Abstract
CD45 is the prototypic transmembrane protein tyrosine phosphatase (PTP), which is expressed on all nucleated hematopoietic cells and plays a central role in the integration of environmental signals into immune cell responses. Here we report an alternative function for the intracellular domain of CD45. We dis-covered that CD45 is sequentially cleaved by serine/metalloproteinases and γ-secretases during activation of human monocytes and granulocytes by fungal stimuli or phorbol 12-myristate 13-acetate but not by other microbial stimuli. Proteolytic processing of CD45 occurred upon activation of monocytes or granulocytes but not of T cells, B cells, or dendritic cells and resulted in a 95-kDa fragment of the cytoplasmic tail of CD45 (ct-CD45). ct-CD45 was released from monocytes and granulocytes upon activation-induced cell death. Binding studies with ct-CD45 revealed a counter-receptor on preactivated T cells. Moreover, T-cell proliferation induced by dendritic cells or CD3 antibodies was inhibited in the presence of ct-CD45. Taken together, the results of our study demonstrate that fragments of the intracellular domain of CD45 from human phagocytes can function as intercellular regulators of T-cell activation.
Introduction
CD45 (PTPRC, leukocyte common antigen, B220, T200) was one of the first signaling molecules identified on leukocytes.1-7 Humans with certain mutations in CD45 or mice that are deficient in CD45 develop a serve-combined immunodeficiency phenotype, systemic lupus erythematosus, and many other diseases.8-13 CD45 is expressed on all nucleated hematopoietic cells and is therefore widely used as a pan-leukocyte marker molecule. CD45 is a prototypic transmembrane protein tyrosine phosphatase (PTP), and it is generally accepted today that it sets the threshold of positive and negative signaling events in leukocytes.12-15 Through dephosphorylation and activation of the src-family kinases Lck, Fyn, and Lyn, CD45 is a positive regulator for signaling via the T-cell and B-cell receptors, respectively.16-18 On the other hand, through dephosphorylation of JAK, CD45 has been shown to negatively regulate cytokine receptor signaling and promotes viral infections.19 In addition CD45 is involved in regulating development, adhesion, and apoptosis in leukocytes.12,13
CD45 is an abundant and large cell surface glycoprotein of 180-220 kDa, comprising up to 10% of the leukocyte surface area.20 It exists in several isoforms generated by alternative splicing of exons 4, 5, and 6, each with variously sized extracellular domains.13 These extracellular domains are poorly conserved among species and heavily glycosylated. In contrast, the 707-amino acid (aa) large cytoplasmic part of CD45 is conserved and contains two tandemly duplicated protein tyrosine phosphatase homology (PTP) domains, D1 and D2. Only D1 has phosphatase activity. The function of D2 is unclear and may contribute to stabilize D1.21-25
A growing number of PTPs are now known to undergo posttranslational proteolytic processing.26-30 For instance, the CD45-related receptor-type PTP (RPTP) leukocyte-common antigen-related (LAR) has been reported to be cleaved by the presenilin/γ-secretase complex.27 Cleavage of the intracellular parts of type I cell surface receptors by presenilin/γ-secretase is typically executed after the receptors have undergone ectodomain shedding.26,27,29,30 It is noteworthy that proteolysis by the presenilin/γ-secretase is not only a simple degradation process but frequently generates fragments of cell surface receptors with novel biologic functions.29,30 Whether CD45 is proteolytically processed in leukocytes has not yet been investigated.
Therefore, we used a novel monoclonal antibody (mAb), 8-301, which is directed against the cytoplasmic tail of CD45, as a tool to analyze potential CD45 cleavage fragments in leukocytes and test their immunologic function. We demonstrate in this study that the intracellular PTP-domain of CD45 (ct-CD45) is proteolytically cleaved and released upon phagocyte activation by fungi or phorbol 12-myristate 13-acetate (PMA) and functions as an inhibitor of T-cell activation.
Methods
Media, reagents, and chemicals
Cells were maintained in RPMI 1640 medium (Invitrogen, Paisley, United Kingdom), supplemented with 2 mmol/L l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS; Sigma-Aldrich, St Louis, MO). Recombinant human granulocyte macrophage–colony-stimulating factor and interleukin-4 (IL-4) were kindly provided by Novartis Research Institute (Vienna, Austria). Lipopolysaccharide (LPS) from Escherichia coli (serotype 0127-B8; used at 1 μg/mL), zymosan A (100 μg/mL), β-glucan from Saccharomyces cerevisiae (100 μg/mL), peptidoglycan from Staphylococcus aureus (100 μg/mL), laminarin from Laminaria digitata (5 mg/mL), poly(I:C) (20 μg/mL), PMA (100 nM/L), cycloheximide (2 and 10 μg/mL), propidium iodide, 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF; 200 μmol/L), aprotinin (20 μg/mL), and (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (E64d; 100 μmol/L) were from Sigma-Aldrich. Pam3CSK4 (10 μg/mL) and CpG (ODN2006, used at 0.5 mmol/L) were from Invivogen (San Diego, CA). Annexin V-FITC was from Caltag (Burlingame, CA). Candida albicans (4454M) was obtained from ATCC (Manassas, VA).
Apocynin (used at 1 mmol/L), piceatannol (50 μmol/L), EGTA (5 mmol/L), calpeptin (100 μmol/L), N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal (MG-132; 100 μmol/L), pepstatin (2 μmol/L), PTPase CD45 inhibitor (1 mmol/L), and γ-secretase inhibitor X (5 μmol/L) were from Calbiochem (Darmstadt, Germany). IL-10 was purchased from R&D Systems (Minneapolis, MN). Recombinant, human CD45 (aa 607-1304, 95 kDa), LAR (aa 1275-1613, 37 kDa), and RPTPγ (aa 801-1147, 66 kDa) were from BIOMOL Research Laboratories (Plymouth Meeting, PA). Recombinant CD45 and human serum albumin (Sanofi-Aventis, Vienna, Austria) were labeled with Alexa Fluor 448 from Invitrogen according to the manufacturer's instructions and used for staining at 20 μg/mL. Recombinant S100A4 (metastasin) was kindly provided by N. Ambartsumian (Department of Molecular Cancer Biology, Copenhagen, Denmark). The CytoTox 96 assay for measuring lactate dehydrogenase (LDH) release was obtained from Promega (Madison, WI) and was performed according to the manufacturer's instructions. The SensoLyte FDP protein phosphatase kit was from Anaspec (San Jose, CA).
