Abstract
Cytokines are hypothesized to play a central role in the pathophysiology of IgG-mediated hemolytic transfusion reactions (HTRs), and deeper understanding is required for improving therapy for these events. After establishing well-defined mouse models of HTRs, we tested whether cytokines were involved. Red blood cells (RBCs) from human glycophorin A transgenic (hGPA-Tg) or wild-type (WT) mice were transfused into non-Tg recipients passively immunized with monoclonal antibodies (Mabs). Only transfusions of incompatible RBCs induced IgG-mediated HTRs, exemplified by rapid clearance and hemoglobinuria. Very high plasma levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6), and lower levels of tumor necrosis factor-α (TNF-α), were induced after incompatible transfusion. No significant changes in IL-10, IL-12, or interferon-γ (IFN-γ) levels were observed. The proinflammatory cytokines elaborated in this in vivo mouse model are also implicated in the systemic inflammatory response syndrome (SIRS) and confirm the hypothesis that cytokine storm occurs as a result of HTRs.
Introduction
Immune-mediated hemolysis is a serious complication of blood transfusion.1 Hemolytic transfusion reactions (HTRs) vary in severity from clinically inapparent to systemic inflammatory response syndrome (SIRS), multiorgan failure, and death.2 A leading hypothesis regarding the pathophysiology of IgG-mediated HTRs, in which complement is thought to play a minor role, implicates “cytokine storm.”3
Phagocytosis of IgG-coated red blood cells (RBCs) in vitro induces cytokine secretion, which may cause the clinical symptoms of HTRs.3 Human case reports also suggest that cytokine storm in other settings produces SIRS and multiorgan failure.4 In addition, cytokines were implicated in one human IgG-mediated HTR5 and in patients with autoimmune thrombocytopenic purpura (AITP), who develop hemolysis after receiving anti-Rh(D).6
Although animal HTR models exist,1,7,8 the role of cytokine storm has not been evaluated. We used a murine HTR model involving passive immunization with IgG1 antibodies, because it is well characterized8 and allows for a more controlled experimental design than the use of active immunization. Thus, in our murine HTR models, passive immunization with anti-human glycophorin A (hGPA) monoclonal antibodies (Mabs) led to rapid, dose-dependent clearance of transfused, incompatible hGPA-transgenic (Tg) RBCs.8 IgG1-mediated clearance was markedly impaired in FcγR knockout (KO) mice, but only moderately inhibited in C3 KO mice, suggesting that activating Fcγ receptors are dominant in this process.8 The current study uses this model to test the hypothesis that HTRs lead to cytokine storm.
Methods
Mice
Wild-type (WT) C57BL/6, C3 KO, and FVB/NJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). hGPA-Tg mice were maintained as described.8 Mice were used at 8 to 12 weeks of age.
Procedures were approved by the Institutional Animal Care and Use Committees at Columbia University Medical Center and at Emory University School of Medicine. No human subjects were used in this study.
Antibodies
IgG1 anti-hGPA (6A7 and 10F7)9 and anti-HEL10 Mabs, purified by protein A chromatography (Bio X Cell, West Lebanon, NH), were quality controlled and confirmed to lack lipopolysaccharide (LPS).8 WT C57BL/6 mice were passively immunized by tail-vein injection with phosphate-buffered saline (PBS), 100 μg 6A7, 2 mg 10F7, or 2 mg anti-HEL before transfusion.
Transfusion of fluorescently labeled RBCs
WT FVB/NJ and hGPA-Tg mice were anesthetized and exsanguinated by cardiac puncture. Washed, buffy coat–depleted RBCs were labeled with chloromethylbenzamido 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiO) per manufacturer's instructions (Invitrogen, Carlsbad, CA). Control, WT C57BL/6 RBCs were labeled with 3,3′-dihexadecyloxacarbocyanine perchlorate (DiO), mixed 1:3 with DiI-labeled RBCs, and brought to a 40% hematocrit with LPS-free PBS. Each mouse was transfused with a 500 μL mixture of either (1) DiI-labeled WT FVB/NJ and DiO-labeled WT C57BL/6 RBCs (compatible transfusion), or (2) DiI-labeled hGPA-Tg and DiO-labeled WT C57BL/6 RBCs (incompatible transfusion).
RBC survival
At defined intervals posttransfusion, mice were anesthetized and 25 μL of retroorbital plexus blood was obtained. To determine percent survival of transfused RBCs, the ratio of DiI-labeled to DiO-labeled RBCs in passively immunized mice was compared with the ratio of DiI-labeled to DiO-labeled RBCs in nonimmunized control mice.10 When possible, urine was collected at necropsy at the final, 20 hour time point. Hemoglobinuria was detected using a PowerWave XS (BioTek, Winooski, VT) spectrophotometer.
