Abstract
Despite the evidence for the role of inflammation in cancer initiation, promotion, and progression, the precise mechanism by which the inflammation within tumor is orchestrated by inflammatory cells remains to be determined. Here, we report that tumor-infiltrating mast cells remodel tumor microenvironment and promote tumor growth. Mast cell infiltration and activation in tumors were mainly mediated by tumor-derived stem cell factor (SCF) and its receptor c-Kit on mast cells. Low concentrations of SCF efficiently induced the chemotactic migration of mast cells. Tumor-infiltrating mast cells, activated by higher concentrations of SCF, expressed multiple proinflammatory factors and increased IL-17 expression in tumors. The activity of NF-κB and AP-1 in tumor cells was intensified in the mast cell–remodeled inflammatory microenvironment. SCF-activated mast cells also exacerbated tumor immunosuppression by releasing adenosine and increasing T regulatory cells, which augmented the suppression of T cells and natural killer cells in tumors. These findings emphasize that the remodeling of the tumor microenvironment can actually be initiated by tumor cell–released SCF and suggest that mast cells are not only a participator but also a critical regulator of inflammation and immunosuppression in the tumor microenvironment.
Introduction
Chronic inflammation, a “promoting force” in the tumor microenvironment, has long been known to be commonly braided with the initiation, promotion, and progression of tumorigenesis.1-5 To date, however, it is still incompletely understood how the inflammation in the tumor microenvironment is orchestrated by inflammatory cells. Recently, mast cells were highlighted as not only a major participator but also an important regulator of inflammation,6,7 and their accumulation in tumors has also been well documented,8-13 implying that mast cells may possibly play an important role in orchestrating the inflammation in tumors.
The tumor microenvironment is regarded as a “smoldering” inflammation site in which a lot of cytokines, chemokines, and enzymes mediate the inflammatory process and drive malignant progression.14,15 Among them, TNF-α, IL-6, VEGF, iNOS, Cox-2, and MMP-9 are of particular interest.15-18 Coincidentally, all of them can be produced by mast cells. However, the tumor microenvironment is also characterized by its immunoediting from immunosurveillance to immunosuppression.19 Mast cells have been found to play a critical role in the suppression of immune reactions.20 They not only produce inhibitory cytokine IL-10,21 but they also are essential for the immune tolerance mediated by regulatory T (Treg) cells.22 Thus, mast cell infiltration into tumor may possibly remodel tumor microenvironment and profoundly influence tumor behavior by participating and regulating inflammatory and immune reactions. However, although some studies have shown that mast cells promote tumor angiogenesis and tumor growth because of their properties as inflammatory cells,23-25 the roles of mast cells in tumor progression have been incompletely understood so far. Several key questions remain unclear, especially how mast cells are recruited into the tumor site and whether they can remodel the tumor microenvironment.
Mast cell migration to the tumor site and the following activation may be the prerequisite for their promoting effect on tumors. In this regard, stem cell factor (SCF) is possibly involved, because SCF triggers the c-Kit signaling pathway for the differentiation, migration, maturation, and survival of mast cells.26 In the present study, we investigated the relation of mast cells and SCF in tumor progression and showed that SCF recruited and activated mast cells, the activated mast cells remodeled the tumor microenvironment by intensifying inflammation and immunosuppression, the tumor cell NF-κB and AP-1 activities were augmented, and the suppression of T cells and natural killer (NK) cells was exacerbated in such remodeled microenvironments. These findings provide a new insight into the role of mast cells in tumors and the relation among inflammation, immunosuppression, and tumor.
Methods
Animals and cell lines
BALB/c and C57BL/6 mice, 6 to 8 weeks old, were purchased from the Center of Medical Experimental Animals of Hubei Province (Wuhan, China) and the Center of Experimental Animals of the Chinese Academy of Medical Science (Beijing, China) for studies approved by the Animal Care and Use Committee of Tongji Medical College. Mouse tumor cell lines H22 (hepatocarcinoma), colon-26 (colon cancer), 4T1 (breast cancer), RM1 (prostate cancer), B16 (melanoma), and LLC1 (lung cancer), and human tumor cell lines HepG2 (liver cancer), SNU1 (gastric cancer), Caco2 (colon cancer), A549 (lung cancer), MCF7 (breast cancer), SKOV3 (ovarian cancer), and EC9706 (esophageal cancer) were purchased from the ATCC (Manassas, VA) and the China Center for Type Culture Collection (CCTCC, Wuhan, China) and cultured according to their guidelines.
Generation of bone marrow–derived mast cells
Bone marrow cells were harvested from femurs of mice and cultured in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM HEPES, 50 μM 2-ME, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were cultured in the presence of 10 ng/mL IL-3 (PeproTech, Rocky Hill, NJ), and the nonadherent cells were passaged every 3 days. Four weeks later, the cells were used as mast cells for experiments and referred to as bone marrow–derived mast cells (BMMCs).
Tumor growth experiment
BALB/c mice were inoculated with H22 tumor cells by subcutaneous injection of 105 cells to the left flank. Twelve days later, the mice (n = 8 per group) with tumors approximately 5 × 5 mm2 received 105 BMMCs by intratumor injection or 5 × 106 BMMCs by intravenous injection. When indicated, the mice received intraperitoneal injection of 100 μg of goat anti–mouse SCF neutralizing antibody (IgG; R&D Systems, Minneapolis, MN) or goat IgG isotype control 24 hours and 1 hour before BMMC injection, or they received the intravenous injection of BMMCs mixed with 50 μg rat anti–mouse c-Kit blocking antibody (eBioscience, San Diego, CA) or rat IgG2b isotype control. Tumor growth was monitored by measuring the length (L) and width (W) of tumors. The volume (V) of the tumor was calculated by the formula V = (L × W2)/2.
Analysis of the infiltration of mast cells in tumor tissues
BALB/c mice were inoculated with H22 tumor cells by subcutaneous injection of 105 cells. Twelve days later, 5 × 106 CFSE-labeled BMMCs, with or without antibodies as above, were injected into tumor-bearing mice through the tail vein. The peripheral tumor tissues were surgically excised from mice 24 hours after the injection, and frozen sections were prepared and analyzed by fluorescence microscopy (200×, Leica DMI6000B, Wetzlar, Germany), using HC Plans objective lens and a Leica DFC300 FX camera. Image acquisition and processing were performed using Leica Application Suite software, version 2.3.4.R2.
In vitro migration assay
Fresh H22 tumor tissues were set in the lower chamber, and 6 × 104 BMMCs were set in the upper chamber, in triplicate, of a 24-well transwell apparatus (BD Biosciences, San Jose, CA) and incubated at 37°C for 6 hours. Here, we used the uncoated filter, not the filter coated with fibronectin or other ECM proteins as commonly used,27-30 in the apparatus to avoid the possibility that some tumor-derived factors might affect mast cell migration by influencing the interaction of mast cells with fibronectin or other ECM proteins. Thus, the efficiency of mast cell migration might be not high, but the result was significant and could be used to evaluate the chemotactic effect of SCF on mast cells.31 BMMCs migrating into the lower chamber were enumerated under microscopy, and the migrated cell percentage was calculated. SCF (PeproTech) was used as positive control. Anti-SCF neutralizing antibody, anti–c-Kit antibody, and isotype controls (10 μg/mL) were used in the inhibition test.
Analysis of gene expression by conventional RT-PCR and real-time RT-PCR
Total RNA was extracted from cells with TRIzol reagent (Invitrogen, Carlsbad, CA) or from tissues homogenized in TRIzol according to the manufacturer's instructions. The relative quantity of mRNA was determined by reverse transcription–polymerase chain reaction (RT-PCR: 28 cycles; One-step RT-PCR kit, Qiagen, Valencia, CA). The mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The primer sequences are shown in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
For real-time RT-PCR assays, the cDNA sequences of all detected genes were retrieved from the NCBI database.32 The primers were designed with the Oligo Primer Analysis 4.0 software, and the sequences were blasted (http://www.ncbi.nlm.nih.gov/BLAST/). The primer sequences are shown in Table S2. Total RNA (100 ng) was used for reverse transcription using Superscript II RNase H reverse transcriptase (Invitrogen) in a volume of 25 μL. Then, 2 μL of cDNA was amplified with SYBR Green Universal PCR Mastermix (Bio-Rad, Richmond, CA) in duplicate. For sample analysis, the threshold was set based on the exponential phase of products, and the CT value for samples was determined. The resulting data were analyzed with the comparative CT method for relative gene expression quantification against the housekeeping gene GAPDH.
Western blot analysis
Cell lysates or tumor tissue homogenates (30 μg of total protein) and prestained molecular weight markers were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by the transfer onto nitrocellulose membranes. The membranes were blocked in TBST (Tris-buffered saline with 0.5% Triton X-100) containing 5% nonfat milk and probed with anti–mouse or –human SCF antibodies (R&D Systems). After incubation with the secondary antibody conjugated with horseradish peroxidase, membranes were extensively washed, and the immunoreactivity was visualized by enhanced chemiluminescence according to the manufacturer's protocol (ECL kit; Santa Cruz Biotechnology, Santa Cruz, CA). All antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Assay of soluble SCF and IFN-γ by enzyme-linked immunoabsorbent assay
For the assay of SCF, tumor cells were cultured with or without BMMCs for 24 hours, or 400 mg of tumor tissue was cut into small pieces and cultured in 24-well plate in 1 mL RPMI 1640 medium for 24 hours. The soluble SCF in the supernatants was assessed by Mouse SCF Quantikine ELISA (enzyme-linked immunoabsorbent assay) Kit (R &D Systems) according to the manufacturer's protocol.
For the analysis of the effect of MMP-9 inhibitor on the production of soluble SCF, MMP-9 inhibitor 2-([(4-biphenylsulfonyl) amino]-3-phenyl-propionic acid; Calbiochem, La Jolla, CA) was injected into mice (1 mg/kg, intraperitoneally) 30 minutes before mast cell injection. The control mice received an equal amount of vehicle (1% DMSO). In the in vitro experiments, the inhibitor was added to the culture medium to a final concentration of 0.2 mM.
For the assay of IFN-γ, NK cells were cultured in the presence of IL-2 (50 U/mL) for 24 hours, and IFN-γ in the supernatant was measured by the mouse IFN-γ ELISA Kit (R&D Systems).
Construction of H22 tumor cell line expressing SCF-siRNA
SCF sense and antisense siRNAs were generated using the Silencer siRNA construction kit according to the manufacturer's instruction (Ambion, Austin, TX). After hybridization and purification, the different double-stranded SCF-siRNAs and control siRNA were transiently transfected into H22 tumor cells using GeneSilencer siRNA transfection reagent (Gene Therapy Systems, San Diego, CA). The most efficient SCF-siRNA sequence (cagtcaagtcttacaaggg) and its control siRNA (ctggtcaagtctacaagag) were verified by RT-PCR detection of SCF mRNA 24 hours after transfection and inserted into RNAi-Ready pSIREN-RetroQ–expressing vector with the U6 promoter (Clontech, Mountain View, CA). The recombinant SCF siRNA-expressing plasmids and control plasmids were transfected into H22 tumor cell using FuGENE 6 transfection reagent (Roche, Indianapolis, IN) for stable expression after selection.
MMP-9 assay by gelatin zymography
Active MMP-9 mediates the shedding of membrane-associated SCF.33 To evaluate the influence of mast cell–derived MMP-9 on the release of SCF, the production of MMP-9 by mast cells was analyzed by gelatin zymography as described previously.34 Briefly, proteins prepared from mast cells were separated by 7.5% SDS-PAGE containing 1% gelatin. The gels were incubated in MMP activation buffer containing 50 mM Tris (pH 8.0) and 10 mM CaCl2 at 37°C overnight, and then stained with 1% Coomassie Brilliant Blue R-250 for 3 hours and destained in 10% (vol/vol) methanol and 5% (vol/vol) acetic acid.
Clinical tumor specimens and murine tumor specimens
Clinical tumor specimens were acquired by surgery from untreated cancer patients, which was approved by the Ethical Committee of the Medical Faculty of Tongji Medical College. Informed consent was obtained in accordance with the Declaration of Helsinki from all subjects. Seven types of tumor specimens were collected, including breast cancer, gastric cancer, colon cancer, lung cancer, ovarian cancer, liver cancer, and esophageal cancer. For each type of tumor, specimens were acquired from 4 to 5 patients.
To obtain different murine tumor specimens, mice were inoculated with tumor cells by subcutaneous injection of 105 cells to the left flank. BALB/c mice were inoculated with H22 cells, colon-26 cells, or 4T1 cells, respectively. C57BL/6 mice were inoculated with RM1 cells, B16 cells, or LLC1 cells, respectively. Tumors were surgically dissected for the experiments when tumor size reached approximately 5 × 5 mm2.
Flow cytometric analysis
Mouse tumor cells or human tumor cells were incubated with biotin-conjugated goat anti–mouse SCF or goat anti–human SCF and the corresponding isotype control IgG for 30 minutes at 4°C. After washing, the cells were further incubated with PE-conjugated streptavidin for flow cytometric analysis. All antibodies were purchased from R&D Systems. Variables were acquired on a fluorescence-activated cell sorting Calibur flow cytometer (BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
For IL-17 intracellular staining, tumor-infiltrating immune cells were isolated from tumor tissue,35 and then fixed and permeabilized with Fix/Perm solution (BD Biosciences). The cells were then resuspended in Perm buffer and incubated with FITC-labeled anti–mouse IL-17 antibody or isotype control (eBioscience) at room temperature in the dark for 20 minutes.
For intracellular staining of Foxp3, tumor-infiltrating immune cells were stained with PE-cy3–conjugated anti–mouse CD3. After cellular surface staining, the cells were treated with Fix/Perm solution and restained with FITC-labeled anti–mouse Foxp3 antibody or isotype control (eBioscience).
Assay of the activities of NF-κB and AP-1
BMMCs (5 × 106), with or without anti–c-Kit antibody as indicated, were injected by the tail vein into mice (n = 5 per group) bearing WT H22 tumor or SCF-knockdown H22 tumor. The mice were killed 72 hours later, and tumor cells were isolated from peripheral tumor tissues.35 The nuclear extract was prepared with Nuclear Extraction Kit (Millipore, Billerica, MA). The activities of NF-κB and AP-1 in nuclear extract were determined by NF-κB Assay kit and AP-1 Assay kit (Millipore), respectively, according to the manufacturer's protocol.
Isolation of T cells and NK cells
Tumor tissues from different groups were digested with collagenase and hyaluronidase for 1 hour at 37°C and minced. After lysis of red blood cells, the dissociated cells were underlaid with 5 mL of Lymphocyte-M solution and centrifuged at 1100g for 20 minutes. Tumor-infiltrating lymphocytes were harvested from the interface. T cells were isolated on a T-cell enrichment column (R&D Systems), and NK cells were purified by magnetic sorting with biotinylated-DX5 antibody. T cells and NK cells were also isolated from spleen of normal mice and used in the related experiments.
T-cell proliferation assay
The isolated T cells (104) were cultured in the presence or absence of anti-CD3 and anti-CD28 antibodies (1 μg/mL each) in 96-well culture plates. [3H]-thymidine was added during the last 10 hours of the 72-hour culture, and then the incorporation of [3H]-thymidine was measured to determine T-cell proliferation. The results were expressed as stimulation index (cpm of stimulated cells/cpm of unstimulated cells).
Assay of adenosine
The peripheral tumor tissues were frozen and powdered in liquid nitrogen. The tissue powder (1 mg) was mixed with 1 mL of 0.6 M perchloric acid by vigorous vortexing and sonicated with a microsonicator for 10 seconds at 45 W. Then, the mixture was centrifuged at 11 000g for 20 minutes at 4°C. The supernatant was neutralized with 5 M K2CO3 to pH 7.2, centrifuged at 1100g to remove precipitates, and stored at −80°C until high-performance liquid chromatography (HPLC) analysis. HPLC analysis of adenosine was performed as previously described.36
For the assay of adenosine in the culture supernatant, 1 mL of supernatant was mixed with 30 μL of perchloric acid (4.4 M), and centrifuged at 16 000g for 10 minutes at 4°C. The supernatant was neutralized to pH 7.2 and analyzed by HPLC.
Statistics
Results were expressed as mean values plus or minus SD and interpreted by repeated-measure ANOVA. Differences were considered to be statistically significant when the P value was less than .05.
Results
Mast cells promote tumor growth on the basis of SCF/c-Kit–mediated migration into tumor
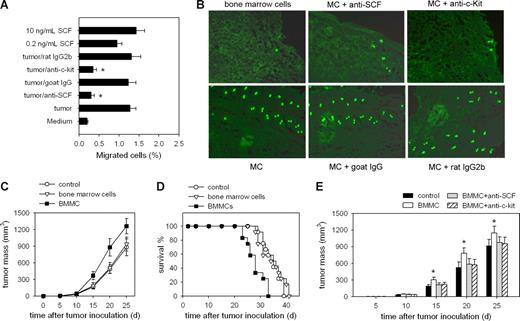
To verify whether the chemotactic migration of mast cells into tumor is the prerequisite for their tumor-promoting activity, we first investigated whether SCF is the main chemokine mediating the migration of mast cells into tumor. The result from transwell assay showed that the migration of BMMCs was induced by the cultured tumor tissues, and the migration was significantly inhibited by SCF neutralizing antibody and c-Kit–blocking antibody (Figure 1A). When CFSE-labeledBMMCs were injected into mice bearing H22 hepatocarcinoma through the tail vein, both anti-SCF and anti–c-Kit antibodies effectively impaired the infiltration of BMMCs into the tumor (Figure 1B), indicating that the migration is mainly mediated by SCF released from tumor tissues. We then injected BMMCs into tumor-bearing mice to analyze the effect of mast cells on tumor growth. The intravenous injection of BMMCs markedly accelerated the growth of the tumor (Figure 1C) and shortened the survival of mice (Figure 1D), both anti-SCF and anti–c-Kit antibodies abolished the tumor-promoting effect of the intravenously injected BMMCs (Figure 1E). These data suggest that SCF/c-Kit axis–triggered mast cell migration into tumor is the prerequisite for their tumor-promoting activity.
Mast cells promote tumor growth with SCF/c-Kit–mediated chemotactic migration as the prerequisite. (A) SCF induces the migration of mast cells (MCs). The migration of MCs in transwell assay was determined in the presence of tumor tissues, antibodies, or SCF. *P < .05 compared with tumor tissue group. (B) Infiltration of circulating MCs into the tumor. CFSE-labeled BMMCs with or without antibodies were injected into tumor-bearing mice by the tail vein. The peripheral tumor tissues were surgically excised from mice 24 hours after the injection, and frozen sections were prepared and analyzed by fluorescence microscopy. (C) Mast cells promote tumor growth. BMMCs were injected into tumor-bearing mice by intravenous injection. Bone marrow cells were used as control. The growth of tumor was monitored. (D) Survival rate follow-up after the intravenous injection of BMMCs. The survival period of tumor-bearing mice in the BMMC injection group was significantly shortened, compared with that in the control groups (n = 12; P < .05, Kaplan-Meier analysis). The data were the representative of 2 independent experiments in which the similar results were obtained. (E) Dependence of tumor-promoting effect of MCs on the SCF/c-Kit axis. BMMCs, with or without antibodies, were injected into tumor-bearing mice by the tail vein. Both of 2 antibodies abolished the tumor-promoting effect of MCs. *P < .05, compared with the control group. Error bars represent SD.
Mast cells promote tumor growth with SCF/c-Kit–mediated chemotactic migration as the prerequisite. (A) SCF induces the migration of mast cells (MCs). The migration of MCs in transwell assay was determined in the presence of tumor tissues, antibodies, or SCF. *P < .05 compared with tumor tissue group. (B) Infiltration of circulating MCs into the tumor. CFSE-labeled BMMCs with or without antibodies were injected into tumor-bearing mice by the tail vein. The peripheral tumor tissues were surgically excised from mice 24 hours after the injection, and frozen sections were prepared and analyzed by fluorescence microscopy. (C) Mast cells promote tumor growth. BMMCs were injected into tumor-bearing mice by intravenous injection. Bone marrow cells were used as control. The growth of tumor was monitored. (D) Survival rate follow-up after the intravenous injection of BMMCs. The survival period of tumor-bearing mice in the BMMC injection group was significantly shortened, compared with that in the control groups (n = 12; P < .05, Kaplan-Meier analysis). The data were the representative of 2 independent experiments in which the similar results were obtained. (E) Dependence of tumor-promoting effect of MCs on the SCF/c-Kit axis. BMMCs, with or without antibodies, were injected into tumor-bearing mice by the tail vein. Both of 2 antibodies abolished the tumor-promoting effect of MCs. *P < .05, compared with the control group. Error bars represent SD.
Tumor cell–derived SCF is responsible for the infiltration of mast cells into the tumor
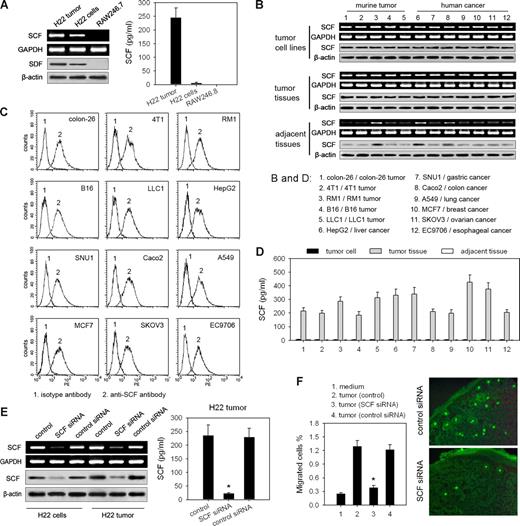
Next, we tested whether SCF is mainly produced by tumor cells. By detecting the expression of SCF, we found that the cultured H22 cells expressed both mRNA and protein of SCF, whereas SCF was not detectable in the culture supernatants (Figure 2A), suggesting that SCF is a membrane-associated protein on tumor cells and might be released in tumor tissue. To confirm this, we additionally detected SCF expression in another 5 murine tumor cell lines, 7 human tumor cell lines, and the corresponding mouse and human tumor tissues. SCF expression was very high in tumor cells and tumor tissues, but very low in the adjacent tissues around the tumor (Figure 2B). All of these tumor cells expressed membrane-associated SCF (Figure 2C), whereas SCF was only detectable in the supernatants of the cultured tumor tissues but not in the supernatants of either tumor cells or adjacent tissues around the tumor (Figure 2D). Thus, we constructed a H22 tumor cell line stably expressing SCF siRNA (Figure 2E). Both SCF expression and SCF release were strikingly decreased in siRNA-expressing tumors (Figure 2E), indicating that the soluble SCF in tumor tissue is mainly released from tumor cells. More importantly, SCF-knockdown tumors could not efficiently attract BMMCs either in vitro or in vivo (Figure 2F), suggesting that mast cell infiltration into the tumor is mainly induced by tumor cell–produced SCF.
Tumor cell–derived SCF is responsible for the infiltration of mast cells into tumor. (A) Assay of SCF expression in H22 tumor or tumor cells. SCF expression was detected by RT-PCR and Western blot, respectively. SCF in the supernatants of the cultured tumor tissue or tumor cells was assayed by ELISA. Mouse monocyte system cell line RAW246.7 was used as the negative control. (B) Assay of SCF expression in tumor cells and tumor tissues. SCF expression in murine tumor cell lines and human tumor cell lines, corresponding murine tumor and specimens from human tumor, and normal tissue adjacent to tumor was detected by RT-PCR and Western blot, respectively. (C) SCF on the surface of different tumor cells was analyzed by flow cytometry. (D) Assay of soluble SCF produced by different tumors. Tumor cell lines, the corresponding tumor tissues, and the adjacent tissues around the tumor were cultured in vitro. SCF in the supernatants was detected by ELISA. (E) Silence of SCF expression in H22 tumor cells by SCF siRNA. SCF expression was detected by RT-PCR and Western blot, respectively. The soluble SCF released from tumor tissue was assayed by ELISA. (F) SCF-knockdown tumor cannot efficiently induce the migration of MCs. SCF-knockdown or control tumor tissues were used for transwell assay of MC migration (left). The infiltration of circulating MCs into SCF-knockdown or control tumor tissue (right) was analyzed using the same protocol as that in Figure 1B. *P < .05, compared with control tumor. Error bars represent SD.
Tumor cell–derived SCF is responsible for the infiltration of mast cells into tumor. (A) Assay of SCF expression in H22 tumor or tumor cells. SCF expression was detected by RT-PCR and Western blot, respectively. SCF in the supernatants of the cultured tumor tissue or tumor cells was assayed by ELISA. Mouse monocyte system cell line RAW246.7 was used as the negative control. (B) Assay of SCF expression in tumor cells and tumor tissues. SCF expression in murine tumor cell lines and human tumor cell lines, corresponding murine tumor and specimens from human tumor, and normal tissue adjacent to tumor was detected by RT-PCR and Western blot, respectively. (C) SCF on the surface of different tumor cells was analyzed by flow cytometry. (D) Assay of soluble SCF produced by different tumors. Tumor cell lines, the corresponding tumor tissues, and the adjacent tissues around the tumor were cultured in vitro. SCF in the supernatants was detected by ELISA. (E) Silence of SCF expression in H22 tumor cells by SCF siRNA. SCF expression was detected by RT-PCR and Western blot, respectively. The soluble SCF released from tumor tissue was assayed by ELISA. (F) SCF-knockdown tumor cannot efficiently induce the migration of MCs. SCF-knockdown or control tumor tissues were used for transwell assay of MC migration (left). The infiltration of circulating MCs into SCF-knockdown or control tumor tissue (right) was analyzed using the same protocol as that in Figure 1B. *P < .05, compared with control tumor. Error bars represent SD.
Activation of mast cells by SCF is necessary for their tumor-promoting effect
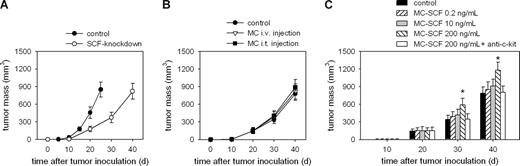
We then investigated the role of SCF in mast cell–mediated tumor promotion. Compared with WT tumors, the inoculation of SCF-knockdown tumor cells generated a much retarded tumor growth. The SCF-knockdown tumor on day 40 after inoculation reached similar size as that of WT tumor on day 25 (Figure 3A), indicating that SCF and mast cells are crucial for tumor development. As expected, the intravenous injection of BMMCs had no effect on the growth of SCF-knockdown tumors (Figure 3B). Unexpectedly, the intratumor injection of BMMCs, untreated or pretreated with low concentration of SCF (0.2 ng/mL or 10 ng/mL), to the SCF-knockdown tumor did not promote tumor growth either (Figure 3B), whereas the injection of BMMCs pretreated with higher concentration of SCF (200 ng/mL) promoted the growth of the tumor (Figure 3C). This finding implies that SCF not only mediates the chemotactic migration of mast cells into tumor but it also activates, at higher concentration, mast cells to generate the tumor-promoting effect.
Activation of mast cells by SCF is necessary for their tumor-promoting effect. (A) SCF-knockdown retards tumor growth. Mice (n = 8 per group) were inoculated with SCF-knockdown H22 cells and control WT H22 cells, respectively. The growth of tumor was monitored. (B,C) Mice (n = 8 per group) were inoculated with SCF-knockdown H22 tumor cells. When tumor size reached approximately 5 × 5 mm2, the mice received BMMCs either by intravenous (i.v.) injection or by intratumor (i.t.) injection (B), or received the intratumor injection of BMMCs pretreated with different concentrations of SCF and anti–c-kit antibody (20 μg/mL) as indicated (C). The growth of the tumor was promoted only by the intratumor injection of MCs pretreated with a higher concentration of SCF (200 ng/mL), which was abolished by anti–c-kit antibody. Error bars represent SD.
Activation of mast cells by SCF is necessary for their tumor-promoting effect. (A) SCF-knockdown retards tumor growth. Mice (n = 8 per group) were inoculated with SCF-knockdown H22 cells and control WT H22 cells, respectively. The growth of tumor was monitored. (B,C) Mice (n = 8 per group) were inoculated with SCF-knockdown H22 tumor cells. When tumor size reached approximately 5 × 5 mm2, the mice received BMMCs either by intravenous (i.v.) injection or by intratumor (i.t.) injection (B), or received the intratumor injection of BMMCs pretreated with different concentrations of SCF and anti–c-kit antibody (20 μg/mL) as indicated (C). The growth of the tumor was promoted only by the intratumor injection of MCs pretreated with a higher concentration of SCF (200 ng/mL), which was abolished by anti–c-kit antibody. Error bars represent SD.
SCF-stimulated mast cells augment the release of SCF from H22 tumor cells
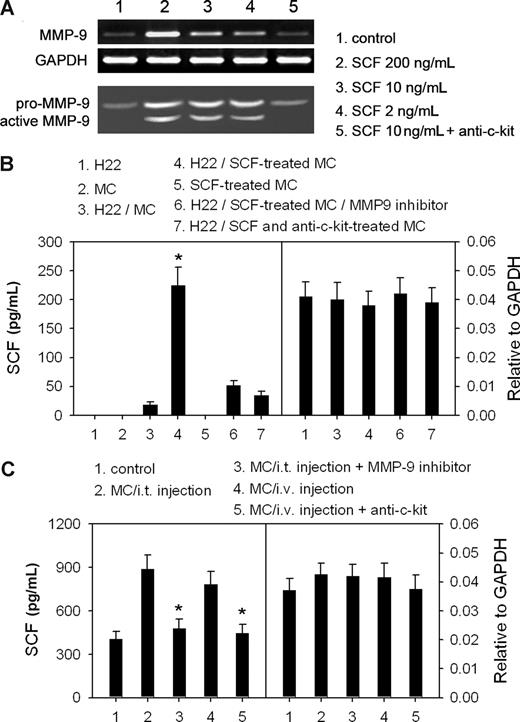
Active MMP-9 can release membrane-bound SCF.33 Coincidentally, mast cells have been found to produce MMP-9 for their migration.37 Therefore, we wondered whether the SCF/c-Kit signal can regulate the expression of MMP-9 in mast cells, therefore promoting the release of SCF from tumor cells. The cultured BMMCs expressed a low level of a latent form of MMP-9. However, as low as 2 ng/mL SCF could stimulate BMMCs to produce more latent MMP-9 and active MMP-9 (Figure 4A). SCF-promoted production of MMP-9 by mast cells could be inhibited by anti–c-Kit antibody (Figure 4A), suggesting that SCF/c-Kit signal promotes the production and activation of MMP-9 in mast cells. In addition, SCF-treated BMMCs significantly increased the concentration of SCF in the supernatant of H22 cells, whereas this effect was inhibited by MMP-9 inhibitor. Moreover, the release of SCF was not increased if BMMCs were pretreated with SCF in the presence of anti–c-Kit (Figure 4B). Under each of the above conditions, SCF mRNA levels were not significantly influenced (Figure 4B). Consistent with the in vitro data, a much higher level of SCF was detected in tumor tissue after intratumor injection or intravenous injection of mast cells (Figure 4C), but SCF mRNA levels were not changed (Figure 4C). In these situations, the increased release of SCF was also suppressed by MMP-9 inhibitor and anti–c-Kit (Figure 4C). Taken together, these data suggest that SCF/c-Kit signal can stimulate mast cells to produce active MMP-9, and the latter then increases the level of soluble SCF in tumor by promoting the release of SCF from tumor cells, thereby favoring the activation of mast cells in tumor.
SCF-stimulated mast cells augment the release of SCF from tumor cells. (A) SCF stimulates the production of active MMP-9 by mast cells (MCs). BMMCs were cultured for 24 hours in the presence of different concentrations of SCF and anti–c-Kit (10 μg/mL). The production of MMP-9 was detected by RT-PCR and gelatin zymography. (B) MC-derived MMP-9 increased the release of SCF from H22 tumor cells. BMMCs were treated with 5 ng/mL of SCF for 4 hours in the absence or presence of 10 μg/mL anti–c-Kit antibody. H22 cells and SCF-treated BMMCs were cultured alone or in 2 chambers separated by semipermeable membrane. SCF in the supernatants was detected by ELISA (left). SCF mRNA was detected by real-time RT-PCR (right). *P < .05, compared with the H22/MC group. (C) Assay of SCF in tumor tissues. Tumor-bearing mice received the intraperitoneal injection of MMP-9 inhibitor and the intratumor (i.t.) injection of MCs, or received the intravenous (i.v.) injection of MCs with anti–c-Kit antibody. The tumor tissues were excised 48 hours after MC injection and cultured in vitro. SCF in the supernatants was detected by ELISA (left). The mRNA level of SCF in tumor tissues was detected by real-time RT-PCR (right). *P < .05, compared with the MC injection groups. Error bars represent SD.
SCF-stimulated mast cells augment the release of SCF from tumor cells. (A) SCF stimulates the production of active MMP-9 by mast cells (MCs). BMMCs were cultured for 24 hours in the presence of different concentrations of SCF and anti–c-Kit (10 μg/mL). The production of MMP-9 was detected by RT-PCR and gelatin zymography. (B) MC-derived MMP-9 increased the release of SCF from H22 tumor cells. BMMCs were treated with 5 ng/mL of SCF for 4 hours in the absence or presence of 10 μg/mL anti–c-Kit antibody. H22 cells and SCF-treated BMMCs were cultured alone or in 2 chambers separated by semipermeable membrane. SCF in the supernatants was detected by ELISA (left). SCF mRNA was detected by real-time RT-PCR (right). *P < .05, compared with the H22/MC group. (C) Assay of SCF in tumor tissues. Tumor-bearing mice received the intraperitoneal injection of MMP-9 inhibitor and the intratumor (i.t.) injection of MCs, or received the intravenous (i.v.) injection of MCs with anti–c-Kit antibody. The tumor tissues were excised 48 hours after MC injection and cultured in vitro. SCF in the supernatants was detected by ELISA (left). The mRNA level of SCF in tumor tissues was detected by real-time RT-PCR (right). *P < .05, compared with the MC injection groups. Error bars represent SD.
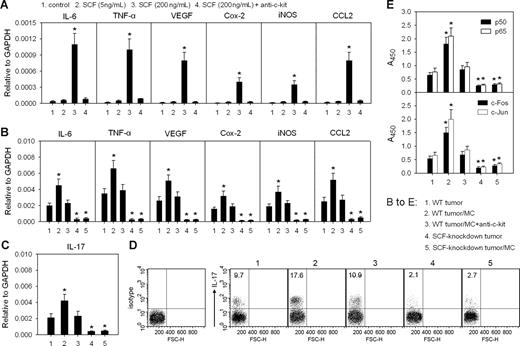
SCF-activated mast cells remodel tumor inflammatory microenvironment
The activated mast cells can release a large number of proinflammatory factors. Here, we found that the expressions of proinflammatory genes encoding IL-6, TNF-α, VEGF, Cox-2, iNOS, and CCL2 were up-regulated in BMMCs in the presence of SCF at high concentration (200 ng/mL) but not low concentration (5 ng/mL; Figure 5A). This up-regulation could be abolished by anti–c-Kit antibody (Figure 5A), indicating that SCF/c-Kit signal activates mast cells. Consistent with in vitro data, the intravenous injection of BMMCs resulted in the significant increase of mRNA levels of the above proinflammatory genes in WT tumor, which was abolished by anti–c-Kit antibody (Figure 5B). The intravenous injection of BMMCs into SCF-knockdown tumor did not significantly change the mRNA levels of these genes in the tumor (Figure 5B). More importantly, the intravenous injection of BMMCs also increased the transcription of IL-17 gene and the amount of IL-17–producing cells in the WT tumor but not in the SCF-knockdown tumor (Figure 5C,D). These data suggest that SCF-activated mast cells remodel tumor inflammatory microenvironment. Because the activities of NF-κB and AP-1, 2 key transcription factors, in tumor cells can be increased by the proinflammatory factors such as TNF-α and others,38 we then further analyzed the influence of the mast cell–remodeled environment on tumor cells by detecting the activities of NF-κB and AP-1 in tumor cells. The result showed that the activities of p65/p50 and c-Jun/c-Fos were significantly increased in the tumor cells isolated from the peripheral tumor tissue after mast cell injection (Figure 5E). Anti–c-Kit antibody hindered the increase of such activities, and the injection of BMMCs into SCF-knockdown tumor did not significantly alter the activities of NF-κB and AP-1 (Figure 5E).
SCF/c-Kit signal induces the mast cell–mediated remodeling of tumor inflammatory microenvironment. (A) Expression of proinflammatory genes in mast cells. Mast cells were cultured in the presence or absence of SCF and anti–c-Kit antibody. The levels of IL-6, TNF-α, VEGF, Cox-2, iNOS, and CCL2 mRNAs were detected by real-time PCR. (B-E) Expression of proinflammatory genes in tumor and the activities of NF-κB and AP-1 in tumor cells. The mice bearing WT H22 tumor received the intravenous injection of mast cells and anti–c-Kit antibody as indicated. The mice bearing SCF-knockdown H22 tumor received the intratumor injection of mast cells. The levels of IL-6, TNF-α, VEGF, Cox-2, iNOS, CCL2, and IL-17 mRNAs in tumor tissues were detected by real-time PCR (B,C). IL-17 expression (IL-17+) cells in immune cells from tumor were analyzed by flow cytometry (D). Numbers on plots are percentages of total cells gated. Tumor cells were isolated from tumor tissue as described in “Assay of the activities of NF-κB and AP-1.” (E). *P < .05, compared with control tumor cells or WT tumor control. Error bars represent SD.
SCF/c-Kit signal induces the mast cell–mediated remodeling of tumor inflammatory microenvironment. (A) Expression of proinflammatory genes in mast cells. Mast cells were cultured in the presence or absence of SCF and anti–c-Kit antibody. The levels of IL-6, TNF-α, VEGF, Cox-2, iNOS, and CCL2 mRNAs were detected by real-time PCR. (B-E) Expression of proinflammatory genes in tumor and the activities of NF-κB and AP-1 in tumor cells. The mice bearing WT H22 tumor received the intravenous injection of mast cells and anti–c-Kit antibody as indicated. The mice bearing SCF-knockdown H22 tumor received the intratumor injection of mast cells. The levels of IL-6, TNF-α, VEGF, Cox-2, iNOS, CCL2, and IL-17 mRNAs in tumor tissues were detected by real-time PCR (B,C). IL-17 expression (IL-17+) cells in immune cells from tumor were analyzed by flow cytometry (D). Numbers on plots are percentages of total cells gated. Tumor cells were isolated from tumor tissue as described in “Assay of the activities of NF-κB and AP-1.” (E). *P < .05, compared with control tumor cells or WT tumor control. Error bars represent SD.
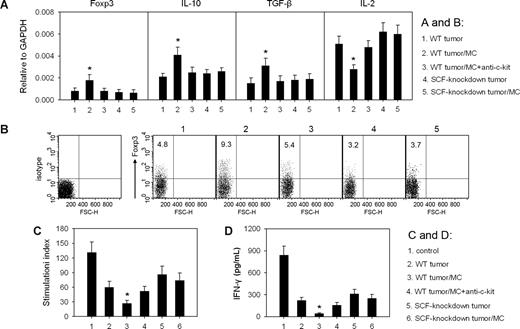
SCF-activated mast cells remodel tumor microenvironment by intensifying immunosuppression
To investigate whether SCF-activated mast cells contribute to tumor immune suppression, we analyzed the expressions of immune-associated genes in tumors. Among them, IL-2 is related to immune activation; IL-10, TGF-β, and Foxp3 are related to immune suppression. After intravenous injection of mast cells into mice, IL-2 mRNA in the tumor was decreased, and the levels of IL-10, TGF-β, and Foxp3 mRNAs were increased (Figure 6A). Consistent with the increased expression of TGF-β and Foxp3, the percentage of Treg cells in total T cells in the tumor was also increased (Figure 6B). In line with this, the intravenous injection of mast cells intensified the suppression of T lymphocytes and NK cells, 2 major types of cytotoxic effector cells, in tumor microenvironment, evaluated by the further decreased response of T cells to the stimulation of anti-CD3 and anti-CD28 antibodies (Figure 6C) and the reduced production of IFN-γ by NK cells (Figure 6D). The administration of anti–c-Kit antibody suppressed the effect of mast cell injection on the gene expression pattern and the activities of T cells and NK cells in the tumor microenvironment (Figure 6A,C,D). However, the injection of mast cells into SCF-knockdown tumors produced a much less effect on the gene expression pattern and the activities of T cells and NK cells (Figure 6A,C,D). Taken together, these data indicate that SCF-activated mast cells can exacerbate the immunosuppression in the tumor microenvironment.
SCF/c-Kit signal activates mast cells to exacerbate the immunosuppression in tumor microenvironment. When tumor size reached approximately 5 × 5 mm2, the mice bearing WT H22 tumor received the intravenous injection of mast cells (MCs) and anti–c-Kit antibody as indicated, and the mice bearing SCF-knockdown tumor received the intratumor injection of MCs. (A) The expression of Foxp3 and cytokine genes in tumor. The levels of Foxp3, IL-10, TGF-β, and IL-2 mRNAs in tumor tissues were detected by real-time PCR 72 hours after the injection of mast cells. (B) Treg cells (Foxp3+) in T cells (gated CD3+ cells) from tumor were analyzed by flow cytometry. Numbers on plots are percentages of total cells gated. (C,D) Mast cells intensify the suppression of T cells and NK cells in tumor. Seventy-two hours after the injection of mast cells, T cells and NK cells were isolated from the tumor. The proliferation of T cells (C) and the production of IFN-γ by NK cells (D) were determined as described in “Assay of soluble SCF and IFN-γ by enzyme-linked immunosorbent assay.” T cells and NK cells isolated from normal spleen were used as control. *P < .05, compared with WT tumor control. Error bars represent SD.
SCF/c-Kit signal activates mast cells to exacerbate the immunosuppression in tumor microenvironment. When tumor size reached approximately 5 × 5 mm2, the mice bearing WT H22 tumor received the intravenous injection of mast cells (MCs) and anti–c-Kit antibody as indicated, and the mice bearing SCF-knockdown tumor received the intratumor injection of MCs. (A) The expression of Foxp3 and cytokine genes in tumor. The levels of Foxp3, IL-10, TGF-β, and IL-2 mRNAs in tumor tissues were detected by real-time PCR 72 hours after the injection of mast cells. (B) Treg cells (Foxp3+) in T cells (gated CD3+ cells) from tumor were analyzed by flow cytometry. Numbers on plots are percentages of total cells gated. (C,D) Mast cells intensify the suppression of T cells and NK cells in tumor. Seventy-two hours after the injection of mast cells, T cells and NK cells were isolated from the tumor. The proliferation of T cells (C) and the production of IFN-γ by NK cells (D) were determined as described in “Assay of soluble SCF and IFN-γ by enzyme-linked immunosorbent assay.” T cells and NK cells isolated from normal spleen were used as control. *P < .05, compared with WT tumor control. Error bars represent SD.
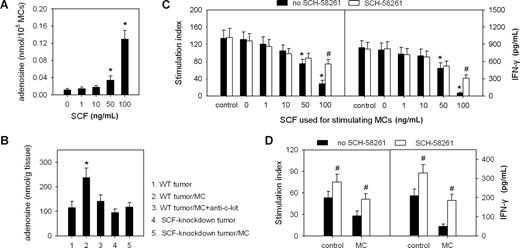
SCF-activated mast cells release adenosine in the tumor microenvironment
The cultured BMMCs may release adenosine after stimulation.36 Recently, adenosine has been reported to inhibit the production of IL-2 and IFN-γ by T cells.39,40 Thus, SCF-activated mast cells might release adenosine to directly suppress T cells and NK cells. To test this, we first detected the release of adenosine by BMMCs in the presence of SCF. SCF could stimulate BMMCs to release adenosine in a dose-dependent manner (Figure 7A). However, the intravenous injection of mast cells into mice bearing WT tumor increased the level of adenosine in tumor, which was suppressed by anti–c-Kit antibody, whereas the intratumor injection of mast cells did not increase the level of adenosine in the SCF-knockdown tumor (Figure 7B). We next investigated the effect of mast cell–released adenosine on T-cell proliferation driven by anti-CD3 and anti-CD28 antibodies and the production of IFN-γ by NK cells in response to IL-2 stimulation. Both T-cell proliferation and the production of IFN-γ by NK cells were inhibited in the presence of the supernatants of mast cells stimulated with 100 ng/mL of SCF (Figure 7C). The inhibitory effect was significantly relieved by adenosine receptor A2A antagonist SCH-58261 (Sigma-Aldrich, St Louis, MO; Figure 7C). We then used SCH-58261 to interfere with adenosine signaling pathway in vivo. The results showed that SCH-58261 not only relieved the suppression of T cells and NK cells after the injection of mast cells, but it also augmented the activities of T cells and NK cells in control tumor (Figure 7D).
SCF-activated mast cells release adenosine to suppress the immune response. (A) Assay of adenosine released by BMMCs after the stimulation with SCF. BMMCs were cultured in the absence or presence of SCF for 48 hours. The adenosine in the supernatant was assayed as described in “Assay of adenosine.” (B) Assay of adenosine in tumor tissues. The mice bearing WT H22 tumor received the intravenous injection of mast cells and anti-c-Kit antibody as indicated. The mice bearing SCF-knockdown H22 tumor received the intratumor injection of mast cells. Seventy-two hours later, the adenosine in tumor tissues was assayed as described in “Assay of adenosine.” *P < .05, compared with the 0 ng/mL SCF group or the WT tumor group. (C,D) Mast cell–produced adenosine inhibits T cells and NK cells. Splenic T cells and NK cells were cultured with the culture supernatant of SCF-stimulated mast cells or control SCF medium in the presence or absence of adenosine receptor A2A antagonist SCH-58261 (C). The T cells and NK cells from tumor were isolated from the mice bearing WT H22 tumor 72 hours after the intratumor injection of mast cells or control bone marrow cells with or without SCH-58261 (D). The proliferation of T cells (left) and the production of IFN-γ by NK cells (right) were determined as described in “Assay of soluble SCF and IFN-γ by enzyme-linked immunosorbent assay.” *P < .05, compared with the 0 ng/mL SCF, control, or WT tumor groups; #P < .05, compared with the no-SCH-58261 group. Error bars represent SD.
SCF-activated mast cells release adenosine to suppress the immune response. (A) Assay of adenosine released by BMMCs after the stimulation with SCF. BMMCs were cultured in the absence or presence of SCF for 48 hours. The adenosine in the supernatant was assayed as described in “Assay of adenosine.” (B) Assay of adenosine in tumor tissues. The mice bearing WT H22 tumor received the intravenous injection of mast cells and anti-c-Kit antibody as indicated. The mice bearing SCF-knockdown H22 tumor received the intratumor injection of mast cells. Seventy-two hours later, the adenosine in tumor tissues was assayed as described in “Assay of adenosine.” *P < .05, compared with the 0 ng/mL SCF group or the WT tumor group. (C,D) Mast cell–produced adenosine inhibits T cells and NK cells. Splenic T cells and NK cells were cultured with the culture supernatant of SCF-stimulated mast cells or control SCF medium in the presence or absence of adenosine receptor A2A antagonist SCH-58261 (C). The T cells and NK cells from tumor were isolated from the mice bearing WT H22 tumor 72 hours after the intratumor injection of mast cells or control bone marrow cells with or without SCH-58261 (D). The proliferation of T cells (left) and the production of IFN-γ by NK cells (right) were determined as described in “Assay of soluble SCF and IFN-γ by enzyme-linked immunosorbent assay.” *P < .05, compared with the 0 ng/mL SCF, control, or WT tumor groups; #P < .05, compared with the no-SCH-58261 group. Error bars represent SD.
Discussion
Our present findings strongly suggest that mast cells infiltrating into tumor produce a protumor effect by remodeling tumor microenvironment. Given that the expression of SCF was found in all the tumor cell lines or tumor tissues of different tumor types tested in this study, such remodeling process may be general in most types of tumors.
During the development of tumor, circulating mast cells migrate into tumor and form one of the major stromal cell populations. Although various factors may induce the chemotactic migration of mast cells, our data in this report suggest that SCF/c-Kit signal is mainly responsible for the migration of mast cells into tumor, because mast cells failed to migrate into SCF-knockdown tumor, and anti–c-Kit antibody abolished the migration of mast cells into WT tumor. Except for mast cell migration and adhesion to extracellular matrix components, the SCF/c-Kit signal is also critical for the survival and functional activation of mast cells and the secretion of mediators from mast cells.41 Importantly, our data show that SCF acts on mast cells in a dose-dependent manner. Low dose of SCF induces both the chemotactic migration of mast cells and the production of active MMP-9 by mast cells, whereas higher concentration of SCF is required for the activation and mediator-secretion of mast cells. The accumulation and activation of mast cells in tumor can be intensified by a vicious feedback circle between mast cells and SCF. SCF-stimulated mast cells produce MMP-9 which not only facilitates the migration of mast cells into tumor but also augments the release of SCF from tumor cells. The increased release of SCF is in favor of both the accumulation and further activation of mast cells.
Inflammation is a fundamental character of the tumor microenvironment.2-4 Numerous proinflammatory factors existent in the tumor microenvironment that influence the growth and metastasis of tumors. Many of them are produced by mast cells. Mast cells were thought to be detrimental to tumor cells by releasing IL-4, TNF-α, and others to induce the apoptosis of tumor cells.42-44 However, much evidence obtained in recent years indicates that these inflammatory cytokines are beneficial to tumors.18-20 In addition, other proinflammatory factors and inflammation-related enzymes also benefit tumor development. Our previous studies have shown that IL-6, iNOS, and CCL2 have the protumor effects through different mechanisms.45,46 TNF-α, VEGF, and Cox-2 also play important roles in tumor initiation and progression.18 In this study, we found that the SCF/c-Kit axis is crucial for the production of these factors and enzymes in tumor, because the transcription activities of these genes in SCF-knockdown tumors were very low. However, mast cells, relying on the SCF/c-Kit signal, make the main contribution to the production of these factors and enzymes in tumors. Mast cells remodeled the tumor inflammatory microenvironment by not only producing the above factors but also increasing the production of IL-17, a critical proinflammatory cytokine.47,48 The increased expression of these genes is obviously beneficial to tumor development. SCF-stimulated production of VEGF by mast cells is consistent with the previous report that mast cells can promote tumor angiogenesis.49,50 Moreover, the proinflammatory factors such as TNF-α and others can increase the activities of NK-κB and AP-1 in tumor cells.38 Our data show that the activities of NK-κB and AP-1 were indeed increased in the tumor cells in the tumor microenvironment remodeled by mast cells. The activated NF-κB and AP-1 may favor the proliferation of tumor cells by inducing the expression of cyclins, the survival of tumor cells by the blockade of apoptosis, and the invasiveness of tumor cells by inducing the production of MIF and EMMPRIN.51,52
Immunosuppression is another basic feature of tumor microenvironment. Although immune surveillance works at an early stage of tumorigenesis, the established tumors primarily induce immune tolerance,53 resulting in the shift of the immune balance from activation to tolerance induction. An absolute immune suppression is usually generated in the tumor microenvironment at the later stage of tumor. Our data in this report indicate that mast cells play important roles in such immune balance shifting. The infiltration of mast cells into the tumor significantly increased the expressions of TGF-β, IL-10, and Foxp3 in the tumor. Consistently, Treg cells in the tumor microenvironment was also increased, because CD4+CD25− T cells can be converted into CD4+CD25+ regulatory T cells by TGF-β–induced expression of transcription factor Foxp3.54 Both the infiltration of mast cells and the increase of Treg cells can explain the increased expression of IL-10, a cytokine-mediating complex immunosuppressive effect. Given that Treg cells release IL-9 to activate mast cells for immune suppression,25 here we propose a novel partnership between mast cells and Treg cells in the tumor microenvironment: they activate each other and cooperate to suppress immune responses.
Adenosine release from the stimulated mast cells was found 23 years ago.36 However, its connection with tumor progression has not yet been elucidated. Our data show that mast cells augment the release of SCF by tumor cells, and then the increased SCF in turn stimulates the release of adenosine by mast cells. Adenosine not only inhibits the production of IFN-γ and IL-2 by CD4+ T cells39,40 but it also suppresses the migration of mast cells.55 The latter may partly explain the accumulation of mast cells around the vasculature in the peripheral region of tumor tissue. Thus, mast cells localizing around tumor vasculature may form the first layer of barrier, where the tumor-infiltrating T cells initially accept the immunosuppressive signal, adenosine, leading to the attenuation of immune attack on tumor cells.
In summary, the findings in this report disclose the complex relation between mast cells, tumor cells, and other immune cells in the tumor microenvironment. The remodeling of the tumor microenvironment can actually be initiated by tumor cell-released SCF. The following events include the mutual influence between mast cells and tumor cells, mast cells and other immune cells. Therefore, the SCF/c-Kit pathway is not only very important for the remodeling of tumor microenvironment but also a very important target for tumor therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Development Program (973) for Key Basic Research (2002CB513100) of China and the National Natural Science Foundation of China (No. 30771974, No. 30772589).
Authorship
Contribution: B.H. designed and conducted research, analyzed data, and wrote the paper; Z.L., G.-M.Z., D.L., C.S., B.L., Y.L., and Y.Y. conducted research and analyzed the data; J.U., H.X., and Z.-H.F. designed the research, analyzed the data, and wrote the paper. All authors have reviewed and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zuo-Hua Feng or Bo Huang, Department of Biochemistry and Molecular Biology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, The People's Republic of China; e-mail: fengzhg@public.wh.hb.cn or tjhuangbo@hotmail.com.