Abstract
Complement activation on human platelets is known to cause platelet degranulation and activation. To evaluate how normal platelets escape complement attack in vivo, we studied the fate of murine platelets deficient in 2 membrane complement regulatory proteins using an adoptive transfer model. We show here that deficiency of either decay-accelerating factor (DAF) or complement receptor 1–related gene/protein y (Crry) on murine platelets was inconsequential, whereas DAF and Crry double deficiency led to rapid clearance of platelets from circu-lation in a complement- and macrophage-dependent manner. This finding contrasted with the observation on erythrocytes, where Crry deficiency alone resulted in complement susceptibility. Quantitative flow cytometry revealed that DAF and Crry were expressed at similar levels on platelets, whereas Crry expression was 3 times higher than DAF on erythrocytes. Antibody blocking or gene ablation of the newly identified complement receptor CRIg, but not complement receptor 3 (CR3), rescued DAF/Crry-deficient platelets from complement-dependent elimination. Surprisingly, deficiency of CRIg, CR3, and other known complement receptors failed to prevent Crry-deficient erythrocytes from complement-mediated clearance. These results show a critical but redundant role of DAF and Crry in platelet survival and suggest that complement-opsonized platelets and erythrocytes engage different complement receptors on tissue macrophages in vivo.
Introduction
Complement, a part of the innate immune system, is spontaneously activated by the alternative pathway on susceptible surfaces with inflammatory and cytolytic consequences.1 To prevent complement activation, autologous cells express several complement regulatory proteins on their plasma membrane.2 These proteins, working in conjunction with fluid-phase complement inhibitors, ensure that complement activation does not occur on autologous cells under normal physiologic conditions. In humans, these membrane regulators of complement include decay-accelerating factor (DAF), membrane cofactor protein (MCP), complement receptor 1 (CR1), and CD59. DAF inhibits complement activation by preventing the formation and accelerating the decay of C3 and C5 convertases.3 MCP acts as a cofactor for factor I–mediated degradation of surface-bound C3b and C4b,4 and CD59 works by inhibiting the assembly of the membrane attack complex (MAC).5 CR1 regulates complement activation by displaying both cofactor and decay-accelerating activities.6
Corresponding genes encoding DAF, CD59, and MCP have also been identified in the rodent.2,7 Interestingly, the mouse has 2 DAF (daf-1, daf-2)2,7 and 2 CD59 (cd59a, cd59b) genes,2,7 with daf-1 and cd59a representing the true murine homolog of human DAF and CD59, respectively.7,8 In addition, the mouse MCP gene, unlike its human counterpart, is expressed only in the testis.9 However, a rodent-specific membrane complement regulatory protein referred to as Crry (complement receptor 1–related gene/protein y) was identified.10 Crry has both cofactor and decay-accelerating activities and is broadly expressed in the mouse.11 Given these properties, Crry is considered a functional homolog of human MCP.7
Platelets and other cells of the hematopoietic system are well endowed with membrane complement regulatory proteins.12 Indeed, deficiency of DAF and CD59 on hematopoietic stem cells contributes to the pathogenesis of paroxysmal nocturnal hematoglobinuria, a disease characterized by complement-dependent hemolysis and platelet activation, with anemia and thrombotic attack as the main pathologic manifestations. Several studies have also shown that complement anaphylatoxins C3a and C5a and the membrane attack complex stimulated degranulation and activation of human and animal platelets.13-16 To evaluate the physiologic role of membrane complement regulators on platelets, we studied the fate of murine platelets that are deficient in DAF and Crry with the use of an adoptive transfer model. We describe here that platelets deficient in DAF and Crry, but not DAF or Crry alone, were rapidly eliminated from the circulation in an alternative pathway complement- and nonsplenic macrophage-dependent manner. We further showed that complement-susceptible platelets were removed by the phagocytic receptor CRIg, whereas neither CRIg nor any other known complement receptors appeared to be involved in phagocytosing complement-susceptible erythrocytes. These results show a critical but redundant role of DAF and Crry in protecting platelets from complement attack and suggest that complement-opsonized platelets and erythrocytes engage different complement receptors on tissue macrophages in vivo.
Methods
Mice
The generation and source of daf-1/C3-deficient (DAF−/−/C3−/−), Crry/C3-deficient (Crry−/−/C3−/−), daf-1/CD59a-deficient (DAF−/−/CD59a−/−), daf-1/Crry/C3-deficient (DAF−/−/Crry−/−/C3−/−), factor B-deficient (fB−/−), and CRIg-deficient (CRIg−/−) mice were described previously.17-22 JH-deficient mice (JH−/−, B-cell deficient)23 were kindly provided by Dr R. Eisenberg (University of Pennsylvania, Philadelphia). The following mice were obtained from The Jackson Laboratories (Bar Harbor, ME): C57BL/6 wild-type mice, complement receptor 3 (CR3)–deficient (CD11b−/−) mice, C4 knockout (C4−/−) mice, C5-deficient mice (C5−/−) harboring a natural mutation, FcRγ-chain knockout (FcRγ−/−) mice (deficient in both FcγRI and FcγRIII),24 and C3 knockout (C3−/−) mice (backcrossed to C57BL/6 background for 6 generations and further backcrossed in-house for 5 more generations). Unless specified, male mice aged 8 to 12 weeks were used as recipients in all transfusion experiments. Mice were maintained under specific pathogen-free conditions. All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania.
Antibodies
The following antibodies and reagents were purchased from BD PharMingen (San Diego, CA): purified rat anti–mouse Crry mAb, phycoerythrin (PE)–conjugated streptavidin, PE-conjugated hamster anti–mouse DAF (CD55) mAb, allophycocyanin (APC)–conjugated streptavidin, PE-conjugated mouse anti–mouse IgG1 mAb, purified rat anti–mouse C3a and C5a mAbs, biotinylated rat anti–mouse C3a and C5a mAbs, purified recombinant mouse C3a and C5a, horseradish peroxidase (HRP)–conjugated avidin, and PE-conjugated Armenian hamster anti–mouse CD61 mAb. PE-conjugated mouse anti–rat IgG2a mAb was purchased from Southern Biotechnology Associates (Birmingham, AL). PE-conjugated rat anti–mouse integrin αIIbβ3 mAb was from EMFRET Analytics GmbH & Co KG (Würzburg, Germany). Rat anti–mouse CD11b mAb (M1/70) was from BioLegend (San Diego, CA). Mouse anti–rabbit GAPDH mAb (6C5) was from Santa Cruz Biotechnology Inc (Santa Cruz, CA). HRP-conjugated rabbit anti–mouse IgG fraction of antiserum was from Sigma-Aldrich (St Louis, MO). The murine CD59a mAb (CD59.3)25 was a gift from Dr Paul Morgan (Cardiff University, Cardiff, United Kingdom). Goat polyclonal antibodies against human factor H (cross-reactive with mouse and rat factor H)26 were from Quidel (San Diego, CA). PE-conjugated donkey anti–goat IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). HRP-conjugated rabbit anti–goat IgG was from Bio-Rad (Hercules, CA). Biotinylated goat anti–mouse C3 antibodies were prepared as described.27 CRIg mAb was generated by immunizing CRIg-deficient mice with a CRIg-Fc fusion protein prepared as described.21 Clone 14G8 was selected based on its ability to block binding of C3 proteins and C3-opsonized particles to CRIg (data not shown). Hybridomas for the rat anti–mouse CR1/2 mAb 7G6 was kindly provided by Dr M. Carroll (Harvard Medical School, Boston, MA). Hybridoma for the hamster anti–mouse complement receptor 4 (CR4) mAb N418 was purchased from ATCC (Manassas, VA). Monoclonal antibodies 7G6 and N418 were produced and purified as previously described.28,29
Isolation of murine platelets
Mouse blood was collected from the inferior vena cava with a heparinized syringe and diluted (1:1) with modified Tyrode buffer (137 mM NaCl, 20 mM HEPES, 5.6 mM glucose, 0.1% BSA, 1 mM MgCl2, 2.7 mM KCl, 12 mM NaHCO3, 3.3 mM Na2PO4, pH 7.4).30 Platelet-rich plasma (PRP) was isolated by centrifugation at 200g for 8 minutes. Platelets were collected from PRP by centrifugation at 1200g for 8 minutes, washed, and resuspended with Tyrode buffer. Activation of platelets was assessed by fluorescence-activated cell sorting (FACS) analysis for activated αIIbβ3 integrin expression.31 All platelet manipulations were performed at room temperature (RT).
FACS analysis of DAF, Crry, CD59, and factor H on murine platelets and erythrocytes
Murine platelets were identified by gating platelets in forward and side scattering and by expression of CD61.32 To detect Crry expression, platelets (107 in 50 μL) were stained with purified rat anti–mouse Crry mAb, followed by PE-conjugated mouse anti–rat IgG2a mAb. To detect DAF expression, platelets were stained directly with PE-conjugated anti–mouse DAF (CD55) mAb. To detect CD59a, platelets were stained with a murine CD59a mAb (CD59.3) followed by PE-conjugated rat anti–mouse IgG1 mAb. To detect factor H expression, platelets and erythrocytes (107 in 50 μL) were stained with goat polyclonal anti–human factor H, followed by PE-conjugated donkey anti–goat IgG. All stainings and washings were carried out in FACS buffer (0.1% albumin and sodium azide in phosphate-buffered saline). Incubation with antibody was performed at RT for 1 hour. Samples were analyzed on a FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Data were acquired with the CellQuest software (Becton Dickinson) and analyzed with the FlowJo software (TreeStar, Ashland, OR).
We determined the number of DAF, Crry, and CD59a molecules expressed on mouse platelets by FACS using QuantiBRITE beads (Becton Dickinson) as described previously for erythrocytes.27,33 Briefly, platelets were stained with PE-labeled primary or secondary mAbs with known antibody/PE molecular ratios. The number of DAF, Crry, or CD59a molecules per platelet was estimated based on the platelet mean fluorescence intensity (MFI) and a standard curve of MFI generated by a set of 4 precalibrated PE-labeled fluorescent beads.27,33
Assessment of platelet half-life and C3 deposition in vivo
To determine the survival of transfused platelets in vivo, isolated platelets from donor mice were labeled ex vivo with carboxy fluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) in 1 mL PBS containing 5 μM of CFSE. The labeled platelets were then introduced into various recipient mice by the tail vein. Blood samples were collected 10 minutes after platelet infusion in 1 mL of 0.2 M EDTA-PBS and at various time points thereafter as indicated. Platelets were isolated by centrifugation and analyzed directly by FACS for CFSE staining, and the percentage of CFSE-positive platelets was determined at each time point. For analysis of in vivo C3 deposition on platelets, isolated platelets were stained with biotinylated goat anti–mouse C3 antibodies, followed by APC-conjugated streptavidin. C3 deposition on platelets was determined by FACS and expressed as MFI. Assessment of erythrocyte half-life in vivo was carried out as previously described.18,19,27
In vitro complement deposition assays
Washed platelets and erythrocytes (107 in 100 μL) were incubated with mouse plasma/serum (at 10%, 20%, 40%, and 80%) in modified Tyrode buffer at 37°C for 20 or 60 minutes. After incubation, cells were washed 3 times with PBS and stained with biotinylated goat anti–mouse C3 and APC-conjugated streptavidin. In some experiments they were costained with PE-conjugated rat anti–mouse integrin αIIbβ3 mAb as an activation marker. The extent of C3 deposition and level of αIIbβ3 expression on platelets was determined by FACS.
C3a/C3a-desArg and C5a/C5a-desArg enzyme-linked immunoabsorbent assay
Washed platelets (107 in 100 μL) were incubated with 40% wild-type mouse plasma (in 100 mM EGTA-MgCl2) in modified Tyrode buffer at 37°C for 30 minutes. After incubation, cells were spun down, and the supernatants were assayed for the levels of C3a/C3a-desArg and C5a/C5a-desArg by sandwich enzyme-linked immunoabsorbent assay (ELISA). ELISA plates were coated overnight at 4°C with rat anti–mouse C3a (clone I87-1162) or C5a (clone I52-I486), which also recognized C3a-desArg and C5a-desArg, respectively. After blocking with assay diluents (BD PharMingen) at RT for 1 hour, plasma samples or C3a/C5a standards (150 pg to 300 ng/mL for C3a, 75 pg to 150 ng/mL for C5a in assay diluents) were added to the plates and incubated at RT for 1 hour. Plates were washed and incubated with biotinylated rat anti–mouse C3a (clone I87-419) or C5a (clone I52-278) for 1 hour and then developed with avidin-conjugated HRP.
Retroviral vector-mediated transgenic expression of Crry
A 1.3-kb mouse Crry cDNA was amplified by reverse transcription–polymerase chain reaction (PCR) from C57/BL6 mouse spleen cDNAs with 5′-CTGCA-GGTAAAACGTTGTTTGAGAACGGTG-3′ and 5′-GAATTCCGTGCTGGGCTAGTGGT-3′ as primers. The cDNA was cloned into the PCR 2.1 vector (Invitrogen) and then subcloned at EcoRI sites into MigR1, a bicistronic retroviral vector carrying a green fluorescence protein marker downstream of encephalomyocarditis virus internal ribosomal entry site in tandem with the transgene.34 (MigR1 vector was kindly provided by Dr W.S. Pear, University of Pennsylvania). Production of Crry-encoding retrovirus and transduction of mouse bone marrow stem cells were carried as described previously.27
In vivo macrophage depletion
To deplete macrophages, mice were intravenously injected with clodronate-liposomes (2 mg/20 g).35,36 This treatment is known to deplete splenic, hepatic, and bone marrow macrophages within 24 hours.35,36 Liposome-encapsulated PBS was used as a negative control. After 24 hours of clodronate treatment, more than 90% of the macrophages in spleen and bone marrow were shown to be eliminated, as indicated by F4/80 staining by FACS (data not shown). To exclude the possibility that clodronate-liposomes may affect systemic complement activity, sera from the treated mice were analyzed using an in vitro zymosan-induced complement activation assay.27 Neither clodronate-liposomes nor PBS-liposomes were found to affect systemic complement activity (data not shown).
Splenectomy
Splenectomy was performed under Avertin anesthesia. An incision was made on the abdomen over the spleen. The splenic artery was ligated with the nonabsorbable suture Dermalon (Tyco Healthcare, Norwalk, CT). After the connective tissues were cut away, the spleen was removed. The incision was closed with nonabsorbable sutures, and the skin was closed with 7.5-mm MICHEL wound clips (Roboz Surgical Instrument, Gaithersburg, MD). Mice were used as transfusion recipients 2 weeks after surgery.
In vivo anti-CRIg, CR1/2, CR3, and CR4 treatment
To block CRIg function in vivo, 12 mg/kg of rat anti–mouse CRIg mAb was injected subcutaneously daily, starting from one day before the transfusion experiment. To block CR1/2 function in vivo, 200 μg of rat anti–mouse CR1/2 mAb (7G6) was injected intravenously 24 hours before the transfusion experiment.28 To block CR3 function in vivo, 200 μg of rat anti–mouse CD11b mAb (M1/70) was injected intravenously 2 hours before the transfusion experiment.37 To block CR4 function in vivo, 500 μg of hamster anti–mouse CR4 mAb (N418) was injected intraperitoneally 24 hours before the transfusion experiment.29
Western blot analysis
Washed platelets and red blood cell (RBC) ghosts were solubilized in 1% Nonidet P-40. The platelet and RBC proteins (240 μg total protein/lane) along with human and mouse serum (0.5 μL/lane) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and then transferred to a polyvinylidene diflouride membrane (Millipore, Billerica, MA). Western blot analysis was performed as described previously,38 using polyclonal goat anti–human factor H (1:5000) and a murine GAPDH (1:200) mAb.
Results
Expression of DAF, Crry, and CD59a on murine platelets
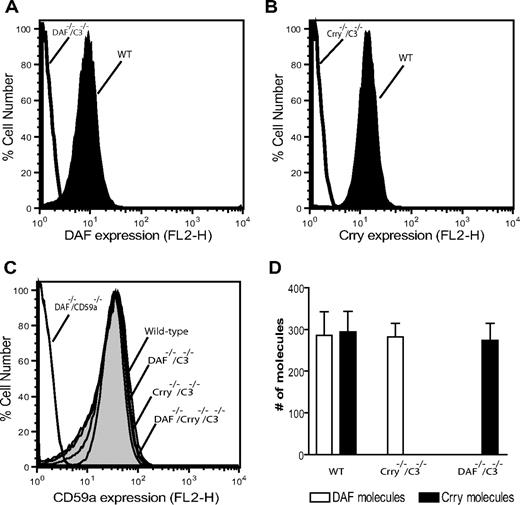
We determined by FACS analysis the expression of DAF, Crry, and CD59a on wild-type (WT) mouse platelets. As negative controls, we used platelets from DAF−/−C3−/−, Crry−/−/C3−/−, and DAF−/−/CD59a−/− mice. Previous work has shown that Crry gene knockout caused embryonic lethality, and viable Crry−/− mice could only be obtained on a C3−/− background.17 As shown in Figure 1A,B, abundant expression of DAF and Crry was detected on WT mouse platelets, but both proteins were absent from DAF−/−C3−/− or Crry−/−/C3−/− mouse platelets, respectively. Similarly, high-level expression of CD59a was detected on WT but not on DAF−/−/CD59a−/− mouse platelets (Figure 1C). Furthermore, deficiency of DAF, Crry, or both on platelets did not cause a compensatory change in CD59a expression (Figure 1C). Using quantitative FACS analysis, we estimated that the number of DAF, Crry, and CD59 expressed on mouse platelets was 286 plus or minus 98, 294 plus or minus 86, and 614 plus or minus 32 molecules/platelet, respectively. No reciprocal compensatory change in the expression of DAF and Crry on Crry−/−/C3−/− or DAF−/−C3−/− platelets was noted (Figure 1D).
Expression of DAF, Crry, and CD59 on murine platelets. FACS analysis of DAF (A) and Crry (B) on wild-type mouse platelets. Platelets from DAF−/−/C3−/− and Crry−/−/C3−/− mice were used as negative controls. (C) FACS analysis of CD59a levels on WT (614 ± 32 molecules/cell) and various knockout mouse platelets. Platelets from DAF−/−/CD59a−/− mice were used as negative controls. (D) Comparison of the number of DAF and Crry molecules on WT, DAF−/−/C3−/−, and Crry−/−/C3−/− mouse platelets. WT and Crry−/−/C3−/− mouse platelets expressed 286 (± 98) and 282 (± 57) DAF molecules/platelet, respectively. WT and DAF−/−/C3−/− mouse platelets expressed 294 (± 86) and 274 (± 71) Crry molecules/platelet, respectively. Error bars represent SEM.
Expression of DAF, Crry, and CD59 on murine platelets. FACS analysis of DAF (A) and Crry (B) on wild-type mouse platelets. Platelets from DAF−/−/C3−/− and Crry−/−/C3−/− mice were used as negative controls. (C) FACS analysis of CD59a levels on WT (614 ± 32 molecules/cell) and various knockout mouse platelets. Platelets from DAF−/−/CD59a−/− mice were used as negative controls. (D) Comparison of the number of DAF and Crry molecules on WT, DAF−/−/C3−/−, and Crry−/−/C3−/− mouse platelets. WT and Crry−/−/C3−/− mouse platelets expressed 286 (± 98) and 282 (± 57) DAF molecules/platelet, respectively. WT and DAF−/−/C3−/− mouse platelets expressed 294 (± 86) and 274 (± 71) Crry molecules/platelet, respectively. Error bars represent SEM.
DAF/Crry-deficient but not DAF-deficient or Crry-deficient mouse platelets had a shortened half-life in vivo
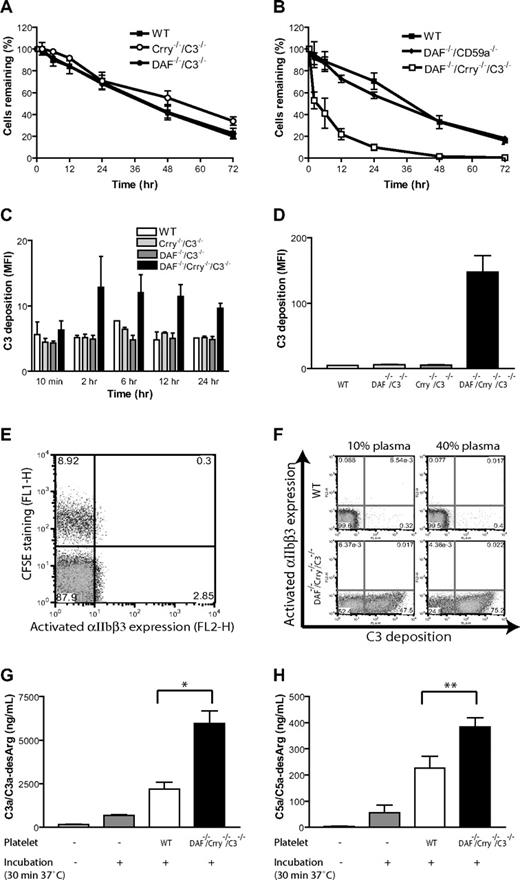
To determine the respective roles of DAF and Crry in protecting platelets from complement attack in vivo, we transferred CFSE-labeled WT, DAF-deficient (from DAF−/−/C3−/− mice), Crry-deficient (from Crry−/−/C3−/− mice), or DAF/Crry-deficient (from DAF−/−/Crry−/−/C3−/− mice) platelets into WT recipients and monitored their fate. We detected no significant effect on platelet half-life by either DAF or Crry deficiency (Figure 2A). However, platelets deficient in both DAF and Crry were more rapidly eliminated from the circulation (Figure 2B). In contrast to DAF/Crry double deficiency, DAF/CD59 double deficiency had no consequence on platelet survival (Figure 2B). Correspondingly, we detected significant C3 deposition only on DAF/Crry-deficient platelets in vivo or when they were incubated with normal mouse serum in vitro (Figure 2C,D). These results suggested that DAF and Crry played a critical but redundant role in protecting platelets from complement attack. Interestingly, we detected no surface expression of activated αIIbβ3 integrin or up-regulation of P-selectin, markers of platelet activation, on DAF/Crry-deficient platelets either in vivo or in vitro (Figure 2E,F; unpublished data), suggesting that C3-opsonized platelets did not display an activated phenotype. Consistent with surface deposition of C3 on DAF/Crry-deficient platelets, we detected significantly increased levels of C3a/C3a-desArg and C5a/C5a-desArg in the plasma sample incubated with DAF/Crry-deficient platelets (Figure 2G,H). We did not detect any increase in plasma C3a/C3a-desArg and C5a/C5a-desArg in vivo after platelet infusion (data not shown), presumably because of rapid pharmacokinetic and/or volume of distribution effect. Addition of CD59a- and CD59b-blocking antibodies to in vitro assays also failed to up-regulate P-selectin or to induce activated αIIbβ3 integrin (data not shown), indicating that lack of platelet activation was not due to CD59-dependent inhibition of MAC formation on the platelet surface.
Complement susceptibility of WT and mutant mouse platelets. (A) Normal survival of transfused WT (■; n = 4), Crry−/−/C3−/− (○; n = 5), and DAF−/−/C3−/− (●; n = 4) mouse platelets in WT recipients; n refers to the number of recipient mice. Platelets were pooled from 2 to 3 donor mice. (B) Accelerated elimination of DAF−/−/Crry−/−/C3−/− (□; n = 4) but not DAF−/−/CD59a−/− (♦; n = 4) mouse platelets compared with WT (■; n = 3) mouse platelets. In panels A and B, platelets were pooled from 2 to 3 donor mice. Blood samples were taken at 10 minutes after labeled platelets were intravenously infused, and the percentage of labeled platelets at this time point was taken as 100% for later reference. (C) FACS analysis of C3 deposition on donor platelets from panel A (WT, Crry−/−/C3−/−, DAF−/−/C3−/−) and panel B (DAF−/−/Crry−/−/C3−/−). MFI indicates mean fluorescence intensity. C3 deposition on DAF−/−/Crry−/−/C3−/− platelets at 12 hours and 24 hours were significantly higher than C3 deposition on any other platelets (P < .05, Student t test). (D) FACS analysis of C3 deposition on WT, DAF−/−/C3−/−, Crry−/−/C3−/−, and DAF−/−/Crry−/−/C3−/− mouse platelets in vitro. Washed platelets were incubated with mouse serum (20% diluted in Tyrode buffer) at 37°C for 30 minutes. Similar results were obtained with 40% serum. Three mice were used in each group. Triplicate assays for each mouse were performed. (E) FACS analysis of activated αIIbβ3 integrin on CFSE-labeled DAF−/−/Crry−/−/C3−/− platelets 2 hours after transfer into WT recipients. (F) FACS analysis of activated αIIbβ3 integrin on WT and DAF−/−/Crry−/−/C3−/− mouse platelets after in vitro incubation (30 minutes, 37°C) with 10% or 40% WT mouse plasma (in 10 mM EGTA-MgCl2). Numbers on plots are percentages of cells in gates. (G) ELISA analysis of C3a/C3a-desArg concentration in mouse plasma after in vitro incubation with WT or DAF−/−/Crry−/−/C3−/− mouse platelets. Intact plasma (not incubated) and plasma incubated in the absence of added platelets were used as controls. All plasma samples, including controls, were diluted to 40% in 10 mM EGTA-MgCl2 before assay. (H) ELISA analysis of C5a/C5a-desArg concentration in mouse plasma after in vitro incubation. Experimental conditions were the same as panel G. Error bars represent mean (± SEM) of triplicate assays. *P < .001, **P < .05, Student t test.
Complement susceptibility of WT and mutant mouse platelets. (A) Normal survival of transfused WT (■; n = 4), Crry−/−/C3−/− (○; n = 5), and DAF−/−/C3−/− (●; n = 4) mouse platelets in WT recipients; n refers to the number of recipient mice. Platelets were pooled from 2 to 3 donor mice. (B) Accelerated elimination of DAF−/−/Crry−/−/C3−/− (□; n = 4) but not DAF−/−/CD59a−/− (♦; n = 4) mouse platelets compared with WT (■; n = 3) mouse platelets. In panels A and B, platelets were pooled from 2 to 3 donor mice. Blood samples were taken at 10 minutes after labeled platelets were intravenously infused, and the percentage of labeled platelets at this time point was taken as 100% for later reference. (C) FACS analysis of C3 deposition on donor platelets from panel A (WT, Crry−/−/C3−/−, DAF−/−/C3−/−) and panel B (DAF−/−/Crry−/−/C3−/−). MFI indicates mean fluorescence intensity. C3 deposition on DAF−/−/Crry−/−/C3−/− platelets at 12 hours and 24 hours were significantly higher than C3 deposition on any other platelets (P < .05, Student t test). (D) FACS analysis of C3 deposition on WT, DAF−/−/C3−/−, Crry−/−/C3−/−, and DAF−/−/Crry−/−/C3−/− mouse platelets in vitro. Washed platelets were incubated with mouse serum (20% diluted in Tyrode buffer) at 37°C for 30 minutes. Similar results were obtained with 40% serum. Three mice were used in each group. Triplicate assays for each mouse were performed. (E) FACS analysis of activated αIIbβ3 integrin on CFSE-labeled DAF−/−/Crry−/−/C3−/− platelets 2 hours after transfer into WT recipients. (F) FACS analysis of activated αIIbβ3 integrin on WT and DAF−/−/Crry−/−/C3−/− mouse platelets after in vitro incubation (30 minutes, 37°C) with 10% or 40% WT mouse plasma (in 10 mM EGTA-MgCl2). Numbers on plots are percentages of cells in gates. (G) ELISA analysis of C3a/C3a-desArg concentration in mouse plasma after in vitro incubation with WT or DAF−/−/Crry−/−/C3−/− mouse platelets. Intact plasma (not incubated) and plasma incubated in the absence of added platelets were used as controls. All plasma samples, including controls, were diluted to 40% in 10 mM EGTA-MgCl2 before assay. (H) ELISA analysis of C5a/C5a-desArg concentration in mouse plasma after in vitro incubation. Experimental conditions were the same as panel G. Error bars represent mean (± SEM) of triplicate assays. *P < .001, **P < .05, Student t test.
Transgenic expression of Crry rescued DAF/Crry-deficient platelets in vivo
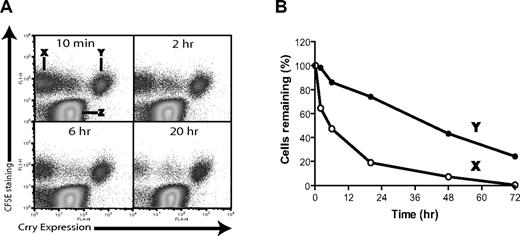
To confirm that the shortened half-life of DAF/Crry-deficient platelets in vivo was indeed due to membrane complement regulator deficiency, we restored Crry expression to the hematopoietic cells of DAF−/−/Crry−/−/C3−/− mice by retroviral vector-mediated Crry gene transduction of bone marrow stem cells. Approximately 25% of the platelets from the transgenic mice became Crry-positive (data not shown) and expressed 6228 (± 290) Crry molecules/platelet. Platelets were harvested from these mice, labeled with CFSE, and then transfused into WT mouse recipients. As shown in Figure 3A, the transfused platelets were CFSE positive (population X and Y) and distinguishable from the endogenous platelets (population Z). We observed that Crry-positive platelets (population Y) survived normally, whereas most Crry transgene-negative platelets (population X) disappeared from the circulation during a 20-hour period (Figure 3A,B). Thus, transgenic expression of Crry on DAF/Crry-deficient platelets rescued them from accelerated elimination, further showing a direct causal relation between shortened platelet half-life and membrane complement regulator deficiency.
Rescue of DAF−/−/Crry−/−/C3−/− mouse platelets by retroviral transgenic expression of Crry. (A) Tracking by FACS of the survival of nontransgenic (population X) and Crry-transgenic (population Y) DAF−/−/Crry−/−/C3−/− platelets in WT recipients. Donor platelets were labeled with CFSE and were distinguishable from endogenous WT platelets (population Z). (B) Plot of elimination kinetics of populations X and Y platelets in panel A. The percentage of platelets remaining at each time point was normalized to the percentage of platelets remaining at 10 minutes after transfusion.
Rescue of DAF−/−/Crry−/−/C3−/− mouse platelets by retroviral transgenic expression of Crry. (A) Tracking by FACS of the survival of nontransgenic (population X) and Crry-transgenic (population Y) DAF−/−/Crry−/−/C3−/− platelets in WT recipients. Donor platelets were labeled with CFSE and were distinguishable from endogenous WT platelets (population Z). (B) Plot of elimination kinetics of populations X and Y platelets in panel A. The percentage of platelets remaining at each time point was normalized to the percentage of platelets remaining at 10 minutes after transfusion.
Elimination of DAF/Crry-deficient platelets required alternative pathway complement
To directly test the hypothesis that DAF/Crry-deficient platelets were eliminated by a complement-dependent mechanism, we monitored the survival of transfused DAF/Crry-deficient platelets in C3−/−, C4−/−, fB−/−, C5−/−, JH−/−, and FcRγ−/− mice. C3−/− mice lacked overall complement activity, whereas C4−/−, fB−/−, and C5−/− mice lacked the classical, alternative, or the terminal pathway of complement, respectively. JH−/− mice were deficient in B cells and produced no antibodies, and FcRγ−/− mice lacked FcRI and FcRIII.24 We found that C3 and fB deficiency but not C4, C5, JH, or FcRγ deficiency rescued DAF/Crry-deficient platelets from accelerated elimination (Figure 4A). Thus, abnormal clearance of mutant mouse platelets from the circulation required participation of the alternative, but not the classical or terminal complement pathway, and it did not involve FcR-mediated phagocytosis. In support of this conclusion, we found that C3 opsonization of DAF/Crry-deficient platelets in vitro was dependent on fB but not on C4 (Figure 4B).
Role of complement, macrophage, and complement receptors in the elimination of DAF/Crry-deficient platelets. (A) DAF−/−/Crry−/−/C3−/− platelets had accelerated elimination in C4−/−, C5−/−, JH−/−, and FcRγ−/− mice but not in fB−/− and C3−/− mice (n = 3 for each type of recipients). (B) FACS analysis of C3 deposition on DAF−/−/Crry−/−/C3−/− mouse platelets (n = 3) after in vitro incubation with 20% WT, C4−/−, fB−/−, or C3−/− mouse serum in Tyrode buffer, 5 mM MgCl2-EGTA, or 10 mM EDTA. Similar results were obtained with 40% serum. Sera were pooled from 3 to 4 mice of each genotype. Error bars represent means ± SEM of triplicate assays. (C) Treatment of recipient mice with clodronate-liposomes (■; n = 4) but not PBS-liposomes (□; n = 4, negative control) prevented accelerated elimination of DAF−/−/Crry−/−/C3−/− platelets. C3−/− mice (●; n = 4) were used as control recipients for the rescue experiment. (D) Splenectomy (SPX) did not prevent DAF−/−/Crry−/−/C3−/− platelets from accelerated elimination. DAF−/−/Crry−/−/C3−/− platelets transfused into splenectomized WT recipients (■; n = 3) had similar half-life to those transfused into sham-operated WT mice (□; n = 3), and both had accelerated elimination compared with WT platelets transfused into splenectomized WT recipients (●; n = 3). (E) Blockade of CR3 in WT mice by anti-CR3 (M1/70) (■; n = 3) or CR3 gene knockout (▴; n = 3) did not rescue DAF−/−/Crry−/−/C3−/− platelets from accelerated elimination. Blockade of CR4 in CR3−/− mice by anti-CR4 (N418) (○; n = 2) and blockade of CR1/2 in WT mice by anti–CR1/2 (7G6) (▾; n = 3) also did not prevent the elimination. WT (□; n = 3) and C3−/− (●; n = 3) were used as control recipients. (F) Blockade of CRIg in WT mice by anti-CRIg (14G8.2) (●; n = 3) or CRIg gene knockout (■; n = 3) prevented DAF−/−/Crry−/−/C3−/− platelets from accelerated elimination. WT (□; n = 3) and C3−/− (○; n = 3) were used as control recipients. All error bars represent mean (± SEM).
Role of complement, macrophage, and complement receptors in the elimination of DAF/Crry-deficient platelets. (A) DAF−/−/Crry−/−/C3−/− platelets had accelerated elimination in C4−/−, C5−/−, JH−/−, and FcRγ−/− mice but not in fB−/− and C3−/− mice (n = 3 for each type of recipients). (B) FACS analysis of C3 deposition on DAF−/−/Crry−/−/C3−/− mouse platelets (n = 3) after in vitro incubation with 20% WT, C4−/−, fB−/−, or C3−/− mouse serum in Tyrode buffer, 5 mM MgCl2-EGTA, or 10 mM EDTA. Similar results were obtained with 40% serum. Sera were pooled from 3 to 4 mice of each genotype. Error bars represent means ± SEM of triplicate assays. (C) Treatment of recipient mice with clodronate-liposomes (■; n = 4) but not PBS-liposomes (□; n = 4, negative control) prevented accelerated elimination of DAF−/−/Crry−/−/C3−/− platelets. C3−/− mice (●; n = 4) were used as control recipients for the rescue experiment. (D) Splenectomy (SPX) did not prevent DAF−/−/Crry−/−/C3−/− platelets from accelerated elimination. DAF−/−/Crry−/−/C3−/− platelets transfused into splenectomized WT recipients (■; n = 3) had similar half-life to those transfused into sham-operated WT mice (□; n = 3), and both had accelerated elimination compared with WT platelets transfused into splenectomized WT recipients (●; n = 3). (E) Blockade of CR3 in WT mice by anti-CR3 (M1/70) (■; n = 3) or CR3 gene knockout (▴; n = 3) did not rescue DAF−/−/Crry−/−/C3−/− platelets from accelerated elimination. Blockade of CR4 in CR3−/− mice by anti-CR4 (N418) (○; n = 2) and blockade of CR1/2 in WT mice by anti–CR1/2 (7G6) (▾; n = 3) also did not prevent the elimination. WT (□; n = 3) and C3−/− (●; n = 3) were used as control recipients. (F) Blockade of CRIg in WT mice by anti-CRIg (14G8.2) (●; n = 3) or CRIg gene knockout (■; n = 3) prevented DAF−/−/Crry−/−/C3−/− platelets from accelerated elimination. WT (□; n = 3) and C3−/− (○; n = 3) were used as control recipients. All error bars represent mean (± SEM).
DAF/Crry-deficient platelets were phagocytosed by nonsplenic macrophages
To determine whether DAF/Crry-deficient platelets were phagocytosed by macrophages after complement opsonization, we depleted macrophages from WT recipients by clodronate-liposome treatment. As previously reported,35,36 macrophages in the spleen and bone marrow were shown to be largely (> 90%) depleted at 24 hours after intravenous injection of clodronate-liposomes (data not shown). Importantly, we found that DAF/Crry-deficient platelets were protected from accelerated elimination when transfused into clodronate-liposome–treated mice, displaying a half-life that was indistinguishable from platelets transfused into C3−/− mice (Figure 4C). In contrast, mice treated with control liposomes rapidly eliminated DAF/Crry-deficient platelets as usual (Figure 4C). This result strongly suggested that DAF/Crry-deficient platelets were phagocytosed by macrophages. To examine whether splenic or other tissue macrophages such as liver Kupffer cells were responsible, we compared the survival of DAF/Crry-deficient platelets transfused into splenectomized WT mice or sham-operated controls. Figure 4D shows that splenectomy did not prevent the rapid elimination of DAF/Crry-deficient platelets. No difference in clearance kinetics was observed between platelets transfused into splenectomized and sham-operated mice, suggesting that they were primarily removed by nonsplenic macrophages.
Phagocytosis of DAF/Crry-deficient platelets was mediated by CRIg
Both CR3 and the recently discovered complement receptor CRIg are capable of mediating phagocytosis of complement-opsonized particles, and pathogens and both are expressed on tissue macrophages.21,39 To determine whether phagocytosis of DAF/Crry-deficient platelets was mediated by these receptors, we transfused DAF/Crry-deficient platelets into mice that lacked functional CR3 or CRIg as a result of antibody blocking or gene deletion.21,22,40,41 As shown in Figure 4E, antibody blocking of CR3 in WT mice or genetic disruption of CR3 (CD11b gene knockout) had no effect on the accelerated elimination of DAF/Crry-deficient platelets. Moreover, antibody blocking of CR1/2 in WT mice or CR4 in CR3−/− mice did not rescue the transfused DAF/Crry-deficient platelets. In contrast, antibody blocking of CRIg in WT mice or genetic inactivation of the CRIg gene completely prevented DAF/Crry-deficient platelets from rapid elimination (Figure 4F). Thus, macrophage CRIg but not CR3, CR1/2, or CR4 mediated phagocytosis of DAF/Crry-deficient platelets.
Complement-opsonized platelets and erythrocytes engage different macrophage receptors
We previously showed that Crry- and DAF/Crry-deficient mouse erythrocytes were susceptible to complement attack in vivo.18,19 To extend the current study and establish the roles of macrophages and CRIg in the clearance of regulator-deficient erythrocytes, we treated recipient mice with clodronate-liposomes or performed splenectomy before erythrocyte transfusion. Figure 5A,B shows that, similar to regulator-deficient platelets, the elimination of DAF/Crry-deficient erythrocytes was also critically dependent on nonsplenic macrophages. Unexpectedly, however, we found that neither CR3 nor CRIg deficiency protected DAF/Crry-deficient erythrocytes from complement-mediated clearance (Figure 5C). Furthermore, antibody blocking of CRIg, CR4, and CR1/2 in CR3−/− mice also failed to rescue DAF/Crry-deficient erythrocytes from accelerated elimination (Figure 5D), suggesting that the failure to prevent complement-dependent phagocytosis of the mutant mouse erythrocytes was not due to redundancy between any of the known complement receptors.
Role of macrophage and complement receptors in the elimination of DAF−/−/Crry−/−/C3−/− erythrocytes. (A) Treatment of recipient mice with clodronate-liposomes (■; n = 4) but not PBS-liposomes (□; n = 4, negative control) prevented accelerated elimination of DAF−/−/Crry−/−/C3−/− erythrocytes. C3−/− mice (●; n = 4) were used as control recipients. (B) Splenectomy (SPX) did not prevent DAF−/−/Crry−/−/C3−/− erythrocytes from accelerated elimination. DAF−/−/Crry−/−/C3−/− erythrocytes transfused into splenectomized WT recipients (■; n = 3) had similar half-life to those transfused into sham-operated WT mice (□; n = 3), and both were rapidly eliminated. WT erythrocytes transfused into splenectomized WT recipients (●; n = 3) remained stable. (C) DAF−/−/Crry−/−/C3−/− mouse erythrocytes were not rescued in CRIg−/− (□; n = 4) or CR3−/− (▴; n = 3) mice. WT (■; n = 3) and C3−/− (●; n = 3) mice were used as control recipients. (D) Neither blockade of CRIg by antibody (16G8.2) in CR3−/− mice (▴; n = 2) nor blockade of CRIg, CR1/2, CR4 by antibody (16G8.2), (7G6), and (N418) all in CR3−/− mice (▵; n = 3) did not rescue DAF−/−/Crry−/−/C3−/− erythrocytes. WT (■; n = 2) and C3−/− (●; n = 2) mice were used as control recipients. All error bars means represent (±SEM).
Role of macrophage and complement receptors in the elimination of DAF−/−/Crry−/−/C3−/− erythrocytes. (A) Treatment of recipient mice with clodronate-liposomes (■; n = 4) but not PBS-liposomes (□; n = 4, negative control) prevented accelerated elimination of DAF−/−/Crry−/−/C3−/− erythrocytes. C3−/− mice (●; n = 4) were used as control recipients. (B) Splenectomy (SPX) did not prevent DAF−/−/Crry−/−/C3−/− erythrocytes from accelerated elimination. DAF−/−/Crry−/−/C3−/− erythrocytes transfused into splenectomized WT recipients (■; n = 3) had similar half-life to those transfused into sham-operated WT mice (□; n = 3), and both were rapidly eliminated. WT erythrocytes transfused into splenectomized WT recipients (●; n = 3) remained stable. (C) DAF−/−/Crry−/−/C3−/− mouse erythrocytes were not rescued in CRIg−/− (□; n = 4) or CR3−/− (▴; n = 3) mice. WT (■; n = 3) and C3−/− (●; n = 3) mice were used as control recipients. (D) Neither blockade of CRIg by antibody (16G8.2) in CR3−/− mice (▴; n = 2) nor blockade of CRIg, CR1/2, CR4 by antibody (16G8.2), (7G6), and (N418) all in CR3−/− mice (▵; n = 3) did not rescue DAF−/−/Crry−/−/C3−/− erythrocytes. WT (■; n = 2) and C3−/− (●; n = 2) mice were used as control recipients. All error bars means represent (±SEM).
DAF/Crry-deficient platelets were more susceptible to alternative pathway complement attack than DAF/Crry-deficient erythrocytes
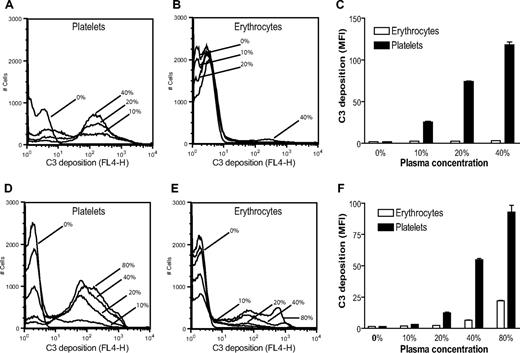
Although both DAF/Crry-deficient platelets and erythrocytes were susceptible to complement-dependent elimination in vivo, we found that DAF/Crry-deficient platelets were significantly more prone to C3 opsonization than DAF/Crry-deficient erythrocytes when exposed to wild-type mouse serum. As shown in Figure 6A-C, after 20 minutes of incubation in 10% to 40% wild-type mouse plasma, DAF/Crry-deficient platelets, but not DAF/Crry-deficient erythrocytes, were heavily opsonized with C3. In contrast, notable C3 deposition on DAF/Crry-deficient erythrocytes was observed only after 60 minutes of incubation in 40% or 80% plasma (Figure 6D-F).
DAF−/−/Crry−/−/C3−/− platelets are more sensitive to alternative pathway complement attack than DAF−/−/Crry−/−/C3−/− erythrocytes. FACS analysis of C3 deposition on DAF−/−/Crry−/−/C3−/− platelets and erythrocytes after 20 minutes (A-C) or 60 minutes (D-F) of incubation with various concentrations of wild-type mouse plasma in 10 mM EGTA-MgCl2. MFI indicates mean fluorescence intensity. Bars in panels C and F represent means (± SEM) of triplicate assays.
DAF−/−/Crry−/−/C3−/− platelets are more sensitive to alternative pathway complement attack than DAF−/−/Crry−/−/C3−/− erythrocytes. FACS analysis of C3 deposition on DAF−/−/Crry−/−/C3−/− platelets and erythrocytes after 20 minutes (A-C) or 60 minutes (D-F) of incubation with various concentrations of wild-type mouse plasma in 10 mM EGTA-MgCl2. MFI indicates mean fluorescence intensity. Bars in panels C and F represent means (± SEM) of triplicate assays.
Apart from DAF and Crry, the plasma protein factor H also confers host cell protection from alternative pathway complement attack,42 and previous work from others has suggested the presence of a membrane form of factor H on murine platelets.26 We wondered wether a similar membrane-associated form of factor H might be present on the mouse erythrocytes and whether different levels of such a protein might have accounted for the differential sensitivity of DAF/Crry-deficient platelets and erythrocytes to alternative pathway complement. Although we detected by FACS analysis cross reactivity with anti–human factor H antibodies both on platelet and erythrocyte surfaces (Figure 7A,B), subsequent Western blot analysis only confirmed the presence of factor H on the mouse platelets (Figure 7C). Thus, membrane-associated factor H was an unlikely determinant for the observed differential sensitivity of DAF/Crry-deficient platelets and erythrocytes to alternative pathway complement.
Factor H is constitutively present on mouse platelets but not erythrocytes. Although FACS analysis detected reactivity to anti–human factor H antibodies on the mouse platelets (A) and erythrocytes (B), Western blot analysis confirmed fH association with platelets but not with erythrocytes (C right). Factor H in human and mouse sera were used as a positive control for antibody reactivity (C left), and GAPDH signal was used as a loading control for platelet and erythrocyte proteins. The positions of molecular weight markers (in kilodaltons) were indicated on the right side of panel C.
Factor H is constitutively present on mouse platelets but not erythrocytes. Although FACS analysis detected reactivity to anti–human factor H antibodies on the mouse platelets (A) and erythrocytes (B), Western blot analysis confirmed fH association with platelets but not with erythrocytes (C right). Factor H in human and mouse sera were used as a positive control for antibody reactivity (C left), and GAPDH signal was used as a loading control for platelet and erythrocyte proteins. The positions of molecular weight markers (in kilodaltons) were indicated on the right side of panel C.
Discussion
We found here that DAF and Crry played an equally important but redundant role in protecting murine platelets from alternative pathway complement-mediated elimination. This observation contrasted with previous findings that Crry played a more critical role than DAF on mouse erythrocytes.18,19 This discrepancy could be explained by the relative levels of DAF and Crry on platelets and erythrocytes. We showed in this study that DAF and Crry were expressed at a similar level on the platelets, whereas Crry level was 3 times higher than DAF on erythrocytes.27 Considering the size differences between the 2 cell types, the relative density of DAF and Crry on the platelet surface was calculated to be much higher than that on erythrocytes. Overall, these data supported the conclusion that when expressed at a sufficiently high level, either DAF or Crry is capable of controlling alternative pathway complement activation on the cell surface.
Previous studies with human and animal platelets have shown that deposition of the membrane attack complex or stimulation with C3a/C5a led to platelet degranulation and activation.13-16 It was notable that, despite increased C3a and C5a release and abundant surface C3 deposition on DAF/Crry-deficient platelets, we observed no P-selectin or activated αIIbβ3 integrin expression on these platelets, suggesting that they did not have an activated phenotype. Neutralization of CD59a and CD59b on DAF/Crry-deficient platelets with blocking mAbs during in vitro complement activation assays moderately increased surface MAC staining (data not shown) but likewise did not induce P-selectin or activated αIIbβ3 integrin expression. It is possible that murine platelets are less sensitive than human platelets to MAC-induced degranulation and activation. Alternatively, a higher number of MAC molecules on the platelet surface are needed to cause platelet activation, a requirement that could not be met in vivo because of CD59 activity or in vitro because of low terminal complement activity in conventionally prepared mouse plasma. Separately, lack of C3a/C5a-stimulated platelet activation may be explained by the absence of C3a and C5a receptors on murine platelets. Consistent with earlier reports in human platelets,43,44 we detected no C5aR or C3aR expression on mouse platelets by FACS (data not shown).
Through the use of gene knockout mice, we established that the accelerated elimination of DAF/Crry-deficient platelets was dependent on the alternative but not the classical pathway of complement. We also showed that the terminal complement system and the FcR pathway were not involved. These results pointed to complement receptor (CR)–mediated phagocytosis as a potential mechanism for their removal from the circulation. Indeed, we detected abundant C3 opsonization of DAF/Crry-deficient platelets and showed that macrophage depletion rescued DAF/Crry-deficient platelets from accelerated elimination. Macrophages from the spleen and liver (Kupffer cells) are known to express several complement receptors, including CR345 and the recently identified CRIg.21 Notably, we found that splenectomy had no effect on the accelerated elimination of DAF/Crry-deficient platelets and erythrocytes, suggesting that the regulator-deficient cells were phagocytosed primarily by nonsplenic macrophages, most likely liver Kupffer cells. This finding seemed to contradict the previous observation that CFSE-labeled Crry-deficient erythrocytes accumulated in the spleen but not in the liver of the recipient mice.19 It is possible that both splenic macrophages and Kupffer cells were involved, but the relatively small size of the spleen makes its contribution to the total phagocytic activity in vivo negligible. Alternatively, but not mutually exclusively, Kupffer cells may process and degrade the ingested erythrocytes at a higher rate and more efficiently, explaining why we detected no CFSE label in the recipient mouse liver in the earlier experiment.19
With the use of blocking antibodies and gene knockout mice, we identified the recently discovered complement receptor CRIg as the critical macrophage phagocytic receptor for DAF/Crry-deficient platelets. Thus, CRIg served as a complement receptor not only for complement-opsonized pathogens21 but also for complement-opsonized autologous cells. Given the propensity of apoptotic cells to trigger complement activation and C3 ligand deposition, it will be of interest to determine whether CRIg might be involved in the processing of apoptotic cells, thereby potentially playing a role in preventing systemic autoimmunity. The finding that functional deficiencies of CRIg, CR1/2, CR3, and CR4 failed to prevent complement-dependent elimination of DAF/Crry-deficient erythrocytes was intriguing and raised the possibility of additional complement receptor(s) yet to be identified. It is also conceivable that DAF/Crry-deficient erythrocytes were removed by a complement-dependent but CR-independent mechanism. For example, complement attack may have exposed cryptic recognition molecules on the “stressed” erythrocytes, leading to their accelerated elimination through non–CR-dependent mechanisms.
Why do complement-opsonized platelets and erythrocytes engage different phagocytic receptors in vivo? One obvious difference between platelets and erythrocytes is their size. The calculated volume of an erythrocyte is 6.5 times that of a platelet (mean erythrocyte volume, 45.7 fL; mean platelet volume, 7.01 fL).46 However, previous studies have shown that CRIg is capable of mediating the phagocytosis of complement-opsonized sheep erythrocytes in vitro,21 suggesting that cell size is not a critical determinant. A more likely explanation is that the amount and nature of C3 ligand(s) deposited on DAF/Cry-deficient platelets and erythrocytes were considerably different. Indeed, we found that DAF/Crry-deficient platelets were much more susceptible to C3 opsonization than were DAF/Crry-deficient erythrocytes in vivo and in vitro, and previous experiments have shown that dimeric murine CRIg has a relatively low affinity for monomeric C3b/iC3b.21,22 We also probed the nature of C3 fragments deposited on the mutant platelets and erythrocytes using 2 anti–C3 antibodies, mAb 2/11 (recognizes C3b, iC3b, C3c) and mAb 2/16 (recognizes C3, iC3b, C3c).47 We found that mAb 2/11 and mAb 2/16 detected similar levels of C3 deposition on platelets but that mAb 2/11 detected more C3 staining than mAb 2/16 on erythrocytes (unpublished data). These results indicated that the composition of the C3 ligands deposited on the 2 cell types was indeed different. The mechanism underlying differential complement opsonization of the 2 cell types is not clear. MCP, another important membrane C3 complement regulator, is known to be expressed only in the mouse testis9 and is therefore unlikely to be responsible for the differential sensitivity. We also explored constitutive association of fH, a fluid-phase C3 regulator,42 with mouse platelets and erythrocytes and excluded its level as a possible contributor. Nevertheless, it is possible that fluid-phase fH may have a higher affinity for C3b deposited on erythrocytes than that on platelets because of differences in cell surface constituents (eg, sialic acid) that favor fH-host cell interaction during complement activation.48,49
In summary, we have established a critical but redundant role of DAF and Crry in protecting murine platelets from alternative pathway complement attack. We further showed that phagocytosis of complement-opsonized platelets was mediated by CRIg on nonsplenic macrophages, whereas neither CRIg nor CR3 were essential for the clearance of complement-susceptible erythrocytes. These results suggest that the level of DAF or Crry expression on a given cell type is a critical determinant for its overall activity and relevance. They also show the complexity in complement receptor-mediated phagocytosis and raise the question as to whether there might be selectivity in CRIg-mediated phagocytosis of foreign pathogens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Skip Brass for useful discussion, Dr Paul Morgan for anti–CD59 mAbs, Dr John Lambris for anti–C3 and Dr Hector Molina for Crry−/−/C3−/− founder mice.
This work was supported by the National Institutes of Health (Bethesda, MD; grants AI-44970, AI-49344, and AI-63288) and by an American Heart Association (Dallas, TX) predoctoral fellowship from the Pennsylvania/Delaware Affiliate (D.D.K.).
National Institutes of Health
Authorship
Contribution: D.D.K. designed and performed research, analyzed data, and wrote the paper; T.M. and Y.K. performed research and analyzed data; R.A.S. contributed research reagents; M.V.L.C. contributed research reagents and helped in data interpretation; W.-C.S. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Chao Song, Institute for Translational Medicine and Therapeutics and Department of Pharmacology, University of Pennsylvania School of Medicine, Rm 1254 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: song@spirit.gcrc.upenn.edu.