Cytokine measurements
Cytokines (interleukin [IL]-6, IL-10, monocyte chemoattractant protein-1 [MCP-1], interferon [IFN]-γ, tumor necrosis factor [TNF]-α, and IL12-p70) were quantified using the Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences, San Diego, CA). Plasma, obtained by retroorbital phlebotomy at various time points (2 hours, 7-9 hours, 17-21 hours) after HTR, was analyzed at a 1:2 and/or 1:10 dilution. Flow cytometry data, acquired with a FACSCan flow cytometer (BD Biosciences),11 was analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistical analysis
One-way ANOVA was used to determine significant differences in cytokine levels and RBC survival using Prism (Graphpad Software; San Diego, CA). A P value of less than .05 was considered significant.
Results and discussion
Transfused incompatible RBCs are rapidly cleared in passively immunized mice
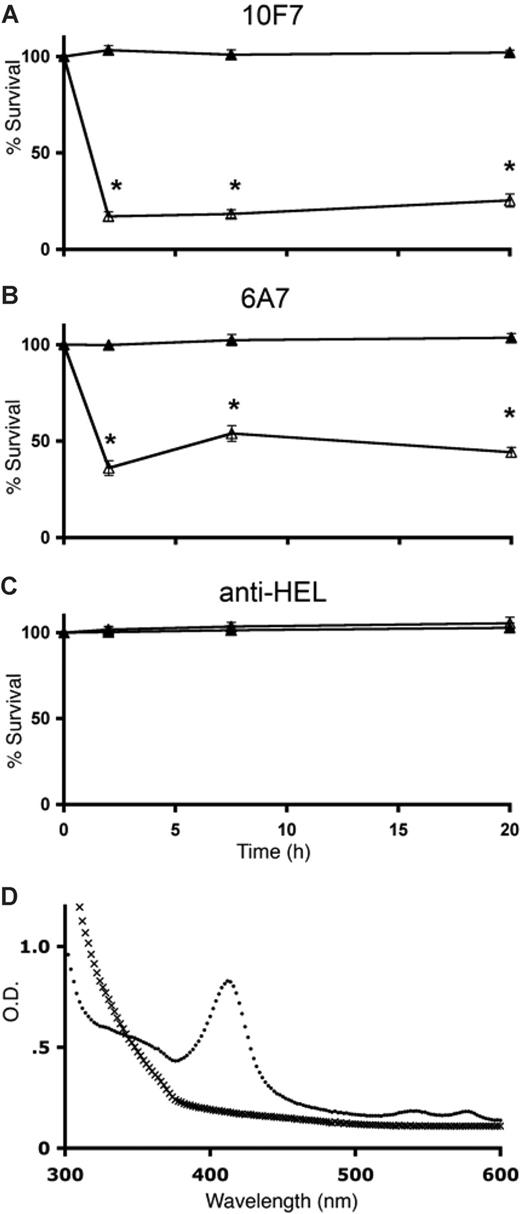
Transfused, incompatible hGPA-Tg RBCs were cleared within 2 hours by mice passively immunized with anti-hGPA Mabs (Figure 1A,B). In contrast, transfused WT FVB/NJ RBCs circulated normally (Figure 1A,B). Passive immunization with an irrelevant, isotype-matched control Mab did not affect RBC survival (Figure 1C). Hemoglobinuria, detected by a prominent Soret band, was found in 3 of 4 mice receiving incompatible transfusions, but not in any mice receiving compatible RBCs (n = 9; Figure 1D).
IgG-mediated clearance of transfused incompatible hGPA-Tg RBCs. WT C57BL/6 mice were passively immunized with 2 mg 10F7 anti-hGPA IgG1 Mab (A), 100 μg 6A7 anti-hGPA IgG1 Mab (B), or 2 mg anti-HEL IgG1 Mab (C). Mice were then transfused with DiO-labeled WT C57BL/6 RBCs (as an internal negative control) and with either DiI-labeled incompatible hGPA-Tg RBCs (△) or DiI-labeled compatible WT FVB/NJ RBCs (▲). RBC survival, as percentage of PBS control (ie, nonimmunized) mice, was quantified by flow cytometry at the indicated time points. Five mice were evaluated at each time point; the means plus or minus 1 SD are provided. The results shown are from one representative experiment (from a total of 4 replicates). *P < .05. Urine was obtained at the 20-hour time point from 4 mice receiving incompatible hGPA-Tg RBC transfusions (●) and 9 mice receiving compatible WT FVB/NJ RBC transfusions (x). Representative spectra from one mouse from each group are shown (D).
IgG-mediated clearance of transfused incompatible hGPA-Tg RBCs. WT C57BL/6 mice were passively immunized with 2 mg 10F7 anti-hGPA IgG1 Mab (A), 100 μg 6A7 anti-hGPA IgG1 Mab (B), or 2 mg anti-HEL IgG1 Mab (C). Mice were then transfused with DiO-labeled WT C57BL/6 RBCs (as an internal negative control) and with either DiI-labeled incompatible hGPA-Tg RBCs (△) or DiI-labeled compatible WT FVB/NJ RBCs (▲). RBC survival, as percentage of PBS control (ie, nonimmunized) mice, was quantified by flow cytometry at the indicated time points. Five mice were evaluated at each time point; the means plus or minus 1 SD are provided. The results shown are from one representative experiment (from a total of 4 replicates). *P < .05. Urine was obtained at the 20-hour time point from 4 mice receiving incompatible hGPA-Tg RBC transfusions (●) and 9 mice receiving compatible WT FVB/NJ RBC transfusions (x). Representative spectra from one mouse from each group are shown (D).
Approximately 20-fold more 10F7 than 6A7 was required to induce similar degrees of clearance in vivo (Figure 1) and phagocytosis by mouse macrophages12 in vitro (E.A.H., S.A.S., S.L.S. unpublished data, November 2007). This may result from differences in specificity, affinity, or effector function. For example, although both are IgG1 Mabs, their glycosylation differs (E.A.H., D.A.S., S.L.S., unpublished data, June 2007), and Fc glycosylation modulates effector function.13 Future studies will evaluate the effect of Fc glycosylation on IgG-mediated HTRs.
Some mouse and human IgG antibodies fix complement via the classical pathway, thereby causing intravascular hemolysis, hemoglobinuria, and renal damage.14,15 For example, we previously showed that clearance of incompatible RBCs by 10F7 was moderately impaired in C3 KO mice.8 In the current study, we evaluated whether complement was required for cytokine storm in IgG-induced HTRs (see below; E.A.H., J.C.Z., S.L.S., unpublished data, April 2008). Interestingly, hemoglobinuria was seen in some C3 KO mice after clearance of incompatible RBCs by 10F7 or 6A7 (not shown). Finding IgG-induced hemoglobinuria in C3-deficient mice is surprising, but may result from the ability of some anti-hGPA antibodies to destabilize RBC phospholipid bilayers, thereby inducing hemolysis.16 In addition, the ability of thrombin to cleave C5 in C3 KO mice may provide another explanation for this phenomenon.17
IgG-mediated HTRs induce cytokine storm
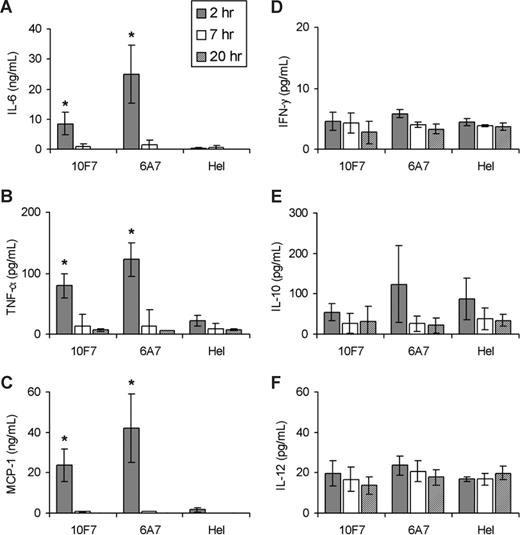
Plasma levels of 6 classical pro- and antiinflammatory cytokines, which prior in vitro studies2 suggested were relevant for IgG-mediated HTRs, were measured at various times after initiation of HTRs. Significantly increased levels of the chemokine MCP-1 and the proinflammatory cytokines IL-6 and TNF-α were detected at 2 hours after incompatible transfusion (Figure 2A-C); these returned to baseline by 7 to 9 hours after transfusion, at the latest. IL-10, IL-12, and IFN-γ levels remained low at all tested time points (Figure 2D-F). In addition, 6A7 and 10F7 induced similar cytokine profiles in C3 KO and WT mice (E.A.H., J.C.Z., S.L.S., unpublished data, April 2008). Therefore, cleavage of the C3 component of complement is not required for cytokine storm. Future studies will determine in detail what role, if any, the complement system plays in producing cytokine storm in this setting.
Plasma cytokine concentrations in C57BL/6 mice at 2, 7, and 20 hours after transfusion. WT C57BL/6 mice were passively immunized with 2 mg 10F7 anti-hGPA IgG1 Mab, 100 μg 6A7 anti-hGPA IgG1 Mab, or 2 mg anti-HEL (irrelevant, isotype-matched control) IgG1 Mab. Mice were transfused with DiO-labeled WT C57BL/6 RBCs (as a negative, internal control) and with either DiI-labeled incompatible hGPA-Tg RBCs (data shown) or compatible WT FVB/NJ RBCs (data not shown because all of these results are very similar to the anti-HEL controls). IL-6 (A), TNF-α (B), MCP-1 (C), IFN-γ (D), IL-10 (E), and IL-12p70 (F) concentrations were measured in plasma, as described in “Cytokine measurements” (note that the scale on the y-axis varies from graph to graph and the absence of bars represents results below the limit of detection of the assay). Five mice were evaluated at each time point and the mean plus or minus 1 SD is provided. Data shown are from 1 representative experiment (from a total of 4 replicates). *P < .05 compared with the anti-HEL controls.
Plasma cytokine concentrations in C57BL/6 mice at 2, 7, and 20 hours after transfusion. WT C57BL/6 mice were passively immunized with 2 mg 10F7 anti-hGPA IgG1 Mab, 100 μg 6A7 anti-hGPA IgG1 Mab, or 2 mg anti-HEL (irrelevant, isotype-matched control) IgG1 Mab. Mice were transfused with DiO-labeled WT C57BL/6 RBCs (as a negative, internal control) and with either DiI-labeled incompatible hGPA-Tg RBCs (data shown) or compatible WT FVB/NJ RBCs (data not shown because all of these results are very similar to the anti-HEL controls). IL-6 (A), TNF-α (B), MCP-1 (C), IFN-γ (D), IL-10 (E), and IL-12p70 (F) concentrations were measured in plasma, as described in “Cytokine measurements” (note that the scale on the y-axis varies from graph to graph and the absence of bars represents results below the limit of detection of the assay). Five mice were evaluated at each time point and the mean plus or minus 1 SD is provided. Data shown are from 1 representative experiment (from a total of 4 replicates). *P < .05 compared with the anti-HEL controls.
Qualitatively consistent with these novel in vivo results, previous in vitro IgG-mediated HTR models identified high levels of MCP-1 and low levels of IL-6 and TNF-α.18 MCP-1, important for leukocyte recruitment, activation, and phagocytosis, is secreted by macrophages, lymphocytes, platelets, and endothelial cells.19 IL-6 and TNF-α induce fever, the acute-phase response, and activation of innate and adaptive immunity.3 Both were elevated in an isolated human HTR case,5 and IL-6 was implicated in human febrile nonhemolytic transfusion reactions.20 AITP patients receiving anti-Rh(D) can have elevated IL-6, TNF-α, IL-10, and MCP-1 by 2 hours after treatment.6
In mouse models of IgG-mediated autoimmune hemolytic anemia, RBCs are predominantly cleared by Kupffer cells,21 which also release MCP-1 and IL-6 in trauma-hemorrhage models.19 Thus, Kupffer cells probably clear RBCs and produce cytokines in our model; however, other sources are possible and further studies are necessary.
In general, the array of inflammatory cytokines expressed varies with the nature of the inflammatory insult.22 For example, in mice, all 6 cytokines evaluated in the current study are elevated within 2 hours of LPS infusion.23 In addition, symptom severity correlates with cytokine levels.24 Therefore, ongoing studies, which will provide a detailed understanding of the profile and amplitude of cytokines induced by HTRs, will lead to a deeper understanding of the pathophysiology of IgG-mediated HTRs, thereby leading to the rational design of novel therapeutic interventions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Traci Chadwick for outstanding technical assistance.
This work was supported in part by a grant from the National Institutes of Health (R21 HL987906; S.L.S. and J.C.Z.).
National Institutes of Health
Authorship
Contribution: All authors participated in designing and performing the research; E.A.H., S.A.S., J.C.Z., and S.L.S. controlled and analyzed the data; J.J. performed the statistical analysis; E.A.H. and S.L.S. wrote the paper; and all authors critiqued various drafts and checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven L. Spitalnik, Department of Pathology and Cell Biology, 630 West 168th St, College of Physicians and Surgeons of Columbia University, New York, NY 10023; e-mail: ss2479@columbia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal