To the editor:
Mammalian blood coagulation is triggered when factor VIIa binds productively to tissue factor (TF). TF resides on the cell surface in a cryptic configuration that can rapidly transform into an active configuration upon disruption of the cell. We have suggested that the disulfide bond in the membrane proximal domain (Cys186-Cys209) of TF is involved in de-encryption.1 This bond has an –RHStaple configuration, which is a feature of allosteric disulfides.2
Several observations support a mechanism where the bond is broken in the cryptic form of the protein and activation involves formation of the disulfide.1,3-5 For example, the thiol-alkylating agent, methyl methanethiolsulfonate (MMTS), blocks TF activation and the thiol-oxidising agent, HgCl2, promotes activation.
This proposal was recently questioned by Pendurthie et al.6 These investigators used the human breast carcinoma cell line MDA-MB231 to study TF de-encryption. In this letter we compare and contrast TF de-encryption in MDA-MB231 cells with 3 other human monocytic cell types. Myeloid leukemia HL60 cells7 and monocytic U937 cells8 were differentiated with phorbol myristate acetate (PMA), which also triggers TF expression.9 PMA treatment did not change TF expression in monocytic THP1 cells.
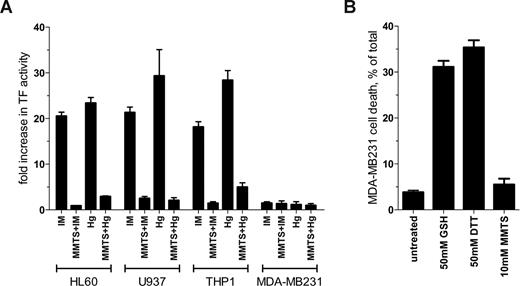
Brief (30 seconds) exposure to ionomycin or HgCl2 resulted in an 18- to 29-fold increase in TF procoagulant activity in HL60, U937, and THP1 cells (Figure 1A). Preincubation of the cells with MMTS reduced TF activation by ionomycin or HgCl2 by at least 83% in all 3 monocytic cells (Figure 1A). In contrast to the results with these monocytic cell lines, TF activity in MDA-MB231 cells was not influenced by ionomycin or HgCl2 treatment (Figure 1A). Preincubation with MMTS also had no effect on TF activity. Procoagulant activity on all cell types was ablated with 10 μg/mL of the anti-TF antibody 9C3 but not 10H10, which blocks TF signaling but not coagulation3 (data not shown).
Comparison of TF de-encryption in different cell types. (A) HL60 and U937 cells were stimulated for 6 hours with 1 μM PMA to induce TF expression. The PMA-treated cell lines and THP1 and MDA-MB231 cells were incubated with 10 μM ionomycin (IM), 100 μM HgCl2 (Hg) or dimethylsulfoxide vehicle control for 30 seconds and TF procoagulant activity measured by clotting time or by analysis of progress curves of factor X activation.1 Both methods of measuring TF procoagulant activity gave equivalent results (data not shown). Cells were also preincubated for 10 minutes with 10 mM (HL60, U937, and MDA-MB231 cells) or 50 mM (THP1 cells) MMTS before the addition of ionomycin or HgCl2. Results are expressed as fold change in TF activity compared with DMSO-treated cells. The bars and errors are the means plus or minus the SE of at least 3 determinations. (B) MDA-MB231 cells at approximately 80% confluence were incubated with 50 mM GSH or DTT or 10 mM MMTS for 15 minutes at 37°C and the cells washed twice with HEPES-buffered saline. Cell viability was determined using CytoTox-One Reagent (Promega, Madison, WI). The bars and errors are the means plus or minus the SE of 3 determinations.
Comparison of TF de-encryption in different cell types. (A) HL60 and U937 cells were stimulated for 6 hours with 1 μM PMA to induce TF expression. The PMA-treated cell lines and THP1 and MDA-MB231 cells were incubated with 10 μM ionomycin (IM), 100 μM HgCl2 (Hg) or dimethylsulfoxide vehicle control for 30 seconds and TF procoagulant activity measured by clotting time or by analysis of progress curves of factor X activation.1 Both methods of measuring TF procoagulant activity gave equivalent results (data not shown). Cells were also preincubated for 10 minutes with 10 mM (HL60, U937, and MDA-MB231 cells) or 50 mM (THP1 cells) MMTS before the addition of ionomycin or HgCl2. Results are expressed as fold change in TF activity compared with DMSO-treated cells. The bars and errors are the means plus or minus the SE of at least 3 determinations. (B) MDA-MB231 cells at approximately 80% confluence were incubated with 50 mM GSH or DTT or 10 mM MMTS for 15 minutes at 37°C and the cells washed twice with HEPES-buffered saline. Cell viability was determined using CytoTox-One Reagent (Promega, Madison, WI). The bars and errors are the means plus or minus the SE of 3 determinations.
Pendurthie et al6 observed an approximately 4-fold increase in TF activity in MDA-MB231 cells when exposed to 100 μM HgCl2 for 15 minutes, 30 times the period of incubation used herein. This relatively modest increase in TF activity is likely due to change in phosphatidylserine exposure, as suggested by the authors. TF de-encryption on HL60 cells could not be explained by this mechanism.1
Pendurthie et al6 also observed an increase in TF activity in MDA-MB231, endothelial, and fibroblast cells after 15 minutes treatment with 20 to 100 mM reduced glutathione (GSH) and dithiothreitol (DTT), and interpreted this result as evidence against the involvement of the Cys186-Cys209 disulfide in TF de-encryption.6 The GSH and DTT effect may be related to the cytotoxicity of these compounds at the concentrations used. GSH or DTT at 50 mM killed 31% and 35% of MDA-MB231 cells, respectively, under the conditions used by Pendurthie et al (Figure 1B). MMTS at 10 mM, on the other hand, had no significant effect on cell viability.
It is apparent from these results that although MDA-MB231 cells express abundant TF, it appears to be constitutively active. This cell line, therefore, is not suitable for the study of TF de-encryption.
Authorship
Contribution: H.P.H.L. performed the experiments and analyzed the data described in the letter. P.J.H. designed the experiments and wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip J. Hogg, UNSW Cancer Research Centre, University of New South Wales, Sydney 2052, Australia; e-mail: p.hogg@unsw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal