobed with β-actin or α-tubulin antibodies to ensure equal loading and transfer of protein. Two additional studies yielded equivalent results. The results of a representative experiment are shown (E,G); 2 additional studies yielded equivalent results.
Induction of Bim by vorinostat plays a functional role in interactions between vorinostat and MK-0457. (A) K562 (top) and LAMA84 (bottom) cells were treated with 5 to 100 nM MK with or without Vor (K562: 2 μM; LAMA84: 1.5 μM) for 48 hours (K562) or 24 hours (LAMA84). (B) IM-resistant K562-R cells were treated with 0.1 to 1 μM MK with or without 1.5 μM Vor for 48 hours. (C) Ba/F3 cells with wt or T315I Bcr/Abl were treated with 0.1 to 0.5 μM MK with or without 1.5 μM Vor for 48 hours. (D) MNCs from bone marrow samples obtained from CML patients 1 and 3 were exposed to 100 to 500 nM MK with or without 1.5 μM Vor for 48 hours. (A-D) After drug treatment, cells were lysed and subjected to Western blot analysis to monitor expression of Bim. (E) K562 cells were stably transfected with constructs encoding siRNA against Bim (siBim) or nonspecific sequence (siCtrl). Cells were exposed to 5 μM of Vor (top panel) or MK (bottom panel) for 48 hours, after which cells were stained with annexin V–FITC and subjected to flow cytometry. (F) K562 cells transfected with siBim or siCtrl were treated with 100 nM MK with or without 2 μM Vor for 48 hours, after which cells were lysed and Western blot analysis was performed to monitor expression of Bim, Bcr/Abl, and phosphohistone H3. (G) Alternatively, cells were stained with DiOC6 (top panels) or annexin V–FITC (bottom panels), respectively, and subjected to flow cytometry. Each lane was loaded with 30 μg (A-C,F) or 100 μg (D) of protein; blots were stripped and reprobed with β-actin or α-tubulin antibodies to ensure equal loading and transfer of protein. Two additional studies yielded equivalent results. The results of a representative experiment are shown (E,G); 2 additional studies yielded equivalent results.
Finally, to assess the functional role of Bim induction by vorinostat on potentiation of MK-0457 lethality, K562 cells stably transfected with Bim siRNA were used. Interestingly, these cells were highly resistant to apoptosis induced by a high concentration (eg, 5 μM) of vorinostat but not MK-0457 (Figure 7E). Notably, vorinostat failed to up-regulate Bim expression in K562 cells transfected with Bim siRNA (Figure 7F). Moreover, Bim knockdown by siRNA substantially protected cells from mitochondrial damage and apoptosis by the vorinostat/MK-457 regimen, manifested by loss of Δψm and annexin V positivity, respectively (Figure 7G). In addition, blockade of vorinostat/MK-0457–induced apoptosis by Bim siRNA failed to prevent down-regulation of Bcr/Abl and inhibition of histone H3 phosphorylation by this regimen (Figure 7F), arguing against the possibility that these events are secondary to apoptosis. Together, these findings indicate that induction of Bim by vorinostat plays a significant functional role in synergistic interaction between vorinostat and MK-0457.
Discussion
The dual aurora kinase and Bcr/Abl inhibitor MK-0457 represents a third-generation agent for the treatment of Bcr/Abl+ leukemias that targets various drug-resistant Bcr/Abl mutants, particularly the T315I mutation, which confers resistance to both first-generation (eg, IM) and second-generation (eg, dasatinib and nilotinib) kinase inhibitors.16,19 The present results indicate that vorinostat substantially lowers the threshold for MK-0457 lethality (eg, from micromolar levels to low nanomolar levels) in CML cells, including those highly resistant to IM and Ba/F3 cells expressing T315I Bcr/Abl. In contrast to IM, MK-045 binds to Bcr/Abl in its active configuration, and avoids the innermost cavity of the Abl kinase domain, which may account for MK-0457 activity against the T315I mutation.12 However, relatively high concentrations of MK-0457 (eg, ≥ 5 μM) are required to inhibit Bcr/Abl activity in primary CML cells,12 in Ba/F3 cells bearing the T315I mutant,13 or cells expressing other mutations (eg, V299L) conferring dasatinib resistance.17 In light of these findings, the observations that vorinostat, administered at concentrations lower than those clinically achievable (ie, maximum plasma concentration Cmax ≈ 9 μM and ≈ 2.5 μM for 300 mg/m2 per day administered intravenously and 400 mg/d administered orally, respectively)51 significantly potentiate MK-0457 activity in CML cells expressing either wt or diverse Bcr/Abl mutations takes on added significance. Unfortunately, access to mutational analysis of primary samples from patients with IM-resistant CML was not available to determine whether the T315I mutation was present. Studies focusing on primary cells with documented Bcr/Abl mutations, particularly T315I and related mutations, represent an important area for future investigation.
The present study suggests that vorinostat interacts with MK-0457 at least in part by targeting Bcr/Abl. In all likelihood, this involves 2 separate but related processes—inhibition of Bcr/Abl kinase, manifested by dephosphorylation of Bcr/Abl and its downstream target CrkL, followed by down-regulation of the Bcr/Abl protein. The latter phenomenon is unlikely to stem from caspase-dependent degradation given evidence that prevention of apoptosis by Bim siRNA had no effect on Bcr/Abl down-regulation. Previous studies suggested that HDACIs, including vorinostat, potentiated Bcr/Abl kinase inhibitor (eg, IM and dasatinib) lethality at least in part by triggering Bcr/Abl down-regulation.33,34 Pan-HDACIs such as hydroxamic acids (eg, LAQ824 and LBH589) induce Hsp90 acetylation by inhibiting HDAC6, disrupting its chaperone function for multiple client proteins, including Bcr/Abl, which then become susceptible to proteasomal degradation.30,32,52 On the other hand, the mechanism(s) responsible for enhanced early inactivation of Bcr/Abl by the vorinostat/MK-0457 regimen is less clear. One possibility is that dissociation of Bcr/Abl from Hsp90 may render it more sensitive to kinase inhibitors such as MK-0457. Notably, Bcr/Abl down-regulation was, if anything, more pronounced in cells bearing the T315I mutation compared with those expressing wt Bcr/Abl. Such findings are consistent with evidence that mutant oncoproteins are particularly dependent on chaperone function for their stability.53 Finally, while such a mechanism could theoretically apply to aurora kinases, the failure to detect significant down-regulation of aurora kinase proteins argues against this possibility.
It is likely that mechanism(s) other than or in addition to Bcr/Abl inactivation/down-regulation contributes to vorinostat/MK-0457 synergism. For example, multiple groups have described a Bcr/Abl-independent form of IM resistance characterized by Bcr/Abl down-regulation and a reciprocal Lyn overexpression/activation.38,46,47 Notably, these cells are resistant not only to IM38 and dasatinib,39 but also to MK-0457 administered alone, presumably reflecting a loss of dependence on Bcr/Abl signaling for survival. Furthermore, Lyn has been identified as a survival factor and target in CML.54 While the observation that MK-0457 administered at concentrations of 500 nM and higher clearly diminished phospho-Lyn expression without substantially inducing cell death argues against a primary role for Lyn inactivation in MK-0457 actions, the possibility that this action contributes to vorinostat/MK-0457 lethality cannot be excluded.
The finding that the vorinostat/MK-0457 regimen was active in cells that had lost Bcr/Abl dependence strongly implicates other actions in synergistic interactions. In this context, MK-0457, by inhibiting aurora kinases (eg, aurora A and particularly aurora B, manifested by diminished histone H3 phosphorylation at Ser10),55 results in accumulation of polyploid cells with 4N or more DNA content.9 Cells exhibiting such aberrant mitotic progression are more susceptible to cell death, although the precise mechanism(s) responsible for this phenomenon remain uncertain.15 Analogously, HDACIs have been shown to trigger an aberrant mitosis and resulting apoptosis in neoplastic cells with a defective G2 checkpoint.36,56 Interestingly, it has recently been shown that HDACI-treated mitotic cells display inactivation of the mitotic spindle assembly checkpoint (SAC).57 Moreover, HDACIs also disrupt mitotic regulation by modulating aurora B kinase activity.58 Notably, combined treatment with vorinostat and MK-0457 strikingly reduced histone H3 phosphorylation and promoted apoptosis in CML cells that had undergone endoreduplication. An additional implication of these findings, together with the observations that the vorinostat/MK-0457 regimen potently inhibited histone H3 phosphorylation and induced apoptosis in Bcr/Abl-independent K562 cells as well as in parental Ba/F3 cells, is that this strategy may be effective in Bcr/Abl− leukemias. Efforts to test this hypothesis are currently under way.
HDACIs induce apoptosis through diverse mechanisms, including modulation of the expression of Bcl-2 family proteins.59 Recently, attention has focused on the ability of HDACIs to transcriptionally up-regulate Bim, a BH3-only proapoptotic protein of the Bcl-2 family.50 There is abundant evidence that Bim induction by HDACIs plays an important role in HDACI lethality.23,60-62 The observations that exposure of CML cells to vorinostat markedly induced Bim expression, and that Bim knockdown by siRNA dramatically attenuated vorinostat/MK-0457 lethality, argue that Bim induction plays a significant role in synergism between these agents. This notion is consistent with recent findings implicating Bim in the lethal effects of mitotic spindle disruption in malignant lymphoid cells.63 In this context, CML cells characteristically exhibit low basal Bim expression,64,65 and induction of this protein by HDACIs may render such cells vulnerable to mitotic spindle disruption-associated lethality.
In summary, the present findings indicate that a strategy combining the dual aurora and Bcr/Abl kinase inhibitor MK-0457 with the HDACI vorinostat potently induces apoptosis in CML cells, including those bearing the T315I mutation. They also argue that this phenomenon most likely involves multiple related mechanisms, including inhibition of Bcr/Abl signaling, disruption of the mitotic machinery, and induction of Bim. This approach builds upon earlier evidence supporting the concept of simultaneously inhibiting and down-regulating Bcr/Abl as an anti-CML strategy,33,34 and extends it to include a novel agent active against gatekeeper mutations like T315I. An additional advantage of this approach is that cooperative disruption of mitotic progression by aurora kinase inhibitors like MK-04579 and HDACIs57,58 may operate independently of Bcr/Abl inhibition, and could permit extension of this strategy to Bcr/Abl− leukemias. Finally, it is of interest that the vorinostat/MK-0457 regimen was lethal toward primary CML cells, but relatively sparing to normal hematopoietic cells, although such preliminary evidence of in vitro selectivity needs further in vivo validation. Accordingly, studies designed to address these and related questions are currently in progress.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA 93738, and CA 100866 from the National Cancer Institute; award 6045-03 from the Leukemia & Lymphoma Society of America, White Plains, NY; an award from the V Foundation, Cary, NC; and an award from the Department of Defense, Fort Detrick, MD.
National Institutes of Health
Authorship
Contribution: Y.D. designed and performed the research, analyzed the data, and wrote the paper; S.C. designed and performed the research, and analyzed the data; C.A.V. performed the research; X-Y.P. and T.K.N. helped to perform the research; P.D. helped to design the research; and S.G. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University Health Sciences Center, Rm 234, Goodwin Research Laboratories, 401 College St, Richmond, VA 23298; e-mail: stgrant@vcu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal