To the editor:
Erythropoietin production is regulated by the transcription factor hypoxia-inducible factor (HIF). Erythrocytosis with raised erythropoietin levels due to dysregulated HIF activity is recognized as a consequence of a hypomorphic HIFα E3 ubiquitin ligase (VHL) allele (Chuvash polycythemia), inactivating mutations of the HIFα hydroxylase PHD2 and, very recently, activating mutations of HIF2α.1-3 We report that erythrocytosis in a large kindred originally reported in 19794 is due to an activating HIF2α mutation. Two affected individuals developed severe pulmonary hypertension, a hitherto unrecognized consequence of mutations in the pathway.
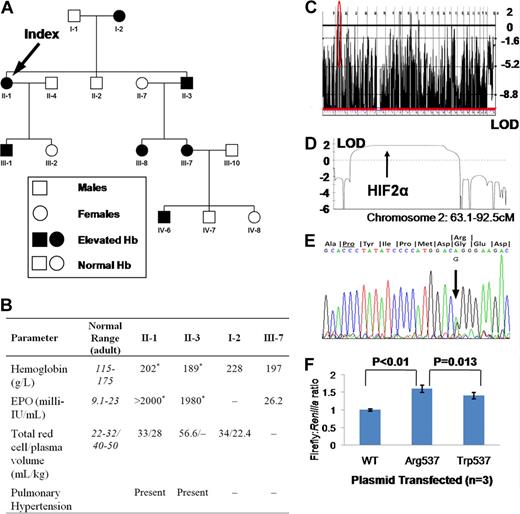
This study was approved by the Hammersmith and Queen Charlotte's and Chelsea Hospitals local research ethics committee. Informed consent was obtained in accordance with the Declaration of Helsinki. At presentation, affected family members (Figure 1A) exhibited erythrocytosis (hemoglobin up to 228 g/L) with elevated total red cell volume and reduced plasma volume (Figure 1B). Other hematological parameters, serum biochemistry, arterial oxygenation, and oxygen-hemoglobin dissociation were normal. Serum erythropoietin was elevated, rising to more than 2000 milli-IU/mL. II-1 and II-3 both had documented pulmonary arterial (PA) hypertension in their sixth decade without any evidence of thromboembolism. A genome-wide single nucleotide polymorphism screen was compatible with linkage to HIF2α (Logarithm or odds ratio [LOD] 1.81, Figure 1C,D) and resequencing disclosed heterozygosity for a G → A substitution at position 2097 (Figure 1E), predicting a glycine-to-arginine change at residue 537 of the protein (HIF2αArg537), present in all affected and no unaffected individuals. Expression of HIF2αArg537 in HepG2 (a hepatoma cell line) cells revealed increased activation of a hypoxia response element-luciferase reporter construct compared with wild-type (Figure 1F). HIF2αArg537 was more active than HIF2αTrp537, consistent with a more severe phenotype in this family.
Data and findings for selected family members. (A) Family tree. (B) Hematological parameters in selected family members. I-2 was diagnosed with “heart failure” at age 62; echocardiography was not available. *Not contemporaneous measurements. – indicates data not available. (C) Genome-wide linkage to autosomal dominant erythrocytosis. (D) Parametric LOD score at region circled in red in panel C. (E) Sequencing trace of showing G/A heterozygosity at base 2097, predicting a change from glycine to arginine at residue 537, 6 codons from critical hydroxyl acceptor proline (underlined). (F) HRE-Firefly:Renilla luciferase activity following cotransfection of a plasmid containing the coding sequence of HIF2α with or without mutations shown. Results shown are means plus or minus SD of 3 independent experiments, each performed in triplicate.
Data and findings for selected family members. (A) Family tree. (B) Hematological parameters in selected family members. I-2 was diagnosed with “heart failure” at age 62; echocardiography was not available. *Not contemporaneous measurements. – indicates data not available. (C) Genome-wide linkage to autosomal dominant erythrocytosis. (D) Parametric LOD score at region circled in red in panel C. (E) Sequencing trace of showing G/A heterozygosity at base 2097, predicting a change from glycine to arginine at residue 537, 6 codons from critical hydroxyl acceptor proline (underlined). (F) HRE-Firefly:Renilla luciferase activity following cotransfection of a plasmid containing the coding sequence of HIF2α with or without mutations shown. Results shown are means plus or minus SD of 3 independent experiments, each performed in triplicate.
These findings confirm that an activating HIF2α mutation dysregulates erythropoietin production in humans. That HIF2α plays a central role in regulating erythropoietin is supported by several observations. First, in hepatoma and neuroblastoma cell lines, erythropoietin mRNA was suppressed by siRNA-mediated silencing of HIF2α, but not HIF1α.5 Second, in VhlR/R mice homozygous for a mutation equivalent to that underlying Chuvash polycythemia in humans, there was erythrocytosis in normoxia associated with stabilization of HIF2α, but not HIF1α.6 Third, reduced oxygen delivery to the kidney in rats activates HIF2α, but not HIF1α in the fibroblasts which produce erythropoietin.7
This report provides the first evidence that genetic HIF activation could cause severe pulmonary hypertension in later life. This is plausible; although clinically important pulmonary hypertension is not evident in Chuvash polycythemia, there is a detectable increase in resting PA pressure and an exaggerated hypoxic pulmonary vasoconstriction response.8,9 There is also experimental support for a critical role of HIF2α in the pulmonary vasculature since hif2α+/− mice are protected from right ventricular hypertrophy in hypoxia.10
These findings have several implications. First, there should be a high index of suspicion for pulmonary hypertension in other kindreds with activation of the HIF pathway. Second, inhibitors of PHD enzymes, which are in late stage clinical trials for treatment of anemia, may cause pulmonary hypertension. Third, it raises the possibility that polymorphic variation in HIF2α contributes to the marked differential susceptibility to erythrocytosis, reduced plasma volume, and pulmonary hypertension in humans at high altitude.
Authorship
The authors are grateful to the family and the family's physicians, and thank Dr C. Mein for performing the SNP genotyping and Mr T. Bhattacharyya for technical assistance.
This work was supported by a UK Medical Research Council Fellowship (D.P.G.) and a British Heart Foundation programme grant (P.H.M.).
Contribution: D.P.G. and P.H.M. designed research, analyzed data, and wrote the paper. D.P.G. and S.K.H. carried out research. E.G.D.T. and C.D.L.R. contributed, looked after the patients, and read and approved the paper.
Conflict-of-interest disclosure: P.H.M. is a scientific founder and director of ReOx, Ltd, which aims to develop PHD inhibitors. The remaining authors declare no competing financial interests.
Correspondence: Patrick Maxwell, Division of Medicine, The Rayne Institute, 5 University Street, London WC1E 6JF, United Kingdom; e-mail: p.maxwell@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal