Abstract
In idiopathic thrombocytopenic purpura (ITP), platelets are destroyed in the spleen, liver, and bone marrow (BM) by autoantibodies and cytotoxic T cells. In a DNA microarray screen of peripheral blood T cells, we found that VLA-4, CX3CR1, and CXCR4, involved in T-cell homing, had increased expression in ITP patients compared with controls. However, we only found increased protein expression of VLA-4 on T cells from peripheral blood by flow cytometry. To address a possible recruitment of T cells into the organs involved in platelet destruction, we analyzed T cells in BM. In BM, T-cell surface expression of VLA-4 and CX3CR1 was increased in ITP patients compared with controls. Furthermore, the number of CD3+ T cells in BM, but not in blood, was increased in ITP patients compared with controls. This finding was confirmed by immunohistochemistry of BM biopsies. The number of regulatory T cells (CD4+/CD25bright) was decreased in the BM of ITP patients, whereas Fas expression was increased. In conclusion, ITP is associated with accumulation and activation of T cells in the BM. Recruitment of T cells into the target organ (eg, BM) is plausible and may be facilitated through increased VLA-4 and CX3CR1 expression. These molecules might serve as new treatment targets in ITP.
Introduction
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disease where platelets are destroyed prematurely, mainly in the spleen, liver, and bone marrow. Besides the well-known autoantibody-mediated destruction of platelets in the reticuloendothelial systems,1-4 several T-cell abnormalities have been identified in ITP. CD4+ T-helper cells from ITP patients have been shown to secrete interleukin 2 (IL-2) upon stimulation with autologous platelets.5,6 Clonal expansion of CD4+ T-helper cells has also been reported,7 and it has been shown that T cells from ITP patients can proliferate in vitro by GPIIb/IIIa stimulation.8,9 We have previously demonstrated that platelets in ITP can be destroyed directly by CD8+ T cell–mediated cytotoxicity,10 and that activation-induced cell death (AICD) of T cells is impaired.11 A CD8+ T cell–mediated platelet lysis in ITP has also been reported by Zhang and coworkers.12 We and others have shown that ITP during active phase is associated with a Th1 cytokine profile,13,14 that is, secretion of interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), whereas remission is associated with an elevated level of transforming growth factor beta 1 (TGF-β1),14,15 that is, a Th3 profile.
Recent studies of immune responses have revealed that cells respond to an activation signal with waves of coordinated gene expression that can be monitored by global expression profiling using DNA microarray technology.16 The components of these responses are probably the key to understanding the specific mechanisms that lead to phenotypic differentiation. In the present study, we analyzed genes and proteins involved in T-cell trafficking, using DNA microarray technology and flow cytometry in chronic ITP patients.
Methods
Subjects
In this study, a total of 26 chronic ITP patients were identified from our roster of ITP patients. All patients were treated at the Hematology Section at Sahlgrenska University Hospital in Gothenburg, Sweden. Besides a thorough history and physical examination, a bone marrow examination on biopsy and/or aspiration material was performed in all patients at presentation, together with sonography or scintigraphy of the spleen and full blood counts. Antibody screen for a rheumatic condition and tests for hepatitis C and HIV were also undertaken in most cases. To be included in the present studies, the patients had to have an unequivocal diagnosis of chronic ITP and had to consent to the proposed studies. The criteria for chronic ITP were (1) isolated thrombocytopenia lasting for more than 6 months and a platelet count less than 100 × 109/L, (2) normal bone marrow examination, (3) normal-sized spleen, and (4) no other cause for the thrombocytopenia. Fresh blood was collected from 10 patients and fresh bone marrow, for flow cytometry, from 6 patients. In 17 patients bone marrow biopsies, obtained at diagnosis and before treatment, were identified retrospectively and analyzed by immunohistochemistry. The patients' characteristics are given in Table 1. Twenty healthy individuals, 14 females and 6 males (aged 36 ± 6.4 [SD] years) were used as controls. All studies were approved by the local ethics committee at University of Gothenburg and informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Characteristics of ITP patients
| ID . | Age, y . | Sex . | ITP duration,* y . | Plc,† ×109/L . | Treatment . | Analysis . |
|---|---|---|---|---|---|---|
| 1 | 32 | F | 3 | 58 | None | FC, ELISA |
| 2 | 33 | M | 0.5 | 47 | Prednisone 20 mg qd, IVIGs | FC, ELISA |
| 3 | 36 | F | 0.5 | 70 | None | FC, ELISA |
| 4 | 53 | M | 45 | 53 | None | FC, ELISA |
| 5 | 45 | M | 4 | 11 | None | FC, ELISA |
| 6 | 44 | M | 26 | 35 | None | FC, ELISA, MA |
| 7 | 59 | M | 31 | 11 | Prednisone 5 mg qd | MA |
| 8 | 53 | F | 2 | 45 | Prednisone 5 mg qd | MA, IHC |
| 9 | 28 | F | 5 | 50 | None | MA |
| 10 | 44 | F | 5 | 33 | Prednisone 10 mg qd | MA |
| 11 | 19 | F | 16 | 5 | None | IHC |
| 12 | 76 | F | 6 | 34 | None | IHC |
| 13 | 64 | F | 5 | 28 | None | IHC |
| 14 | 19 | F | 16 | 0 | None | IHC |
| 15 | 55 | F | 15 | 1 | None | IHC |
| 16 | 61 | M | 15 | 18 | None | IHC |
| 17 | 71 | F | 12 | 1 | None | IHC |
| 18 | 52 | M | 10 | 4 | None | IHC |
| 19 | 67 | M | 15 | 8 | None | IHC |
| 20 | 44 | F | 11 | 1 | None | IHC |
| 21 | 17 | F | 10 | 8 | None | IHC |
| 22 | 73 | F | 10 | 50 | None | IHC |
| 23 | 31 | F | 10 | 79 | None | IHC |
| 24 | 60 | M | 8 | 44 | None | IHC |
| 25 | 22 | F | 10 | 41 | None | IHC |
| 26 | 35 | F | 4 | 47 | None | IHC |
| ID . | Age, y . | Sex . | ITP duration,* y . | Plc,† ×109/L . | Treatment . | Analysis . |
|---|---|---|---|---|---|---|
| 1 | 32 | F | 3 | 58 | None | FC, ELISA |
| 2 | 33 | M | 0.5 | 47 | Prednisone 20 mg qd, IVIGs | FC, ELISA |
| 3 | 36 | F | 0.5 | 70 | None | FC, ELISA |
| 4 | 53 | M | 45 | 53 | None | FC, ELISA |
| 5 | 45 | M | 4 | 11 | None | FC, ELISA |
| 6 | 44 | M | 26 | 35 | None | FC, ELISA, MA |
| 7 | 59 | M | 31 | 11 | Prednisone 5 mg qd | MA |
| 8 | 53 | F | 2 | 45 | Prednisone 5 mg qd | MA, IHC |
| 9 | 28 | F | 5 | 50 | None | MA |
| 10 | 44 | F | 5 | 33 | Prednisone 10 mg qd | MA |
| 11 | 19 | F | 16 | 5 | None | IHC |
| 12 | 76 | F | 6 | 34 | None | IHC |
| 13 | 64 | F | 5 | 28 | None | IHC |
| 14 | 19 | F | 16 | 0 | None | IHC |
| 15 | 55 | F | 15 | 1 | None | IHC |
| 16 | 61 | M | 15 | 18 | None | IHC |
| 17 | 71 | F | 12 | 1 | None | IHC |
| 18 | 52 | M | 10 | 4 | None | IHC |
| 19 | 67 | M | 15 | 8 | None | IHC |
| 20 | 44 | F | 11 | 1 | None | IHC |
| 21 | 17 | F | 10 | 8 | None | IHC |
| 22 | 73 | F | 10 | 50 | None | IHC |
| 23 | 31 | F | 10 | 79 | None | IHC |
| 24 | 60 | M | 8 | 44 | None | IHC |
| 25 | 22 | F | 10 | 41 | None | IHC |
| 26 | 35 | F | 4 | 47 | None | IHC |
Plc indicates platelet count; FC, flow cytometry; ELISA, enzyme-linked immunosorbent assay of human fractalkine; qd, every day; IVIGs, intravenous immunoglobulins; MA, microarray analysis; and IHC, immunohistochemistry of stored bone marrow biopsy collected at initial diagnosis.
Patients 1 through 10: disease duration at time of enrollment in the indicated studies; patients 11 to 26: disease duration at time of retrospective analysis of bone marrow biopsy obtained at disease presentation.
Patients 1 through 10: platelet count at enrollment in the indicated studies; patients 11 to 26: platelet count at disease presentation and bone marrow biopsy, prior to therapy.
Preparation of peripheral blood mononuclear cells and T lymphocytes for microarray analysis
T cells were isolated by immunomagnetic cell sorting, according to the manufacturer's protocol (magnetic-activated cell sorting [MACS]; Miltenyi Biotec, Surrey, United Kingdom). Briefly, peripheral blood mononuclear cells (PBMCs) were prepared from 200 mL heparinized blood from each of 5 chronic ITP patients with active disease (ie, platelet count < 50 × 109 cells/L) and 5 healthy controls by density gradient centrifugation using Ficoll. Two of these ITP patients had glycoprotein IIb (GPIIb)/IIIa antibodies and one had GPIb/IX antibodies detectable using the monoclonal antibody immobilization of platelet antigens (MAIPA) technique17 ; the remaining 2 ITP patients were negative. Monocytes were removed by anti-CD14+ magnetic microbeads, and T cells were isolated from the remaining cells using anti-CD3+ magnetic microbeads. Removing monocytes before the positive selection of T cells increased the purity. The purity of the final T-cell preparation was 93.6% to 96.2%.
Preparation of RNA and hybridization to DNA microarrays
RNA was isolated from the CD3+ T-cell preparations using the method of Chomczynski and Sacchi,18 followed by RNeasy cleanup (Qiagen, Hilden, Germany). The RNA concentration was measured spectrophotometrically with an A260/A280 ratio of 1.8 to 2.0, and the quality was verified by agarose gel electrophoresis. The RNA from the 5 individuals in each group (ie, ITP patients and controls) was pooled in equal amounts. Each pool was analyzed in duplicates as described.10 Briefly, RNA was transcribed into cDNA (Invitrogen, Carlsbad, CA), and biotin-labeled cRNA (Enzo, Farmingdale, NY) was resynthesized. Hybridization to DNA microarrays (Human Genome U95A array version 2; Affymetrix, Santa Clara, CA) and detection of hybridized target cRNA were performed according to the Affymetrix Gene Chip Expression Analysis manual.19 Quality of the cDNA-synthesis and in vitro transcription was assessed by hybridization to Test2-arrays (Affymetrix).
Data analysis
Scanned output files were visually inspected for hybridization artifacts and then analyzed with MicroarraySuite 5.0 software (Affymetrix). To identify regulated genes, the duplicate arrays were scaled to an average intensity of 500 and compared in a crosswise fashion20 using a change call parameter in the MicroarraySuite 5.0 software. These experiments comply with Minimum Information About a Microarray Experiment (MIAME),21 and all files have been deposited in the Gene Expression Omnibus database (GSE574).22
Flow cytometry
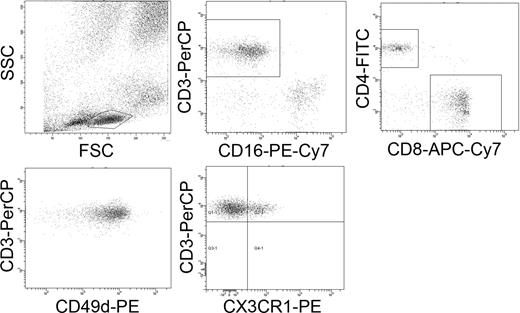
A routine protocol for preparation of PBMCs, from EDTA anticoagulated blood and bone marrow specimens, by Ficoll separation was used. T-cell expression of CX3CR1, VLA-4, CXCR4, Fas, and CD25 was analyzed in a 5-color combination using anti–CD3-peridine-chlorophyll-Cy5.5 (PerCP), anti–CD4-fluorescein isothiocyanate (FITC), anti–CD8-allophycocyanin-Cy7 (APC-Cy7), CD16-phycoerythrin-Cy7 (PE-Cy7), and one of the following antibodies: anti–CX3CR1-PE, anti–CD49d-PE (VLA-4), anti–CD184-PE (CXCR4), anti–CD95-PE (Fas), or anti-CD25-PE. The number of T cells, B cells, and monocyte/macrophages in blood and bone marrow was determined by a 5-color combination using anti–CD3-PerCP, anti–CD19-FITC, anti–CD45-APC-Cy7, anti–CD16-PE-Cy7, and anti–CD14-APC. Naive T cells were identified using antibodies against CD11a-APC and CD45Ra-FITC. All monoclonal antibodies (MoAbs) were from Becton Dickinson Bioscience (San Diego, CA) except the antibody for CX3CR1 that was from Nordic Biosite (Täby, Sweden). The samples were analyzed with a FACSCanto (Becton Dickinson, Mountain View, CA), and data were analyzed using the FACSDiva software (Becton Dickinson). First a gate for lymphocytes was set using forward and side scatter, followed by a gate for CD3 and CD16 identifying T cells as CD3+/CD16−. T-helper cells were identified as CD3+/CD4+ and cytotoxic T cells, as CD3+/CD8+. Figure 1 shows dot plots and gating performed in the 5-color fluorescence-activated cell sorting (FACS) analysis.
Five-color flow cytometry. Representative dot plots showing identification of T cells, T-helper cells, cytotoxic T cells, and the expression of VLA-4 (CD49d; MFI) and CX3CR1 (percentage positive cells).
Five-color flow cytometry. Representative dot plots showing identification of T cells, T-helper cells, cytotoxic T cells, and the expression of VLA-4 (CD49d; MFI) and CX3CR1 (percentage positive cells).
Analysis of fractalkine (CX3CL1) levels in blood and bone marrow plasma
The levels of fractalkine were analyzed in EDTA-anticoagulated blood and bone marrow plasma obtained from 6 ITP patients and 7 healthy individuals using a commercial available assay for human fractalkine (R&D Systems Europe, Abingdon, United Kingdom).
Immunohistochemistry of bone marrow biopsies
In 17 ITP patients, a high-quality bone marrow biopsy was available from the diagnostic workup at presentation and before start of treatment. All these patients have been prospectively followed for at least 6 months and fulfilled the criteria of chronic ITP. Biopsies obtained from 7 healthy subjects served as controls. Sections from the paraffin-embedded biopsies (5 μm thick) were stained with hematoxylin-eosin and immunostained with antibodies against CD3, CD4, CD8, and CD20 using the Dako Envision System in a Techmate Horizon Autostainer (Glostrup, Denmark). The number of positively stained cell profiles was enumerated in a Nikon Optiphot-2 microscope at a magnification of 720× (Tokyo, Japan). At least 15 fields of vision, corresponding to a specimen area of 1.2 mm2, were examined.
Statistics
Unless otherwise stated, mean values plus or minus SEM are reported. Differences between groups were evaluated using Student t test. A P value of .05 or less was considered significant.
Results
DNA microarray analysis
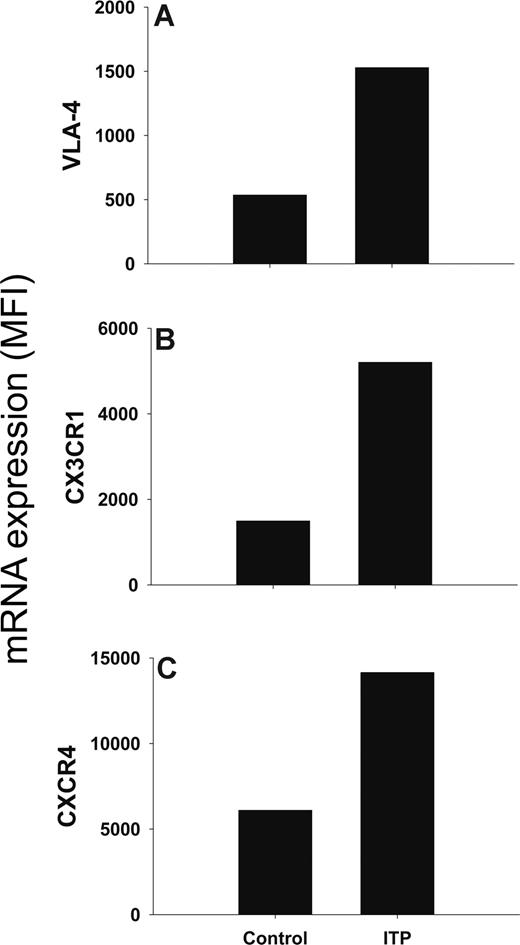
We investigated the expression of molecules involved in T-cell homing in ITP patients and controls by DNA microarray analysis of RNA from peripheral blood T cells. We found that the mRNA expression of the integrin VLA-4, and the chemokine receptors CX3CR1 and CXCR4 was increased in ITP patients compared with controls (Figure 2).
mRNA expression of VLA-4, CX3CR1, and CXCR4 is increased in peripheral blood T cells of ITP patients. The results are presented as mean DNA microarray expression of (A) VLA-4, (B) CX3CR1, and (C) CXCR4. Data are from 2 separate cDNA synthesis and microarray hybridizations from pooled RNA, isolated from peripheral blood T cells of 5 ITP patients with active disease (platelet count < 50 × 109 cells/L) and 5 healthy controls.
mRNA expression of VLA-4, CX3CR1, and CXCR4 is increased in peripheral blood T cells of ITP patients. The results are presented as mean DNA microarray expression of (A) VLA-4, (B) CX3CR1, and (C) CXCR4. Data are from 2 separate cDNA synthesis and microarray hybridizations from pooled RNA, isolated from peripheral blood T cells of 5 ITP patients with active disease (platelet count < 50 × 109 cells/L) and 5 healthy controls.
Flow cytometric analysis of homing receptors, activation markers, and regulatory T cells in blood and bone marrow of ITP patients and controls
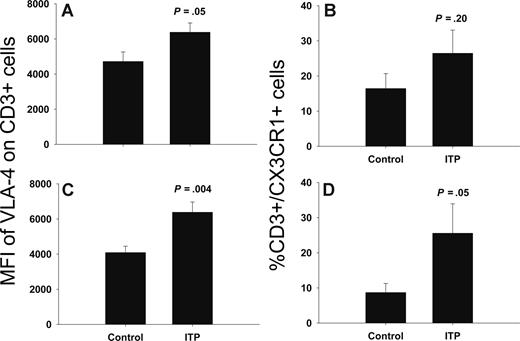
In peripheral blood, there was no statistically significant difference in the percentage of CX3CR1-positive T cells (16.4% ± 4.2% vs 26.5% ± 6.6%, controls and ITP, respectively; P = .20) or mean fluorescence intensity of CXCR4 on T cells (889 ± 183 vs 1034 ± 461, controls and ITP, respectively; P = .78). However, the mean fluorescence intensity of VLA-4 on peripheral blood T cells was increased in ITP compared with controls (Figure 3; 4719 ± 540 vs 6385 ± 525, controls and ITP, respectively; P = .05). Furthermore, in bone marrow, we found an increased percentage of CD3+/CX3CR1+ cells (8.7 ± 2.6% vs 25.6 ± 8.4%, controls and ITP, respectively; P = .05) and increased mean fluorescence intensity of VLA-4 on T cells (4091 ± 359 vs 6387 ± 576, controls and ITP, respectively; P = .004) from ITP patients compared with controls (Figure 3). The surface expression of CXCR4 on T cells was not statistically significant between ITP patients and controls (687 ± 170 vs 880 ± 371, controls and ITP, respectively; P = .65).
Surface expression of VLA-4 and CX3CR1 is increased in bone marrow T cells of ITP patients. Flow cytometric analysis of VLA-4 and CX3CR1 on CD3+ T cells in peripheral blood (A,B) and bone marrow (C,D) from ITP patients (n = 6) and controls (n = 8). Data are presented as means (± SEM).
Surface expression of VLA-4 and CX3CR1 is increased in bone marrow T cells of ITP patients. Flow cytometric analysis of VLA-4 and CX3CR1 on CD3+ T cells in peripheral blood (A,B) and bone marrow (C,D) from ITP patients (n = 6) and controls (n = 8). Data are presented as means (± SEM).
Both the mean fluorescence intensity of VLA-4 on CD3+/CD8+ cells and the percentage of CD3+/CD8+/CX3CR1+ cells were increased in ITP patients compared with controls (VLA-4: controls 4500 ± 342 vs ITP 6762 ± 732, P = .03; CX3CR1: controls 14.7% ± 3.8% vs ITP 35.6% ± 9.3%, P = .04).
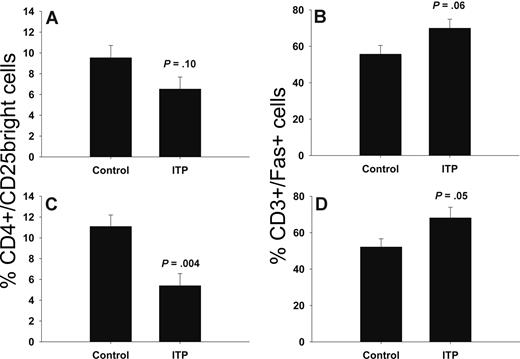
We found that Fas was overexpressed on the surface of T cells from bone marrow (52.2% ± 4.5% vs 68.2% ± 5.8%, controls and ITP, respectively; P = .05) and blood (55.7% ± 4.7% vs 70.0% ± 4.9%, controls and ITP, respectively; borderline significance, P = .06) of ITP patients compared with controls (Figure 4). Furthermore, ITP patients had a reduced number of regulatory T cells (Tregs) in bone marrow (11.1% ± 1.1% vs 5.4% ± 1.2%, controls and ITP, respectively; P = .004), but this was not statistically significant in blood compared with controls (Figure 4; 9.5% ± 1.2% vs 6.5% ± 1.1%, controls and ITP, respectively; P = .10).
Reduced number of CD4+/CD25bright regulatory T cells and increased Fas expression in ITP. Flow cytometric analysis of CD25 on CD3+/CD4+ T-helper cells and Fas on CD3+ T cells in peripheral blood (A,B) and bone marrow (C,D) from ITP patients (n = 6) and controls (n = 8). The results are given as percentage of T cells expressing CD25 and Fas. Data are presented as means (± SEM).
Reduced number of CD4+/CD25bright regulatory T cells and increased Fas expression in ITP. Flow cytometric analysis of CD25 on CD3+/CD4+ T-helper cells and Fas on CD3+ T cells in peripheral blood (A,B) and bone marrow (C,D) from ITP patients (n = 6) and controls (n = 8). The results are given as percentage of T cells expressing CD25 and Fas. Data are presented as means (± SEM).
Analysis of fractalkine levels in ITP patients and controls
We analyzed blood and bone marrow plasma levels of fractalkine in ITP patients and controls. There was no statistically significant difference in blood or bone marrow plasma levels of fractalkine between ITP patients and controls (blood plasma, 10.4 ± 3.6 vs 5.2 ± 1.4 ng/mL, respectively; P = .22; bone marrow plasma, 18.4 ± 8.0 vs 8.9 ± 1.3 ng/mL, respectively; P = .29). However, in both groups, the levels of fractalkine were higher in bone marrow plasma compared with blood plasma (P = .037).
Analysis of T cells, B cells, and monocytes/macrophages in blood and bone marrow of ITP patients and controls
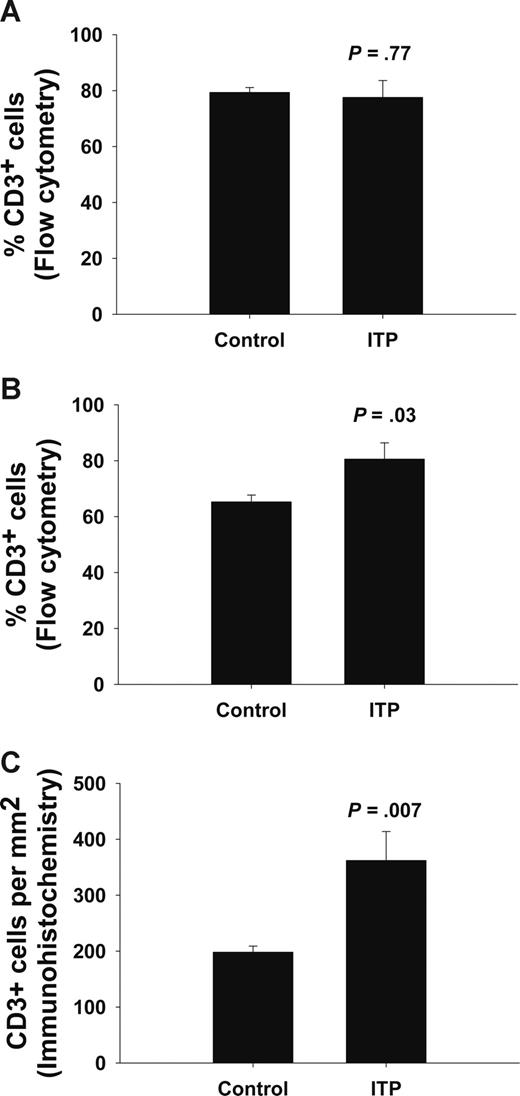
We analyzed the number of T cells, B cells, and monocytes/macrophages in bone marrow by flow cytometry in 6 ITP patients and 6 controls. We found an increased number of T cells (Figure 5; ITP 80.6% ± 5.8%, control 65.3% ± 5.8%; P = .03), but no statistically significant difference in the number of B cells (ITP 5.2% ± 1.4%, control 12.7% ± 3.1%; P = .07) and CD14+ monocytes/macrophages (ITP 3.3% ± 0.94%, control 2.6% ± 0.67%; P = .53) in the bone marrow of ITP patients compared with controls. In blood, however, there was no statistically significant difference in the number of these cell subsets between ITP patients and controls (Figure 5; T cells 77.6% ± 6.0% vs 79.4% ± 1.7%; P = .77; B cells 5.1% ± 1.6% vs 5.5% ± 1.0%; P = .74; monocytes/macrophages 3.5% ± 1.2% vs 4.0% ± 1.2%; P = .78; ITP and controls, respectively). To verify the finding of increased number of T cells in the bone marrow of ITP patients, we performed immunohistochemical staining on retrospectively identified bone marrow biopsies, obtained at the time of initial diagnosis and before treatment, from 17 chronic ITP patients; results were compared with those of bone marrow biopsies from 7 healthy controls. Once more, we found an increased number of T cells (Figure 5; ITP 361 ± 52 vs control 198 ± 11 cells/mm2; P = .007) and no statistically significant difference in the number of B cells (ITP 234 ± 29 vs control 222 ± 30 cells/mm2; P = .80) in the bone marrow sections from ITP patients compared with controls. However, the number of T-helper cells (expressing CD4) or cytotoxic T cells (expressing CD8) were not statistically significant between ITP and control (CD4+: ITP 218 ± 27 vs control 181 ± 30 cells/mm2, P = .46; CD8+: ITP 218 ± 36 vs control 157 ± 21 cells/mm2, P = .30).
Increased number of T cells in bone marrow of ITP patients. Flow cytometric analysis of CD3+ T cells in blood (A) and bone marrow (B) from ITP patients (n = 5) and controls (n = 6). The results are given as percentage of CD3+ cells within the lymphocyte population gated in the forward and side scatter dot plot. Immunohistochemistry (C) and enumeration of CD3+ T cells on bone marrow biopsies obtained from untreated and newly diagnosed ITP patients (n = 17) and controls (n = 7). These results are given as number of CD3+ cells per cross-sectional area (mm2). Data are presented as means plus or minus SEM.
Increased number of T cells in bone marrow of ITP patients. Flow cytometric analysis of CD3+ T cells in blood (A) and bone marrow (B) from ITP patients (n = 5) and controls (n = 6). The results are given as percentage of CD3+ cells within the lymphocyte population gated in the forward and side scatter dot plot. Immunohistochemistry (C) and enumeration of CD3+ T cells on bone marrow biopsies obtained from untreated and newly diagnosed ITP patients (n = 17) and controls (n = 7). These results are given as number of CD3+ cells per cross-sectional area (mm2). Data are presented as means plus or minus SEM.
To investigate the number of naive T cells, we analyzed the surface expression of CD11a and CD45Ra in ITP patients and controls as previously described.23 However, we found no statistically significant difference between ITP patients and controls (data not shown).
Discussion
In chronic ITP, platelets are prematurely destoyed in the spleen, liver, and bone marrow. In the present study, we found an increased number of T cells in bone marrow of ITP patients compared with controls, a finding that emphasizes the importance of T cells in the pathogenesis of the disease. Furthermore, our data also suggest that VLA-4 and CX3CR1 may be of importance in recruiting T cells into the organs where the platelet destruction takes place.
Both the mRNA and protein expression of VLA-4 was increased in peripheral blood T cells from ITP patients compared with controls. Furthermore, protein expression of VLA-4 was also increased in bone marrow T cells in ITP. VLA-4 is known to be expressed on activated T cells24 and promotes trafficking of CD8+ T cells to the bone marrow.25,26 Indeed, in this study we found an increased mean fluorescence intensity of VLA-4 on CD8+ T cells in the bone marrow, suggesting that VLA-4–expressing CD8+ T cells may be recruited into the bone marrow, which is known to participate in platelet destruction. VLA-4 has also been implicated in several diseases, such as multiple sclerosis, Crohn disease, and atherosclerosis. In these conditions, VLA-4 is vital for T-cell recruitment into target tissues. The importance of this reaction is further illustrated by the favorable effect of natalizumab, a monoclonal anti–VLA-4 IgG4 antibody, in multiple sclerosis and Crohn disease.27,28
In addition to the up-regulation of VLA-4, we also found increased expression of CX3CR1 in peripheral T cells from ITP patients by DNA microarray analysis. The increased T-cell expression of CX3CR1 in ITP was confirmed in bone marrow but not in blood by flow cytometry. CX3CR1 is the specific receptor for the chemokine fractalkine (CX3CL1). Fractalkine exist in 2 forms, a membrane-bound form and a soluble form released by proteolytic cleavage.29 We did not find any significant difference in blood or bone marrow plasma levels of fractalkine between ITP patients and control. However, fractalkine levels were elevated in bone marrow plasma, compared with blood, in both groups. Thus, this suggests that, in ITP, the increased expression of CX3CR1 alone is a sufficient chemokine signal for trafficking of T cells from peripheral blood into the bone marrow. CX3CR1 is expressed on natural killer (NK) cells and CD8+ T cells, and most of them are positive for granzyme B.30 CD8+ T cells in bone marrow are known to be mainly central memory cells (TCM's) giving lifelong specificity for antigens.31 Interestingly, CX3CR1 appears to be the main receptor for homing of CD8+ cells in AIDS.32 The interaction between fractalkine and CX3CR1 on effector lymphocytes in endothelium is enough for adhesion and tissue recruitment.33,34 Thus, our findings indicate that CX3CR1 may contribute to the recruitment of T cells, and especially CD8+ cytotoxic cells, to the effector organs for platelet destruction in ITP. An increased number of CD3+/CD8+/CX3CR1+ cells were also observed in bone marrow of ITP patients compared with controls. Together these data indicate that the CD8+ T cells residing in the bone marrow may participate in the destruction of platelets in ITP by T cell–mediated cytotoxicity.10,12,35
Several studies have demonstrated low levels of regulatory T cells (Tregs) in blood of ITP patients.36,37 However, no studies have been performed on bone marrow specimens from ITP patients. We found a trend toward lower levels in blood and significantly lower levels of Tregs in bone marrow from ITP patients compared with controls. Tregs produce transforming growth factor beta 1 (TGFβ1) that suppresses Th1 response, proliferation, activation, and differentiation of T cells.38 The importance of Treg-derived TGFβ1 in autoimmunity is illustrated by the findings that both complete TGFβ1 deletion39 and T cell–specific deletion of TGFβ1 in mice result in multifocal inflammatory disease.38 Our data support the prevailing theory of ITP as a Th1 disease; a low level of TGFβ1 in ITP up-regulates the Th1 response and promotes the destruction of platelets both by autoantibodies and T cell–mediated cytotoxicity. It can also be hypothesized that this mechanism might contribute to the suppressed megakaryocyte production seen in ITP.40
Fas is a marker of T-cell activation,41 and we have previously shown that Fas mRNA is increased in peripheral blood T cells from ITP patients.10 In this study, we could show that Fas expression was increased on T cells from bone marrow of ITP patients compared with controls by flow cytometry. Thus, bone marrow T cells from ITP patients appear to be more activated than those from controls.
Our ITP patients had an increased number of CD3+ T cells in bone marrow but not in peripheral blood compared with controls. The difference was seen both by flow cytometry in 6 patients and by immunohistochemistry of retrospectively identified bone marrow biopsies from 17 patients and can be accounted for by an active recruitment of T cells into the bone marrow in ITP. Because all of the ITP patients were untreated at the time of the bone marrow biopsy that was analyzed by immunohistochemistry, we can rule out that the difference in T-cell number was caused by immunosuppressive treatment. Furthermore, we can also rule out that this was caused by an increase in the production of naive T cells in the BM of ITP patients because the percentage of naive T cells was unchanged between ITP patients and controls.
In conclusion, T cells from ITP patients appear to relocate from peripheral blood and accumulate in the bone marrow, possibly due to an increased surface expression of VLA-4 and CX3CR1. In addition, ITP patients have increased number of activated T cells but fewer regulatory T cells in their bone marrow. We propose that VLA-4 and CX3CR1 may be important for the homing of effector T cells to the organs involved in the destruction of platelets in ITP (eg, spleen, liver and bone marrow). However, whether treatment targeting VLA-4 or CX3CR1 has any effect in ITP remains to be shown.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Margareta Jernås, Rahil Hezaveh, and Iréne Andersson for expert technical assistance.
This work was supported by grants from the Swedish Research Council (529-2004-6512; 521-2006-5103, Stockholm, Sweden), The Swedish federal government under the LUA/ALF agreement (Gothenburg, Sweden), the Sahlgrenska University Hospital Foundation (Gothenburg, Sweden), the Foundations of the National Board of Health and Welfare (Stockholm, Sweden), the Åke Wiberg Foundation (Stockholm, Sweden), the Jeansson Foundations (Stockholm, Sweden), the Tore Nilsson Foundation for Medical Research (Stockholm, Sweden), the Magnus Bergvall Foundation (Stockholm, Sweden), and the Wilhelm and Martina Lundgren Science Foundation (Gothenburg, Sweden).
Authorship
Contribution: B.O. and H.W. designed, performed, and analyzed the experiments, collected and reviewed data, and wrote the paper; B.R. performed the quantitative immunohistochemical analysis; L.C. participated in the microarray design, analysis, and interpretation; S.J. participated in the design and interpretation of flow cytometry.
Conflict-of-interest disclosure: B.O. and H.W. have a patent pending regarding treatment with VLA-4 or CX3CR1 antagonists in ITP. The remaining authors declare no competing financial interests.
Correspondence: Bob Olsson, Department of Internal Medicine, Sahlgrenska University Hospital, Vita stråket 15, SE-413 45, Gothenburg, Sweden; e-mail: bob.olsson@medic.gu.se.