Antibodies and fusion proteins
mAb 8-301 (IgG1) was generated in our laboratory by immunizing BALB/c mice with human T-cell lysates. Murine negative control antibody (Ab) VIAP (calf intestine alkaline phosphatase-specific) was also generated in our laboratory. Anti-CD45 (MEM-28) was from Abcam (Cambridge, United Kingdom). The neutralizing polyclonal anti-IL-10 Ab (clone 25209) was obtained from R&D Systems. Anti- Gp91phox clone 53 was from BD Biosciences (Franklin Lakes, NJ). CD3-mAb (OKT3) was from Ortho Pharmaceutical Corporation (Raritan, NJ). The human CD45- human IgG-Fc fusion proteins were produced in our laboratory in human embryonic kidney 293 cells: Ct-CD45-Ig represents the aa 598-1304 of CD45 (Swiss-Prot P08575), D1-Ig (aa 651-910), the phosphatase-dead D1 C851S-Ig (aa 651-910; cysteine 851 changed to serine), and D2-Ig (aa 942-1226). The ICOS-L-Ig covers the aa 1-101. The control-Ig fusion protein was described previously.31
Cell lines, cell isolation, and stimulation
CD45+ and CD45-deficient (J45.01) Jurkat cells were obtained from ATCC. For isolation of leukocytes, whole blood of healthy donors was separated by standard density gradient centrifugation with Lymphoprep (Nycomed, Oslo, Norway). Approval was obtained from the Medical University of Vienna institutional review board for these studies. Monocytes and T cells were isolated from peripheral blood mononuclear cells using the magnetic cell-sorting technique (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously.32 Dendritic cells (DCs) were generated by culturing purified blood monocytes for 7 days with a combination of granulocyte macrophage–colony-stimulating factor (50 ng/mL) and IL-4 (100 U/mL). Polymorphonuclear neutrophils were isolated from the pellet of the Lymphoprep gradient. Contaminating erythrocytes in polymorphonuclear neutrophils were removed with ammonium chloride lysis buffer (157 mmol/L NH4Cl, 10 mmol/L KHCO3, and 0.1 mmol/L ethylenediaminetetraacetic acid). In the standard stimulation protocol, 2 × 106 cells were incubated in 24-well plates for 90 minutes (unless otherwise noted) in the presence of different stimuli in RPMI 1640 medium + 10% FCS at 37°C. C albicans yeast for monocyte stimulation was grown freshly on Sabouraud agar plates, counted using a Buerker-Tuerk chamber, and different numbers of C albicans were seeded into 24-well plates in RPMI 1640 medium + 10% FCS. For the generation of hyphae, these plates were incubated at 37°C for 2 hours, whereas yeast plates were kept on ice. C albicans yeast and hyphae were either used alive or inactivated in the plate for 1 hour at 65°C. Subsequently, 2 × 106 monocytes/well were added and cocultured for 90 minutes at 37°C.
T-cell proliferation assays
Stimulation of allogeneic, purified T cells with DC was performed as described recently.33 Recombinant ct-CD45, IL-10, and S100A4 were titrated into an allogeneic mixed lymphocyte reaction (MLR) with a fixed number of T cells (1 × 105) and DCs (1 × 104). Experiments were performed in 96-well cell culture plates. Proliferation of T cells was monitored by measuring tritiated thymidine ([methyl-3H]TdR; Valeant Pharmaceuticals, Irvine, CA) incorporation on day 5 of culture. Cells were harvested 18 hours later, and radioactivity was determined on a microplate scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). Assays were performed in triplicate. For proliferation assays with Ig-fusion proteins, plates were coated with both anti–mouse IgG (3 μg/mL; Invitrogen) and anti–human IgG, Fc-specific (3 μg/mL; Jackson ImmunoResearch Laboratories, West Grove, PA) overnight at 4°C, washed, and then incubated with the respective fusion protein at the indicated concentrations plus anti-CD3 (1 μg/mL). After another washing step, T cells (1 × 105/well) were added, and proliferation was measured after 72 hours as described above.
Flow cytometric analysis
For membrane staining, cells (5 × 105) were incubated for 30 minutes at 4°C with unconjugated mAb. After washing, Oregon Green-conjugated goat anti–mouse-Ig from Invitrogen was used as a second-step reagent. For cytoplasmic staining, cells were harvested, fixed with FIX (An der Grub, Kaumberg, Austria) for 20 minutes, washed, and permeabilized for 20 minutes with PERM solution (An der Grub) in the presence of the primary mAb. Oregon Green-conjugated anti–mouse-Ig was used as second-step reagent. Annexin V staining was performed as described previously.34 Flow cytometric analysis was performed using a FACScalibur flow cytometer (BD Biosciences).
Subcellular fractionation of monocytes
After washing in phosphate-buffered saline (PBS), 1 × 108 monocytes were incubated in 1 mL of hypotonic buffer (42 mmol/L KCl, 10 mmol/L HEPES, pH 7.4, 5 mmol/L MgCl2, and protease inhibitors) for 15 minutes at 4°C. Cells were mechanically disrupted by 20 strokes with a tissue homogenizer (S20; Glas-Col, Terre Haute, IN). Nuclei and intact cells were removed by centrifugation at 300g for 10 minutes at 4°C. The cleared postnuclear supernatant was subjected to ultracentrifugation at 100 000g for 1 hour at 4°C. The resulting supernatant was collected as cytoplasmic fraction, and the membrane pellet was solubilized for 30 minutes at 4°C in radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitors).
Immunoaffinity purification and Western blotting
For Western blot (WB) samples 2 × 106 cells were harvested after stimulation and immediately resuspended in 100 μL of reducing SDS sample buffer, frozen in N2, and boiled at 95°C for 5 minutes. For mass spectrometry (MS), immunoprecipitation with mAb 8-301 was performed from lysates produced by solubilization of 2 × 108 monocytes in 1 mL of lysis buffer containing 1% IGEPAL CA-630 (Sigma-Aldrich) supplemented with Complete Protease Inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). For Western Blot, released ct-CD45 was precipitated from cell culture supernatants from 5 × 107 cells cultivated in 10 mL of RPMI 1640 medium + 10% FCS. For immunoprecipitation, 100 μg (for MS) or 10 μg (for WB) mAb 8-301 were loaded onto 7 × 108 or 7 × 107 sheep anti–mouse IgG 2.8 μm Dynabeads (Dynal, Oslo, Norway) as described elsewhere in detail.35 After washing twice in PBS/0.01% BSA, beads were incubated with cell lysate or cell-culture supernatant for 24 hours at 4°C on a rotator. Subsequently, the beads were washed with PBS, and bound protein was eluted into reducing sample buffer by boiling for 5 minutes. Western blot detection limit of precipitated ct-CD45 was at 5 ng. Western blotting was performed under standard conditions using mAbs at 1 μg/mL. Bound mAbs were detected using anti-mouse-Ig-HRP (Dako Denmark A/S, Glostrup, Denmark; 1/10 000) and chemiluminescence detection (SuperSignal; Pierce, Rockford, IL).
Phosphatase assays
SensoLyte phosphatase substrate was prepared according to the manufacturer's instructions. Magnetic beads with precipitated ct-CD45 were directly incubated with SensoLyte phosphatase substrate for 60 minutes. Then the beads were removed using a Dynal MPC-2 magnetic particle concentrator and fluorescence was measured with a VICTOR plate reader (PerkinElmer).
Mass spectrometry
Lysates of monocytes treated with PMA for 90 minutes were precipitated with mAb 8-301 as described above. Gels were silver-stained, the protein lanes were cut out, and the gel pieces were treated with trypsin. For the identification of amino acid sequences, peptides were separated by nano-flow LC (1100 Series LC system; Agilent Technologies, Palo Alto, CA) using the HPLC-Chip technology (Agilent) equipped with a 40-nL Zorbax 300SB-C18 trapping column and a 75 μm × 150 mm Zorbax 300SB-C18 separation column at a flow rate of 400 nL/min, using a gradient from 0.2% formic acid/3% ACN to 0.2% formic acid/50% ACN over 60 minutes. Peptide identification was accomplished by MS/MS fragmentation analysis with an iontrap mass spectrometer (XCT-Ultra; Agilent) equipped with an orthogonal nanospray ion source. The MS/MS data, including peaklist-generation and search engine, were interpreted by the Spectrum Mill MS Proteomics Workbench software (version A.03.02; Agilent), allowing for 2 missed cleavages, and searched against the SwissProt Database for human proteins (version 20061207 containing 15.265 entries), allowing for precursor mass deviation of 1.5 Da, a product mass tolerance of 0.7 Da, and a minimum matched peak intensity (%SPI) of 70%. The scores were essentially calculated from sequence tag lengths but also consider mass deviations. To assess reliability of the peptide scores, we performed searches against the corresponding reverse database.36 Search yielded 5.4% positive hits with peptides scoring more than 9.0, whereas 1.18% positive hits were found with peptides scoring more than 13.0. Consequently, we set the threshold for protein identification to at least one peptide scoring higher than 13.0.
To identify the molecule recognized by mAb 8-301, the 200-kDa band was isolated from monocyte lysates by immunoprecipitation and mass spectrometric analysis was performed. From this band, 28 different peptides of the digest were matched to the leukocyte common antigen precursor (CD45 antigen, P08575) covering 26% (within amino acids 292-1254) of the total sequence of 1304 amino acids.
Results
Molecular characterization of the mAb 8-301-defined antigen
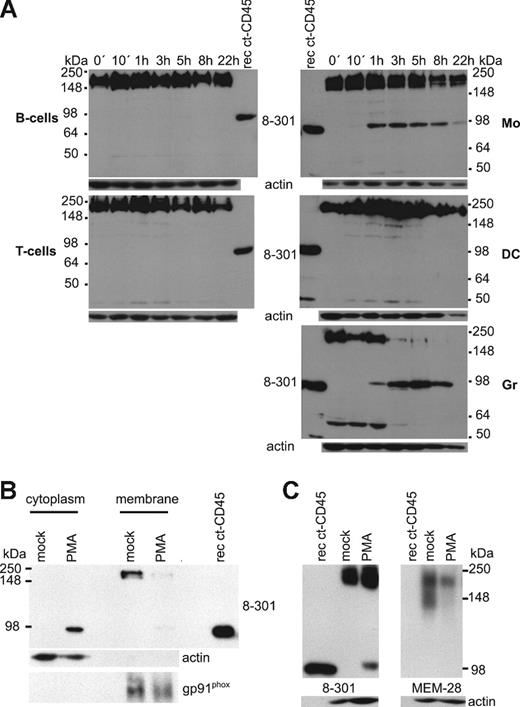
mAb 8-301 was produced in our laboratory by immunization of BALB/c mice with human T-cell homogenates. We could demonstrate by mass spectrometric analysis (for details, see “Mass spectrometry”) and by Western blot that mAb 8-301 is directed against CD45 (Figure 1A).
Molecular characterization of the mAb 8-301-defined antigen. (A) Lysates of CD45+ and CD45-deficient Jurkat cells were used in Western blots to validate the reactivity of mAb 8-301 with CD45 in parallel with the conventional CD45 mAb MEM-28. (B) Flow cytometric analysis revealed that mAb 8-301, in contrast to MEM-28, shows intracellular reactivity with monocytes. The figure shows histogram profiles including isotype-matched control mAb VIAP (open) and mAb 8-301 or MEM-28 (filled). (C) Reactivity of mAb 8-301 with cytoplasmic tails of protein tyrosine phosphatases CD45, RPTPγ, and LAR was tested by immunoblot (left). Loading was controlled by Ponceau S staining of the membrane (right). Representative experiments of at least 2 independent experiments are shown.
Molecular characterization of the mAb 8-301-defined antigen. (A) Lysates of CD45+ and CD45-deficient Jurkat cells were used in Western blots to validate the reactivity of mAb 8-301 with CD45 in parallel with the conventional CD45 mAb MEM-28. (B) Flow cytometric analysis revealed that mAb 8-301, in contrast to MEM-28, shows intracellular reactivity with monocytes. The figure shows histogram profiles including isotype-matched control mAb VIAP (open) and mAb 8-301 or MEM-28 (filled). (C) Reactivity of mAb 8-301 with cytoplasmic tails of protein tyrosine phosphatases CD45, RPTPγ, and LAR was tested by immunoblot (left). Loading was controlled by Ponceau S staining of the membrane (right). Representative experiments of at least 2 independent experiments are shown.
Remarkably, mAb 8-301, in contrast to the conventional CD45 mAb MEM-28, did not show surface recognition (Figure 1B) but had strong intracellular reactivity. To analyze whether mAb 8-301 binds to the cytoplasmic tail of CD45, we tested whether mAb 8-301 recognizes a recombinant CD45 protein representing the cytoplasmic domain (Figure 1C). MAb 8-301 recognized this 95-kDa protein, but not the intracellular domains of other related phosphatases such as RPTPγ and LAR. Thus, mAb 8-301 is directed against the cytoplasmic tail of CD45.
A 95-kDa fragment of CD45 is produced in phagocytes
The phorbol ester PMA, a potent protein kinase C activator, was shown previously to induce cleavage of PTPs such as LAR in different cell lines.26,37 Here we applied mAb 8-301 as a tool to identify proteolytically produced fragments of CD45 in different human leukocyte populations upon PMA stimulation. MAb 8-301 recognized total CD45 in the range of 200 kDa in all tested leukocyte populations (Figure 2A). Furthermore, we could detect a potential CD45 cleavage fragment of 95 kDa in monocytes soon after PMA stimulation. The amount of this protein increased for 3 hours and then declined. Expression of this protein was not restricted to monocytes but was also induced in neutrophil granulocytes after 1 hour, whereas DCs, B cells, T cells, or the myeloid cell lines U937 and THP-1 did not express it (Figure 2A and data not shown). Because the recombinant cytoplasmic CD45 and the potential CD45 fragment have the same size, we speculated that this 95-kDa protein is the cytoplasmic tail of CD45 (ct-CD45). We analyzed the protein sequence of the potential fragment by mass spectrometry. The data presented in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) show that we could identify 11 peptides, all of which aligned to the intracellular part of CD45. Furthermore, we found that upon 3 hours' stimulation, the 95-kDa protein is located in the cytoplasmic and not in the membrane fraction (Figure 2B). The ratio of intact versus cleaved CD45 molecules was determined by densitometric analysis, and we found that 29% (± 6%) and 9% (± 4%) of the CD45 molecules are cleaved upon stimulation by PMA (n = 8) and zymosan (n = 7) in monocytes, respectively, within 90 minutes. In contrast to mAb 8-301, mAb MEM-28 reacted neither with PMA-induced ct-CD45 nor with the recombinant ct-CD45 (Figure 2C). Thus, the 95-kDa protein detected by mAb 8-301 in the cytoplasm of activated phagocytes is ct-CD45.
A 95-kDa fragment of CD45 is produced in monocytes and granulocytes. (A) Assessment of potential CD45 cleavage fragments with the intracellular-reactive CD45 mAb 8-301. B cells, T cells, monocytes (Mo), DCs, and granulocytes (Gr) were stimulated for different time periods with PMA, and lysates were analyzed by Western blot. A representative experiment of 3 independently performed is shown. (B) Membrane and cytoplasmic fractions of resting monocytes and of monocytes, stimulated for 3 hours with PMA, were isolated and analyzed in Western blots with mAb 8-301 for localization of the 95-kDa band. Isolation of membranes was controlled with a gp91phox antibody, and isolation of cytoplasm was controlled with an actin antibody. One representative experiment of 2 independent experiments is shown. (C) MEM-28 does not react with the PMA-induced ct-CD45. A recombinant cytoplasmic CD45 protein covering the total intracellular part (lane 1), resting monocytes (lane 2), and monocytes stimulated for 90 minutes with PMA (lane 3) were analyzed in Western blots with mAb 8-301 and MEM-28.
A 95-kDa fragment of CD45 is produced in monocytes and granulocytes. (A) Assessment of potential CD45 cleavage fragments with the intracellular-reactive CD45 mAb 8-301. B cells, T cells, monocytes (Mo), DCs, and granulocytes (Gr) were stimulated for different time periods with PMA, and lysates were analyzed by Western blot. A representative experiment of 3 independently performed is shown. (B) Membrane and cytoplasmic fractions of resting monocytes and of monocytes, stimulated for 3 hours with PMA, were isolated and analyzed in Western blots with mAb 8-301 for localization of the 95-kDa band. Isolation of membranes was controlled with a gp91phox antibody, and isolation of cytoplasm was controlled with an actin antibody. One representative experiment of 2 independent experiments is shown. (C) MEM-28 does not react with the PMA-induced ct-CD45. A recombinant cytoplasmic CD45 protein covering the total intracellular part (lane 1), resting monocytes (lane 2), and monocytes stimulated for 90 minutes with PMA (lane 3) were analyzed in Western blots with mAb 8-301 and MEM-28.
Fungal stimuli induce ct-CD45 in human monocytes
Sensing of pathogens is the first critical step in launching an immune response. Because phagocytes are especially equipped with pattern recognition receptors, we wondered whether ct-CD45 is also induced by microbial stimuli. It is noteworthy that ct-CD45 was produced upon treatment of monocytes with zymosan, the particulate cell wall extract of S cerevisiae, but not upon treatment with the TLR2 ligands Pam3CSK4 or peptidoglycan (Figure 3A). Furthermore, stimulation via neither TLR3, TLR4, TLR9, poly(I:C), LPS, nor CpG induced ct-CD45. We also tested whether ct-CD45 is produced by the induction of cell death. Inducers of cell death like cycloheximide or staurosporine or freeze/thaw cycles did not lead to ct-CD45 generation (data not shown). Figure 3B shows that zymosan and PMA but not LPS stimulation of monocytes is accompanied by a diminished reactivity of MEM-28 seen by flow cytometry, indicating the release of the extracellular domain. Ct-CD45 generation upon zymosan stimulation of monocytes shows the same kinetics as upon PMA stimulation (Figures 2A, 3C). Stimulation of monocytes with heat-inactivated (Figure 3D) as well as with vital (data not shown) C albicans yeast and hyphae led also to ct-CD45 expression. Phagocytes express and use a variety of cell surface receptors for sensing fungi, in particular the fungal cell wall components β-glucan and mannan. Beside TLR2, mannose receptor (CD206), and complement receptor 3 (CD11b/CD18), Dectin-1, the major β-glucan receptor on myeloid cells, was recently discovered to be an important player in controlling fungal infection.38-41 Because zymosan is mainly composed of β-glucan42 and the TLR2 stimuli Pam3Cys did not induce ct-CD45 (Figure 3A), we tested whether ct-CD45 can be produced upon ligation of Dectin-1. Therefore we used a particulate β-glucan preparation from S cerevisiae, which induced a slight ct-CD45 band (Figure 3E). Furthermore, we investigated whether induction of ct-CD45 by zymosan can be blocked by the soluble β-glucan laminarin from L digitata, which was shown to block activation by zymosan via Dectin-1.38,40,43 We found that laminarin strongly inhibited zymosan-induced production of ct-CD45 (Figure 3E). Ligation of Dectin-1 leads to activation of Syk tyrosine kinase, which is important for zymosan-induced respiratory burst in phagocytes.44 We found that the generation of ct-CD45 by zymosan but not by PMA was blocked by the Syk kinase inhibitor piceatannol (Figure 3F). Thus, we concluded that ligation of Dectin-1 is critically involved in the induction of ct-CD45 by zymosan. Because zymosan and PMA are both able to induce an oxidative burst in phagocytes (data not shown), we tested whether oxidative burst formation is involved in ct-CD45 generation. Remarkably, apocynin, which blocks the oxidative burst by specifically inhibiting the assembly of the functional NADPH-oxidase complex, was indeed able to block ct-CD45 production by zymosan as well as by PMA (Figure 3F).
Fungal stimuli induce ct-CD45 in human monocytes. (A) Human monocytes were stimulated with Pam3CSK4 (P3C), peptidoglycan (PG), zymosan (Zym), poly(I:C) (PI:C), LPS, CpG, or PMA for 90 minutes. Lysates were analyzed by Western blot with mAb 8-301. (B) Binding of MEM-28 to resting monocytes (dashed line) or monocytes treated for 90 minutes with PMA, zymosan, or LPS (black lines) were examined by flow cytometry. Isotype-matched control mAb VIAP (dotted line) was used as nonbinding control. (C) Monocytes treated with zymosan were harvested at different time points and analyzed by Western blot with mAb 8-301. (D) Different numbers of C albicans hyphae and yeast were heat-inactivated and cocultured with monocytes. Lysates were analyzed by Western blot with mAb 8-301 for generation of ct-CD45. (E) Stimulation of monocytes with the Dectin-1 ligand β-glucan (β-Glu) leads to ct-CD45 production. Blocking with laminarin (Lam) also inhibited zymosan-induced ct-CD45. (F) The syk inhibitor piceatannol (picea; 50 μmol/L) strongly inhibited ct-CD45 production by zymosan. NADPH-oxidase inhibitor apocynin (apo; 1 mmol/L) blocked ct-CD45 induced by PMA and zymosan. Data represent 3 independently performed experiments.
Fungal stimuli induce ct-CD45 in human monocytes. (A) Human monocytes were stimulated with Pam3CSK4 (P3C), peptidoglycan (PG), zymosan (Zym), poly(I:C) (PI:C), LPS, CpG, or PMA for 90 minutes. Lysates were analyzed by Western blot with mAb 8-301. (B) Binding of MEM-28 to resting monocytes (dashed line) or monocytes treated for 90 minutes with PMA, zymosan, or LPS (black lines) were examined by flow cytometry. Isotype-matched control mAb VIAP (dotted line) was used as nonbinding control. (C) Monocytes treated with zymosan were harvested at different time points and analyzed by Western blot with mAb 8-301. (D) Different numbers of C albicans hyphae and yeast were heat-inactivated and cocultured with monocytes. Lysates were analyzed by Western blot with mAb 8-301 for generation of ct-CD45. (E) Stimulation of monocytes with the Dectin-1 ligand β-glucan (β-Glu) leads to ct-CD45 production. Blocking with laminarin (Lam) also inhibited zymosan-induced ct-CD45. (F) The syk inhibitor piceatannol (picea; 50 μmol/L) strongly inhibited ct-CD45 production by zymosan. NADPH-oxidase inhibitor apocynin (apo; 1 mmol/L) blocked ct-CD45 induced by PMA and zymosan. Data represent 3 independently performed experiments.
Ct-CD45 is generated by proteolytic cleavage
Because we could detect ct-CD45 in the cytoplasmic fraction (Figure 2B) and found a diminished reactivity of MEM-28 upon stimulation, suggesting the release of the CD45 ectodomain (Figure 3B), we hypothesized that CD45 could be processed by a mechanism including several cleavage steps. In addition, however, we also examined whether ct-CD45 is an as-yet-unknown translational variant. We tested whether inhibition of de novo protein synthesis with cycloheximide could inhibit ct-CD45 generation.45 However, as shown in Figure 4A, ct-CD45 was expressed during monocyte stimulation with zymosan or PMA in the presence of cycloheximide, which completely prevented the induction of typical monocyte activation markers such as sialoadhesin (CD169) analyzed in parallel (data not shown).
The cytoplasmic tail of CD45 is proteolytically cleaved and released into the cytoplasm. (A) Inhibition of de novo protein synthesis by cycloheximide (2 and 10 μg/mL) did not block production of ct-CD45 upon monocyte stimulation with PMA or zymosan. (B) The activity of protease inhibitors AEBSF, aprotinin, EGTA, calpeptin, E64d, MG-132, and pepstatin on production of ct-CD45 in zymosan-stimulated monocytes was tested in Western blots. (C) Membrane and cytoplasmic fractions of resting monocytes and monocytes stimulated for 90 minutes with PMA were isolated and analyzed in Western blots with mAb 8-301. Presence of the serine protease inhibitor AEBSF inhibited production of membrane-bound ct-CD45. The γ-secretase inhibitor X blocked the release of membrane-bound ct-CD45 into the cytosol. Isolation of membranes was controlled with anti-gp91phox, isolation of cytoplasm with an actin antibody. Data represent at least 2 independently performed experiments.
The cytoplasmic tail of CD45 is proteolytically cleaved and released into the cytoplasm. (A) Inhibition of de novo protein synthesis by cycloheximide (2 and 10 μg/mL) did not block production of ct-CD45 upon monocyte stimulation with PMA or zymosan. (B) The activity of protease inhibitors AEBSF, aprotinin, EGTA, calpeptin, E64d, MG-132, and pepstatin on production of ct-CD45 in zymosan-stimulated monocytes was tested in Western blots. (C) Membrane and cytoplasmic fractions of resting monocytes and monocytes stimulated for 90 minutes with PMA were isolated and analyzed in Western blots with mAb 8-301. Presence of the serine protease inhibitor AEBSF inhibited production of membrane-bound ct-CD45. The γ-secretase inhibitor X blocked the release of membrane-bound ct-CD45 into the cytosol. Isolation of membranes was controlled with anti-gp91phox, isolation of cytoplasm with an actin antibody. Data represent at least 2 independently performed experiments.
Because ct-CD45 is not de novo synthesized, we further examined our hypothesis that ct-CD45 is generated by proteolysis from intact CD45 molecules. To test which protease could be involved in ct-CD45 cleavage, we stimulated monocytes in the presence of different protease inhibitors to block generation of ct-CD45. Results presented in Figure 4B demonstrate that induction of ct-CD45 expression was prevented in the presence of serine protease inhibitors AEBSF or aprotinin and the metalloprotease inhibitor ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetraacetic acid (EGTA). The calpain inhibitor calpeptin, the proteasome inhibitor MG-132, the cysteine protease inhibitor E64d, and the aspartyl protease inhibitor pepstatin did not diminish the generation of ct-CD45. Therefore, potential candidates for the extracellular cleavage step are serine proteases and metalloproteases.
Because the molecular weight of the cellular ct-CD45 and the recombinant ct-CD45 covering the full intracellular part of CD45 (Figure 2A) was similar, we hypothesized that cleavage of the intracellular part would occur near the cell membrane. Cleavage of the intracellular domains of type I cell surface receptors is typically executed by the presenilin/γ-secretase complex after the receptors, including some RPTPs, have undergone ectodomain shedding.27,29,30 Thus, we analyzed whether cleavage of the extracellular part of CD45 is followed by intracellular cleavage of ct-CD45 by γ-secretases (Figure 4C). After 90 minutes' stimulation of monocytes with PMA, we found that some ct-CD45 molecules are still linked to the membrane (Figure 4C right panel), whereas others are already released into the cytoplasm (Figure 4C left panel). AEBSF did block generation of membrane-linked as well as cytoplasmic ct-CD45. The release of ct-CD45 from the membrane into the cytoplasm was prevented by the γ-secretase inhibitor X. Therefore, these results demonstrate that sequential cleavage of CD45 molecules by serine proteases and metalloproteases and the presenilin/γ-secretase pathway in the course of activation leads to the generation of ct-CD45 in phagocytes.
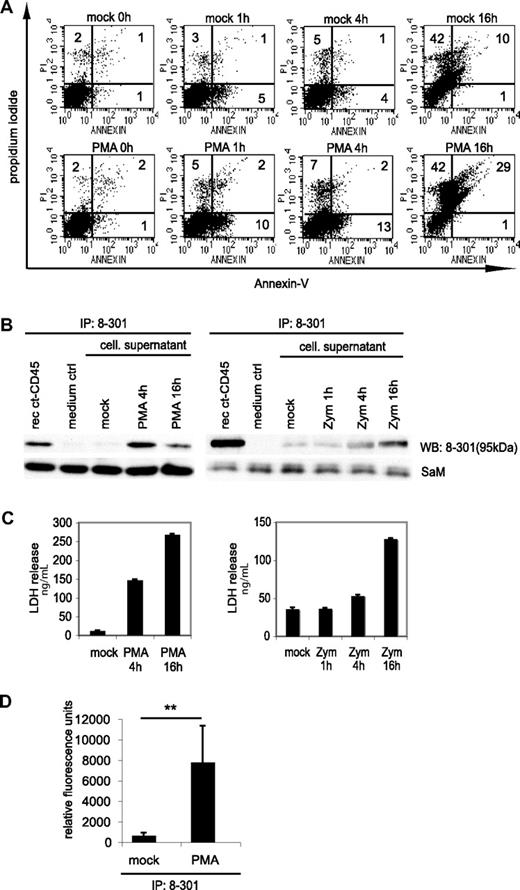
Ct-CD45 is released by phagocytes upon stimulation
Stimulation of phagocytes with zymosan or PMA is known to induce activation-induced cell death.46,47 We indeed found that the number of annexin- and propidium iodide-positive cells increased upon PMA or zymosan treatment (Figure 5A and data not shown). We hypothesized that the observed decrease of ct-CD45 in cell lysates (Figure 2A) might be due to further degradation or release of ct-CD45 from dying cells into the supernatant. Results presented in Figure 5B demonstrate that ct-CD45 was indeed detectable in the supernatant of activated monocytes after 4 to 16 h. We measured by densitometric analysis of Western blots that 1 × 106 monocytes release 859 (± 496) pg and 180 (± 81) pg of ct-CD45 upon stimulation with PMA and zymosan, respectively (n = 3 donors). Granulocytes (1 × 106) release even slightly more ct-CD45 (1.181 ± 308 pg, n = 3 donors) upon stimulation with PMA. The appearance of ct-CD45 in the supernatant of phagocytes correlated with lactate dehydrogenase release, a cytoplasmic protein typically released upon membrane leakage due to cell death (Figure 5C). We found that ct-CD45 isolated from the supernatant of phagocytes retained its phosphatases activity (Figure 5D). Taken together, we concluded that ct-CD45 after stimulation-induced cleavage seems to be released from phagocytes mainly due to cell death.
Ct-CD45 molecules are released into the supernatant upon stimulation of phagocytes with zymosan and PMA. (A) Monocytes stimulated with PMA for different time periods were stained with annexin-V and PI. Percentages of positive cells are indicated. (B) ct-CD45 was isolated from cell culture supernatants of monocytes stimulated with PMA or zymosan by immunoprecipitation with mAb 8-301. As a positive control recombinant ct-CD45 diluted in the same amount of RPMI was precipitated, as a negative control precipitation was performed from RPMI (medium ctrl). Isolated ct-CD45 was immunoblotted and detected by mAb 8-301. As a loading control, the light chain of mAb 8-301 used for precipitation, detected with sheep-anti–mouse-HRP, was used. Data represent at least 2 independently performed experiments. (C) Release of ct-CD45 was accompanied by the release of LDH. The experiment shows the LDH values of the supernatants used for immunoprecipitation in (B). (D) The phosphatase activity of released ct-CD45 was assessed by precipitation of ct-CD45 with mAb 8-301 from supernatants of granulocytes (7 × 107 cells) incubated with or without PMA for 4 hours. The figure shows mean values of relative fluorescent units from 4 different donors of 2 different experiments (± SD). A paired Student t test was performed (**P < .05).
Ct-CD45 molecules are released into the supernatant upon stimulation of phagocytes with zymosan and PMA. (A) Monocytes stimulated with PMA for different time periods were stained with annexin-V and PI. Percentages of positive cells are indicated. (B) ct-CD45 was isolated from cell culture supernatants of monocytes stimulated with PMA or zymosan by immunoprecipitation with mAb 8-301. As a positive control recombinant ct-CD45 diluted in the same amount of RPMI was precipitated, as a negative control precipitation was performed from RPMI (medium ctrl). Isolated ct-CD45 was immunoblotted and detected by mAb 8-301. As a loading control, the light chain of mAb 8-301 used for precipitation, detected with sheep-anti–mouse-HRP, was used. Data represent at least 2 independently performed experiments. (C) Release of ct-CD45 was accompanied by the release of LDH. The experiment shows the LDH values of the supernatants used for immunoprecipitation in (B). (D) The phosphatase activity of released ct-CD45 was assessed by precipitation of ct-CD45 with mAb 8-301 from supernatants of granulocytes (7 × 107 cells) incubated with or without PMA for 4 hours. The figure shows mean values of relative fluorescent units from 4 different donors of 2 different experiments (± SD). A paired Student t test was performed (**P < .05).
Ct-CD45 inhibits T-cell proliferation
Next, we analyzed whether soluble ct-CD45 is able to interact with other immune cells. Using fluorescein-labeled recombinant ct-CD45 molecules for binding studies, we found that ct-CD45 did not bind to resting T cells, monocytes and DC (data not shown) but clearly interacted with preactivated T cells (Figure 6A). Binding of ct-CD45 to activated T cells seems to be mediated via a saturable (Figure 6B) and specific receptor, because it was inhibited in the presence of mAb 8-301 and in the presence of unlabeled ct-CD45. Pretreatment of ct-CD45 with a specific inhibitor of the PTPase domain of CD45 did not block binding to T cells (Figure 6A),48 indicating that the PTPase activity of ct-CD45 is not essential for binding to T cells.
Ct-CD45 has immuno-regulatory function. (A) Binding of ct-CD45 to resting T cells and to T cells activated for 24 hours with PMA/ionomycin was analyzed with Alexa Fluor 488-labeled recombinant ct-CD45 molecules and flow cytometry. Pretreatment of cells with unlabeled recombinant protein or mAb 8-301 but not with PTPase inhibitor-blocked binding of fluorescent ct-CD45 (filled histograms) to T cells. The figure shows overlay histogram profiles including Alexa Fluor 488-labeled human serum albumin used as a negative control (open histogram), which are representative of 3 independent experiments. (B) Binding of increasing amounts of Alexa Fluor 488 labeled recombinant ct-CD45 molecules analyzed by flow cytometry. The figure shows mean values plus or minus SEM of 4 experiments of mean fluorescence intensity. The values of nonspecific staining with Alexa-labeled human serum albumin were subtracted from the values obtained with Alexa-labeled ct-CD45. (C) Addition of recombinant ct-CD45 (▲) inhibits T-cell proliferation induced by allogeneic DC (○). The immunosuppressive cytokine IL-10 (■) and recombinant S100A4 proteins (•) were used as positive and negative control, respectively. Data represent mean cpm (± SEM) of 3 independent experiments. (D) T cells were cultured in the presence of immobilized anti-CD3 alone (mock, taken as 100%), anti-CD3 plus control-Ig (co-Ig), or as positive control anti-CD3 plus ICOS-L-Ig or anti-CD3 plus different CD45-Ig-fusion proteins (all Ig-fusion proteins coated at 0.5 or 2 μg/mL) for 72 hours, and proliferation was measured by [3H]thymidine incorporation. Mean values and SD of 3 different experiments are shown. A paired Student t test was performed (***P < .01; **P < .05). (E) Percentages of annexin- and PI-positive T cells cultured as in (D) with 2 μg/mL Ig-fusion proteins coated. Mean values and SD of 3 different experiments are shown.
Ct-CD45 has immuno-regulatory function. (A) Binding of ct-CD45 to resting T cells and to T cells activated for 24 hours with PMA/ionomycin was analyzed with Alexa Fluor 488-labeled recombinant ct-CD45 molecules and flow cytometry. Pretreatment of cells with unlabeled recombinant protein or mAb 8-301 but not with PTPase inhibitor-blocked binding of fluorescent ct-CD45 (filled histograms) to T cells. The figure shows overlay histogram profiles including Alexa Fluor 488-labeled human serum albumin used as a negative control (open histogram), which are representative of 3 independent experiments. (B) Binding of increasing amounts of Alexa Fluor 488 labeled recombinant ct-CD45 molecules analyzed by flow cytometry. The figure shows mean values plus or minus SEM of 4 experiments of mean fluorescence intensity. The values of nonspecific staining with Alexa-labeled human serum albumin were subtracted from the values obtained with Alexa-labeled ct-CD45. (C) Addition of recombinant ct-CD45 (▲) inhibits T-cell proliferation induced by allogeneic DC (○). The immunosuppressive cytokine IL-10 (■) and recombinant S100A4 proteins (•) were used as positive and negative control, respectively. Data represent mean cpm (± SEM) of 3 independent experiments. (D) T cells were cultured in the presence of immobilized anti-CD3 alone (mock, taken as 100%), anti-CD3 plus control-Ig (co-Ig), or as positive control anti-CD3 plus ICOS-L-Ig or anti-CD3 plus different CD45-Ig-fusion proteins (all Ig-fusion proteins coated at 0.5 or 2 μg/mL) for 72 hours, and proliferation was measured by [3H]thymidine incorporation. Mean values and SD of 3 different experiments are shown. A paired Student t test was performed (***P < .01; **P < .05). (E) Percentages of annexin- and PI-positive T cells cultured as in (D) with 2 μg/mL Ig-fusion proteins coated. Mean values and SD of 3 different experiments are shown.
To examine whether binding of ct-CD45 to T cells has immunoregulatory consequences, we added ct-CD45 proteins to an MLR. We observed that ct-CD45 reduced T-cell proliferation induced by DC. This inhibitory effect of ct-CD45 was comparable with that found with IL-10, the conventional inhibitory factor for T cells, and was not seen with recombinant S100A4 protein used for control (Figure 6C). We also observed this inhibitory function when we stimulated T cells with plate-bound anti-CD3 in the presence of immobilized ct-CD45-Ig fusion proteins (Figure 6D). Inhibition of proliferation was independent of the phosphatase domain of CD45 as a phosphatase-dead D1-Ig protein induced the same effect as active protein. The D2-Ig showed a weaker inhibitory effect than D1-Ig, especially at a lower dose. Figure 6E shows that inhibition of T-cell proliferation by different ct-CD45-Ig proteins was not due to cell death. Ct-CD45 thus represents a novel immune-regulatory factor for human T cells.
Discussion
In this study, we demonstrate an alternative function for CD45. We observed that cytoplasmic tails of CD45 molecules are cleaved and released upon activation of phagocytes and act as cytokine-like factors with negative immunoregulatory function on T cells. ct-CD45 can thereby act as an intercellular regulator between the innate and the adaptive immune system.
We discovered this phenomenon when we studied the existence of post-translationally processed CD45 molecules with a mAb directed against the cytoplasmic part. Ct-CD45 was found in monocytes and neutrophil granulocytes but not in leukocytes or DC upon activation with PMA, the fungal cell wall component zymosan, or C albicans. Ct-CD45 was not detectable after induction of cell death. Together with the finding that ct-CD45 molecules are also generated in the presence of cycloheximide, which blocks ribosomal translation, the rapid appearance of ct-CD45 within 1 hour suggested that it might not be derived from newly synthesized, alternative splice variants of CD45. Alternative splicing of the extracellular part of CD45 is well investigated and responsible for the generation of CD45 isoforms but has not been reported to occur within the conserved intracellular PTP-domains.13,15,45
We could show that cleavage of the cytoplasmic tail from intact CD45 molecules by proteases is responsible for the accumulation of ct-CD45. Proteolysis of other transmembrane and nonreceptor-type PTPs has been reported before in several studies.49-51 Cleavage of the intracellular domains of type I cell surface receptors is typically executed by the presenilin/γ-secretase complex after the receptors, including some RPTPs, have undergone ectodomain shedding.26,27,29,30 For instance, the CD45-related receptor-type PTP LAR has been reported to be cleaved by the γ-secretase pathway.27 The extracellular part of CD45 has been reported to be cleaved by trypsin digestion.52 Here we found that in the course of CD45 processing upon stimulation with PMA or zymosan, sequential cleavage of CD45 molecules by serine proteases and metalloproteases and the presenilin/γ-secretase pathway leads to the generation of ct-CD45 in phagocytes.
The reutilization of intracellular proteins for alternative functions is an emerging theme in cell biology. Release of cytoplasmic or nuclear proteins such as HMGB-1 from dying cells or by atypical (ie Golgi-independent) release mechanisms has been shown to function as a “danger signal” leading to the activation of DCs and consequently promotes adaptive immunity.53-55 We also observed that ct-CD45 seems to be released, but not produced, mainly upon cell death during PMA and zymosan stimulation. Yet atypical release mechanisms may also be involved in the release of ct-CD45. In contrast to HMGB-1, ct-CD45 acted as an inhibitory factor. One could speculate that released ct-CD45 helps to prevent exaggerated immune responses executed by T cells.
How ct-CD45 inhibits T-cell proliferation remains to be determined. However, direct binding to preactivated T cells suggests that there might be a specific receptor for ct-CD45. CD45, and in particular its intracellular part, is known to interact with several proteins, including various protein kinases (eg, Lck and Fyn), cytoskeletal proteins such as fodrin, and intramembrane cell surface receptors such as CD2.13 Thus, one of the already described binding partners for the intracellular part of CD45, if it is expressed on the cell surface, could also serve as an extracellular counter-receptor for ct-CD45 on activated T cells. Casein kinase 2 (CK-2) is an intracellular binding partner of CD45 but has been reported to be expressed upon cell activation on the outside of cell membranes and functions as ectoprotein kinase.56-58 We have investigated whether CK-2 is expressed outside of activated T cells, but mAb 1AD9 against CK-2 showed no extracellular surface reactivity and did not inhibit binding of ct-CD45 to T cells. Thus, CK-2 may not act as a cell surface receptor for ct-CD45 on T cells (data not shown).
Inhibition of T-cell proliferation seems to be independent of the phosphatase activity of CD45, because we found it also with a phosphatase-dead fusion protein. It is noteworthy that inhibition was not only seen upon stimulation with an Ig fusion protein covering the total intracellular part or the D1 domain but also with a D2-Ig protein. Still, the inhibitory activity of D2-Ig was weaker than D1-Ig, especially at lower concentrations. Although it is surprising that both PTP domains are able to act inhibitory, these results are supported by data that the tertiary structures of D1 and D2 are very similar.25
Remarkably, ct-CD45 was only generated in phagocytes like monocytes and granulocytes, but not in T cells, B cells, or DCs. Monocytes and granulocytes are capable of producing a strong respiratory burst. Considering that, together with our observation that ct-CD45 generation was blocked by an inhibitor of NADPH-oxidase, we can speculate that oxidative burst formation is an important factor in the generation of ct-CD45. Phagocytes and their ability to induce an oxidative burst play a central role in the first line of defense against fungi. Yet recent studies also support the idea that phagocytes and factors derived from these innate immune cells such as IL-10 are pivotal in controlling adaptive immune responses against fungi.59,60 These processes are considered central mechanisms to establish a commensal relationship between fungus and host.61 Our finding that activation of human phagocytes with fungal stimuli induces the cleavage and release of ct-CD45 in vitro represents a novel mechanism in the understanding of the complex host-fungus interaction. To investigate the physiologic relevance of ct-CD45 in vivo, we have begun to analyze, by enzyme-linked immunosorbent assay, the ct-CD45 levels in sera from patients with severe infectious diseases, including systemic candidosis, and to test whether ct-CD45 molecules can act immunosuppressive in a murine model of rheumatoid arthritis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Petra Kohl, Christa Zangerle, Edith Bayer, Saro Künig, and Claus Wenhardt for expert technical assistance.
This work was supported by grants FWF-SFB2307 (“Vision Fund”) and APP20266FW from the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung).
Authorship
Contribution: S.K., J.S., S.B., and W.P. performed experiments. C.S. and J.L. generated fusion proteins. O.M. produced antibodies. C.G. and N.G. were responsible for mass spectrometry. M.S. supported writing. S.K. and J.S. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johannes Stöckl, Institute of Immunology, Medical University of Vienna, Borschkegasse 8a, A-1090 Vienna, Austria; e-mail: johannes.stoeckl@meduniwien.ac.at.




![Figure 6. Ct-CD45 has immuno-regulatory function. (A) Binding of ct-CD45 to resting T cells and to T cells activated for 24 hours with PMA/ionomycin was analyzed with Alexa Fluor 488-labeled recombinant ct-CD45 molecules and flow cytometry. Pretreatment of cells with unlabeled recombinant protein or mAb 8-301 but not with PTPase inhibitor-blocked binding of fluorescent ct-CD45 (filled histograms) to T cells. The figure shows overlay histogram profiles including Alexa Fluor 488-labeled human serum albumin used as a negative control (open histogram), which are representative of 3 independent experiments. (B) Binding of increasing amounts of Alexa Fluor 488 labeled recombinant ct-CD45 molecules analyzed by flow cytometry. The figure shows mean values plus or minus SEM of 4 experiments of mean fluorescence intensity. The values of nonspecific staining with Alexa-labeled human serum albumin were subtracted from the values obtained with Alexa-labeled ct-CD45. (C) Addition of recombinant ct-CD45 (▲) inhibits T-cell proliferation induced by allogeneic DC (○). The immunosuppressive cytokine IL-10 (■) and recombinant S100A4 proteins (•) were used as positive and negative control, respectively. Data represent mean cpm (± SEM) of 3 independent experiments. (D) T cells were cultured in the presence of immobilized anti-CD3 alone (mock, taken as 100%), anti-CD3 plus control-Ig (co-Ig), or as positive control anti-CD3 plus ICOS-L-Ig or anti-CD3 plus different CD45-Ig-fusion proteins (all Ig-fusion proteins coated at 0.5 or 2 μg/mL) for 72 hours, and proliferation was measured by [3H]thymidine incorporation. Mean values and SD of 3 different experiments are shown. A paired Student t test was performed (***P < .01; **P < .05). (E) Percentages of annexin- and PI-positive T cells cultured as in (D) with 2 μg/mL Ig-fusion proteins coated. Mean values and SD of 3 different experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2008-02-138131/6/m_zh80160822280006.jpeg?Expires=1765893025&Signature=DXXLkAmJHWryYMugj3YtmUJiqa7K4r55S4U9eoLGfA~Ht8wwSwgLU3Kmg~iMAS65LerEyFyv9gGkP6f4WPat4geHnXd45GbypNhebMoc6HkR4jSZzphSadhkq3fuRxvZ7lZCouDAU0c5AEQSC4lqFhvBDMJ4Wi73NbnSuO-zck~drrdaS-0qMV8VDrxKtZ~dly-9uC1ldoTvCU67huQt1xSc270twS5FAGsYVbYK-1VoPnoz7bE4N8XOBew8WIPSWh-THZq3BDmE2ztiUkw~kYL8NHWj3a-R1ZXmhPsznizRbJ2Fjdl-3AMzW31pxVE1k5xCM5zasOyXbhNIm6n~XA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal