Abstract
The dynamic interplay between dendritic cells (DCs) and human immunodeficiency virus type-1 (HIV-1) is thought to result in viral dissemination and evasion of antiviral immunity. Although initial observations suggested that the C-type lectin receptor (CLR) DC-SIGN was responsible for the trans-infection function of the virus, subsequent studies demonstrated that trans-infection of CD4+ T cells with HIV-1 can also occur through DC-SIGN–independent mechanisms. We demonstrate that a cell surface molecule designated DCIR (for DCimmunoreceptor), a member of a recently described family of DC-expressing CLRs, can participate in the capture of HIV-1 and promote infection in trans and in cis of autologous CD4+ T cells from human immature monocyte-derived DCs. The contribution of DCIR to these processes was revealed using DCIR-specific siRNAs and a polyclonal antibody specific for the carbohydrate recognition domain of DCIR. Data from transfection experiments indicated that DCIR acts as a ligand for HIV-1 and is involved in events leading to productive virus infection. Finally, we show that the neck domain of DCIR is important for the DCIR-mediated effect on virus binding and infection. These results point to a possible role for DCIR in HIV-1 pathogenesis by supporting the productive infection of DCs and promoting virus propagation.
Introduction
For HIV-1 infection to establish, the virus must be first transferred and disseminated from the virus entry sites at the mucosal surfaces to T-cell zones in secondary lymphoid organs, where the virus can productively infect CD4+ T cells. Several hypotheses have been proposed to explain how the virus can cross the mucosal epithelium. For example, it has been postulated that HIV-1 may enter the body through microlesions or can cross the epithelium barrier by different processes such as infection and/or trancytosis of epithelial cells or infection of dendritic cells (DCs) using both DC-specific ICAM3-grabbing nonintegrin (DC-SIGN)–dependent and –independent mechanisms.1,2 Given that cell-free virions do not efficiently cross genital epithelial cells, it has been proposed that HIV-1 uses primarily DCs to penetrate the mucosal epithelium,1,3 and this cell type has been proposed to be the first target in the genital mucosa.4
There is now clear evidence that HIV-1 establishes a complex interplay with DCs. It has been reported that HIV-1 transfer from DCs toward CD4+ T lymphocytes takes place in a biphasic mode.5 In the initial transfer phase (ie, early transfer or trans infectious mode), the virus located within endosomal compartments in DCs is rapidly relocated at the DC/T-cell contact zone. This local concentration of virus between the 2 cell types is referred to as the virologic synapse. This is followed by a second phase (ie, late transfer or cis infectious mode), which is dependent on productive infection of DCs and eventual transfer of progeny virus to surrounding CD4+ T cells. Although involvement of DCs in HIV-1 pathogenesis was discovered very soon after the identification of the virus and several aspects of this complex interplay have been elucidated, there are still fundamental questions that remain to be answered about the multifaceted interactions between HIV-1 and DCs.6 The first crucial event in HIV-1 entry and cis infection of DCs is the binding of the external envelope glycoprotein gp120 to cell surface receptor CD4 and coreceptor (eg, CCR5), a step that results in the formation of a fusion pore between viral and cellular membranes. Recent findings suggest that HIV-1 internalization within DCs is dependent on the association between gp120 and different C-type lectin receptors (CLRs) such as DC-SIGN, mannose receptor (MR) (also known as CD206), langerin (CD207), and syndecan-3.2,7,8 In contrast to the sequence of events initiated by gp120 binding to CD4 and a 7-transmembrane coreceptor, early interactions between gp120 and CLRs will not trigger sufficient conformational changes in the virus envelope spikes to mediate fusion of viral and cellular membranes. Instead, these receptors are thought to facilitate attachment of virions to the cell surface resulting in capture and transmission of HIV-1 to CD4+ T cells in an efficient trans infectious mode (reviewed in Turville et al9 ). Based on previous data, it is expected that a viral entity that is bound to CLRs will be taken up within endolysosomal vacuoles and protected from degradation while remaining in an infectious state for about 2 days, which is the time required for DCs to migrate to lymph nodes where HIV-1 transmission to CD4+ T cells occurs through the virologic synapse.5,10,11
It recently became obvious that DC-SIGN, MR, langerin, and syndecan-3 are not the only receptors on DCs involved in HIV-1 capture and transfer.12,13 Therefore, in an attempt to shed light on the ability of other members of the CLR family to act as attachment factors for HIV-1 on the surface of DCs, we focused our attention on the recently described DCimmunoreceptor (DCIR). This cell surface component has been identified as a prototypic DC-associated CLR that is, as DC-SIGN, down-regulated upon maturation of DCs.14 DCIR, also called C-type lectin superfamily 6 (CLECSF6)15 and LLIR,16 is a novel type II transmembrane molecule of the CLR family containing a consensus intracellular immunoreceptor tyrosine–based inhibitory motif (ITIM),17 an extracellular region that carries a membrane-distal carbohydrate recognition domain (CRD),18 and a neck domain that, by analogy with DC-SIGN, is probably responsible for its oligomerization.19 Additional studies indicated that the neck region of DCIR consists of 23–amino acid repeats. Four different forms of DCIR mRNAs have been cloned that are thought to result from alternative splicing. The longest form was detected in several cell types and tissues,14,15 whereas a form missing the neck domain was cloned from neutrophils.15 Finally, 2 alternatively spliced transmembrane deletion variants were found in DCs.16 DCIR expression is decreased following maturation of DCs induced by various stimuli such as CD40 ligand, lipopolysaccharide, and TNF-α.14 However, no ligand of DCIR has yet been identified and the in vivo function of this receptor remains elusive. Given that DCIR is expressed on cell types known to harbor HIV-1 (eg, DCs and macrophages) and considering that this CLR shares several features with DC-SIGN, we investigated the contribution of DCIR as a putative attachment factor for HIV-1. Importantly, it has been shown that DCIR is expressed at high levels on various antigen-presenting cells (APCs) such as B cells, monocytes, and myeloid DCs, whereas Langerhans cells express low surface levels of DCIR.14 Recently, DCIR was also detected on the surface of plasmacytoid DCs.20 Altogether, these observations provide physiological significance to studies aimed at defining the possible role played by DCIR in interactions between DCs and HIV-1.
In this report, we demonstrate for the first time that DCIR can participate in DC-mediated capture and transmission of HIV-1. The role played by DCIR in virus transfer from immature monocyte-derived DCs to autologous CD4+ T cells was established using 2 distinct but complementary strategies, namely gene silencing and blocking experiments with a specific antibody. The DCIR-mediated transmission of HIV-1 was due to an intracellular storage of intact virions (ie, trans-infection pathway) and also de novo virus production by DCs (ie, cis-infection pathway). We provide evidence that the neck domain of DCIR plays a key role in the process of virus capture and transfer mediated by this CLR. Together, these results indicate that DCIR serves as a portal for HIV-1 infection on DCs.
Methods
Reagents
Recombinant human interleukin-2 (rhIL-2) and efavirenz (EFV) were obtained from the AIDS Repository Reagent Program (Germantown, MD). IL-4 was purchased from R&D systems (Minneapolis, MN), whereas granulocyte macrophage–colony-stimulating factor (GM-CSF) was a generous gift from Cangene (Winnipeg, MB). The culture medium consisted of RPMI-1640 supplemented with 10% fetal bovine serum (FBS), penicillin G (100 U/mL), streptomycin (100 U/mL), primocine (Amaxa Biosystems, Gaithersburg, MD), and glutamine (2 mM).
Antibodies
The phycoerythrin (PE)–labeled anti-DCIR monoclonal antibody (Ab) (clone 216110) was purchased from R&D Systems. A polyclonal anti-DCIR was produced in rabbits following immunization with a peptide called 27P4 corresponding to the COOH-terminal domain of DCIR and more precisely to a region of DCIR spanning amino acids 223 to 237 (ie, LGPQRSVCEMMKIHL).17 The polyclonal anti-DCIR was purified using mAbTrap protein G affinity columns according to the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ). PE-conjugated donkey anti–rabbit immunoglobulin G (IgG) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The FITC-tagged anti–DC-SIGN monoclonal Ab (clone eB-h209) was purchased from eBioscience (San Diego, CA). Hybridomas producing 183-H12-5C and 31-90-25, 2 Abs recognizing different epitopes of the HIV-1 major viral core protein p24, were supplied by the AIDS Repository Reagent Program and ATCC (Manassas, VA), respectively. Abs obtained from these hybridoma cell lines were also purified using mAbTrap protein G affinity columns.
Cells
DCs were generated from purified human monocytes (ie, CD14+ cells). Briefly, peripheral blood was collected from healthy donors with informed consent obtained in accordance with the Declaration of Helsinki, and peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation on a Ficoll-Hypaque density gradient. Next, CD14+ cells were isolated from fresh PBMCs using a monocyte positive selection kit according to the manufacturer's instructions (MACS CD14 micro beads; StemCell Technologies, Vancouver, BC) as described previously.21 To generate immature monocyte-derived dendritic cells (IM-MDDCs), purified monocytes were cultured in complete culture medium that was supplemented every other day with GM-CSF (1000 U/mL) and IL-4 (200 U/mL) for 7 days. The percentage of cells expressing the surface markers CD3 and CD19 was evaluated to assess contamination with T and B cells, respectively. Experiments were performed with cell preparations that contained a minimal amount of contaminants (ie, DC: purity > 95%; CD4+ T cells: purity > 98%). Autologous CD4+ T cells were isolated using a negative selection kit according to the manufacturer's instructions (StemCell Technologies). These cells were activated with phytohemagglutinin L (PHA-L; 1 μg/mL) and maintained in complete culture medium supplemented with rhIL-2 (30 U/mL) at a density of 2 × 106 cells/mL. Raji-CD4 is a B-cell line carrying the Epstein-Barr virus that has been rendered susceptible to HIV-1 infection by stable transfection with a cDNA encoding human CD4. These cells were cultured in RPMI-1640 medium supplemented with 10% FBS along with 1 mg/mL of the selective agent G418 (GIBCO-BRL, Gaithersburg, MD). Human embryonic kidney 293T cells were cultured in Dulbecco modified Eagle medium supplemented with 10% FBS.
Flow cytometric analysis
Cell surface expression of DCIR and DC-SIGN was monitored by flow cytometric analyses (Epics ELITE ESP; Coulter Electronics, Burlington, ON). Before staining, cells were incubated for 15 minutes at 4°C with 10% pooled human sera to block nonspecific binding sites and washed once with phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA). Next, cells were incubated for 45 minutes at 4°C with anti-DCIR (0.25 μg) or anti–DC-SIGN (0.5 μg) monoclonal Ab and then washed twice with PBS and 0.5% BSA. Nonspecific staining was determined using an isotype-matched irrelevant control Ab. After 2 final washes with PBS, cells were fixed in 2% paraformaldehyde and analyzed.
Production of virus stocks
Virions were initially produced upon transient transfection of human embryonic kidney 293T cells as previously described. The infectious molecular clones used in this study included pNL4-3balenv (R5-tropic) and pNL4-3 (X4-tropic). The pNL4-3balenv vector was generated by replacing the env gene of the T-tropic HIV-1 strain, NL4-3, with that of the macrophage-tropic HIV-1 Bal strain, thus resulting in an infectious molecular clone with macrophage-tropic properties (provided by R. Pomerantz, Thomas Jefferson University, Philadelphia, PA).22 Stocks of NL4-3balenv were made upon acute infection of PBMCs that were stimulated initially with PHA-L and maintained in culture medium containing rhIL-2 (used in Figures 3,Figure 4–5). The virus-containing supernatants were harvested at day 7 after infection, filtered through a 0.22-μm cellulose acetate syringe filter, ultracentrifugated, and normalized for virion content using a sensitive in-house double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) specific for the viral p24 protein. In this test, the 183-H12-5C and 31-90-25 Abs are used in combination to quantify p24 levels.23 Preparations of NL4-3 were produced by infecting Raji-CD4 (used in Figures 6,7). Briefly, Raji-CD4 cells (5 × 106 cells) were inoculated with NL4-3 (at a ratio of 1 ng p24 per 105 cells) for 2 hours at 37°C. Cells were then washed extensively to eliminate uninternalized virions and maintained in culture for 6 days.
Nucleofection
The Amaxa cell line nucleofector Kit V (Amaxa, Cologne, Germany) was used to achieve transient transfection of DCIR into Raji-CD4 (program M-13). Briefly, Raji-CD4 cells (10 × 106 cells) were nucleofected with pBK-CMV-DCIR or pBK-CMV-DCIRΔneck (2.5 μg for each plasmid per 10 × 106 cells). These vectors were constructed by subcloning the cDNA coding for full-length human DCIR or a neck-deleted version of DCIR (ie, DCIRΔneck) into the pBK-CMV vector (Stratagene, La Jolla, CA) as a KpnI-Xho fragment. These plasmids were purified using an EndoFree plasmid maxi kit (Qiagen, Mississauga, ON) to obtain DNA of high quality for nucleofection. Raji-CD4 cells were incubated for 5 hours in RPMI-1640 medium supplemented with 20% FBS prior to nucleofection.
Gene silencing of DCIR with siRNAs
Small interfering RNAs (siRNAs) either specific (ie, 5′-ATTTAGGTGGTCTGTCA-3′) or nonspecific for DCIR (ie, 5′-AATTCTCCGAAGGTGTCACGT-3′) were obtained from Qiagen and dissolved in an appropriate buffer. The studied siRNAs were subsequently tested in IM-MDDCs as previously described.21 Controls consisted of cells treated with either oligofectamine alone or oligofectamine and a nonspecific siRNA. Forty hours following transfection, a virus transfer test was carried out. The efficiency of DCIR silencing with the tested siRNA was monitored by flow cytometry.
HIV-1 transfer assay
IM-MDDCs (105 cells in 100 μL) transfected either with the listed siRNAs or preincubated with the polyclonal anti-DCIR (10 μg/1.5 × 105 cells) were exposed to HIV-1 (10 ng p24) for 60 minutes at 37°C. Next, the virus-cell mixture was washed 3 times with PBS to remove unadsorbed virions. In some experiments, cells were also treated with the anti–HIV-1 drug EFV (50 μM). Finally, IM-MDDCs were cocultured with autologous activated CD4+ T cells at a 1:3 ratio in complete RPMI-1640 medium supplemented with IL-2 (30 U/mL) in 96-well plates in a final volume of 200 μL. Virus production was estimated by measuring p24 levels in cell-free culture supernatants.
HIV-1 infection of IM-MDDCs
IM-MDDCs (2 × 105 cells in a final volume of 100 μL) either transfected with the studied siRNAs or preincubated with the polyclonal anti-DCIR were exposed to HIV-1 (20 ng p24) for 2 hours at 37°C. After 3 washes with PBS, cells were maintained in complete RPMI-1640 culture medium supplemented with GM-CSF and IL-4 in 96-well plates in a final volume of 200 μL. Every 3 days and for a period lasting 9 days, half of the medium was removed and kept frozen at −20°C until assayed. Virus production was estimated by measuring p24 levels in culture supernatants by ELISA. Note that in all experiments using the polyclonal anti-DCIR, DCs were pretreated with 10% of pooled human sera to avoid nonspecific reactivity with Fc receptors.
Virus binding and infection assays in Raji-CD4
The role played by DCIR as a putative attachment factor for HIV-1 was assessed using a virus binding test. In brief, Raji-CD4 cells, either negative (ie, parental cell line) or positive for DCIR (3 × 106 cells), were incubated with NL4-3 (300 ng p24) for 60 minutes at 37°C. Next, the virus-cell mixture was washed 3 times with PBS to remove unbound virus and resuspended in PBS containing 1% BSA. The p24 content was determined by our homemade assay. Susceptibility of the studied Raji-CD4 cells to HIV-1 infection was assessed by initially exposing DCIR-negative and DCIR-positive Raji-CD4 (1.5 × 105 cells) to NL4-3 (1.5 ng p24) for 2 hours at 37°C. Thereafter, cells were washed 3 times with PBS to remove nonspecifically bound virions and maintained in culture in 48-well plates in a final volume of 400 μL. Every 3 days after infection and for a period lasting 9 days, half of the medium was removed from each well and kept frozen at −20°C until assayed. Virus production was estimated by measuring p24 levels in cell-free culture supernatants.
Statistical analysis
Statistical analyses were carried out according to the methods outlined by Zar.24 Means were compared using either the Student t test or a single-factor ANOVA followed by Dunnett multiple comparison when more than 2 means were considered. P values of less than .05 were deemed statistically significant. Calculations were performed with the GraphPad Prism software (GraphPad Software, La Jolla, CA).
Results
DC-mediated transmission of HIV-1 is modulated by DCIR
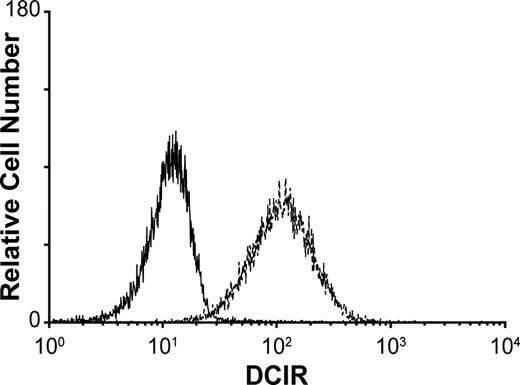
A previous study has demonstrated that DCIR is expressed by all circulating CD14+ monocytes, in DCs derived from CD34+ cord blood progenitors, as well as on the surface of DCs generated in vitro upon culturing blood monocytes with GM-CSF and IL-4 (ie, immature monocyte-derived DCs [IM-MDDCs]).14 This last observation is of high importance because IM-MDDCs are routinely used as an experimental cell system to study characteristics of mucosal myeloid DCs and to define the complexity of interactions between DCs and HIV-1.12 To confirm that DCIR expression is maintained in IM-MDDCs, cell surface expression of DCIR was determined by immunofluorescence staining and flow cytometric analysis. Data depicted in Figure 1 indicate that DCIR is strongly expressed after culturing purified monocytes for 7 days with GM-CSF and IL-4, a treatment known to induce differentiation of monocytes into IM-MDDCs.
Expression of DCIR in IM-MDDCs. Purified monocytes were cultured with GM-CSF and IL-4 for 7 days to derive IM-MDDCs. DCIR expression was determined by flow cytometric analysis after staining with a commercial PE-conjugated anti-DCIR monoclonal Ab. Expression of DCIR is shown as a dotted line, whereas the continuous line represents staining obtained with an isotype-matched irrelevant control Ab. Results shown are representative of 7 independent experiments.
Expression of DCIR in IM-MDDCs. Purified monocytes were cultured with GM-CSF and IL-4 for 7 days to derive IM-MDDCs. DCIR expression was determined by flow cytometric analysis after staining with a commercial PE-conjugated anti-DCIR monoclonal Ab. Expression of DCIR is shown as a dotted line, whereas the continuous line represents staining obtained with an isotype-matched irrelevant control Ab. Results shown are representative of 7 independent experiments.
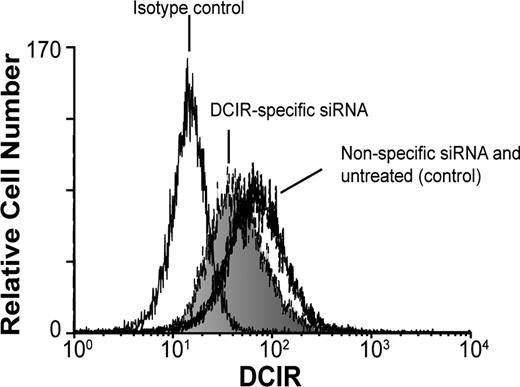
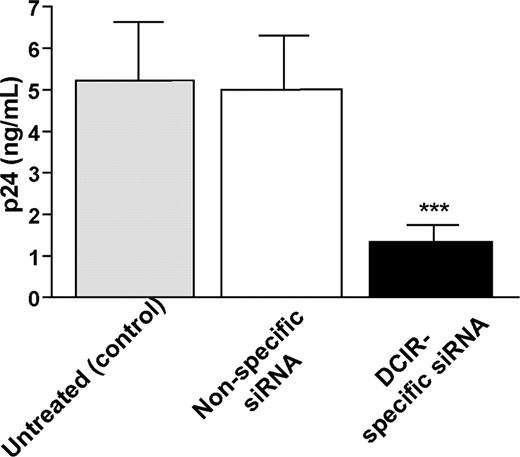
We then asked whether DCIR was involved in HIV-1 uptake by IM-MDDCs and eventual transfer to autologous CD4+ T lymphocytes. To this end, we used 2 different experimental strategies, namely RNA interference (ie, small interfering RNA or siRNA) to reduce DCIR expression in IM-MDDCs and a polyclonal Ab specific for the COOH-terminal end of DCIR. For the virus transfer assay, IM-MDDCs were intentionally exposed to low doses of virions to better reflect the events occurring during mucosal transmission in vivo. Moreover, all virus stocks used in transmission assays were produced upon acute infection of primary human cells (ie, PBMCs) to more closely parallel the natural microenvironment.25 These cells were pulsed with R5-tropic virions (ie, NL4-3Balenv) for 60 minutes at 37°C, before being cocultured with autologous activated CD4+ T cells. The extent of virus transferred to CD4+ T cells was determined by measuring the major core viral protein p24 in culture supernatants. We initially used RNA interference to reduce DCIR expression in IM-MDDCs and analyzed the possible effect on HIV-1 transfer. An approximately 30% decrease in surface expression of DCIR in IM-MDDCs was observed following the use of DCIR-specific siRNA (Figure 2). It should be noted that DC-SIGN expression was not affected by DCIR-specific siRNA, and cell viability was minimally affected upon siRNA treatment (data not shown). Interestingly, transfer of HIV-1 was reduced significantly when IM-MDDCs were transfected with DCIR-specific siRNA but not with a nonspecific control siRNA (Figure 3). Similar observations were made when using another DCIR-specific siRNA (data not shown). To eliminate the possibility that the DCIR-mediated effect on HIV-1 transfer is due to a reduced ability of IM-MDDCs to interact and activate CD4+ T cells, which in turn can modulate the virus transmission process, cellular proliferation was estimated in our coculture experiments. Data from a colorimetric method (ie, MTS cell proliferation assay) indicate that IM-MDDCs transfected either with the DCIR-specific siRNA or nonspecific control siRNA display a comparable capacity to induce proliferation of autologous CD4+ T cells (data not shown).
Decreased DCIR expression in IM-MDDCs by siRNA treatment. IM-MDDCs were either left untreated (control), transfected with a control siRNA, or transfected with a DCIR-specific siRNA. After 40 hours, flow cytometry analysis of DCIR was performed using a combination of PE-labeled anti-DCIR and isotype-matched control Ab. Data shown correspond to a single experiment representative of 7 independent experiments.
Decreased DCIR expression in IM-MDDCs by siRNA treatment. IM-MDDCs were either left untreated (control), transfected with a control siRNA, or transfected with a DCIR-specific siRNA. After 40 hours, flow cytometry analysis of DCIR was performed using a combination of PE-labeled anti-DCIR and isotype-matched control Ab. Data shown correspond to a single experiment representative of 7 independent experiments.
Involvement of DCIR in HIV-1 transmission by IM-MDDCs. IM-MDDCs (1 × 106 cells) were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Next, cells (1 × 105) were pulsed with NL4-3balenv (10 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at day 2 following initiation of the coculture and analyzed for the p24 content. Data shown represent the means (+ SD) of triplicate samples from 3 combined independent experiments. Asterisks denote statistically significant data (***P < .001).
Involvement of DCIR in HIV-1 transmission by IM-MDDCs. IM-MDDCs (1 × 106 cells) were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Next, cells (1 × 105) were pulsed with NL4-3balenv (10 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at day 2 following initiation of the coculture and analyzed for the p24 content. Data shown represent the means (+ SD) of triplicate samples from 3 combined independent experiments. Asterisks denote statistically significant data (***P < .001).
DCIR is involved in trans- and cis-infection pathways
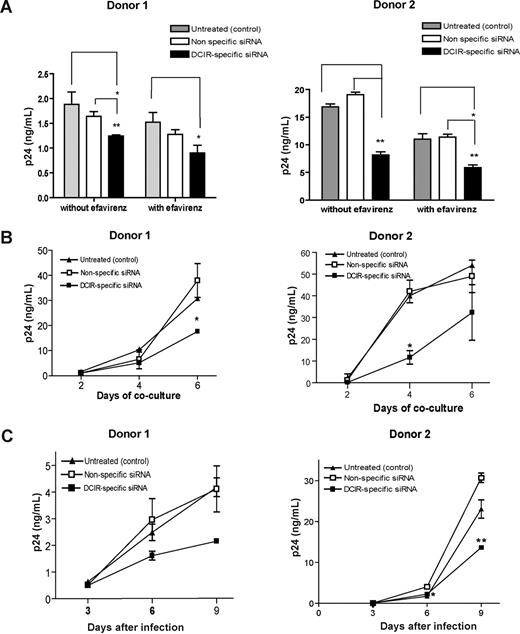
Previously published data demonstrated that HIV-1 is transferred from DCs to CD4+ T cells through both trans- and cis-infection pathways.5 Experiments were therefore carried out to define whether DCIR can contribute to HIV-1 dissemination via trans- and/or cis-infection processes. To solve this issue, virus transfer studies were performed using IM-MDDCs that were initially transfected with the DCIR-specific siRNA and also treated with EFV, a nonnucleoside reverse transcriptase inhibitor. This antiretroviral compound hampers the late transfer (ie, de novo virus production by IM-MDDCs or cis-infection pathway) without affecting the early transfer mode (ie, trans-infection pathway). Results illustrated in Figure 4A demonstrate that the use of the DCIR-specific siRNA led to a reduction in virus transfer corresponding to an average decrease of 30% in absence of EFV compared with an average diminution of 40% in cells treated with EFV. Such a small increment in the reduction of HIV-1 transfer by EFV suggests that DCIR contributes primarily to the early transfer phase (ie, trans-infection pathway) with a small effect on the late transfer (ie, cis-infection pathway). The possible involvement of DCIR in the cis-infection mode was investigated further because of the limited effect of EFV on HIV-1 propagation. To this end, IM-MDDCs treated with the DCIR-specific siRNA were first pulsed with HIV-1 and cultured for 4 days before initiating a coculture with autologous CD4+ T cells. This experimental setup was prompted by the previous demonstration that, in IM-MDDCs, most endosome-associated virus, which is responsible for the early transfer, is degraded within 24 to 48 hours.5,26 The connection between DCIR and the cis-infection pathway was confirmed using this experimental strategy (Figure 4B). Moreover, the use of the DCIR-specific siRNA was found to decrease de novo virus production in IM-MDDCs (Figure 4C), which represents additional evidence that HIV-1 can use DCIR to achieve productive infection of this cell type.
DCIR affects both trans- and cis-infection pathways. (A) IM-MDDCs (1 × 106 cells) were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Next, cells were either left untreated or treated with EFV. IM-MDDCs (2 × 105 cells) were pulsed with NL4–3balenv (20 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at day 2 following initiation of the coculture and analyzed for the p24 content. Data shown represent the means plus or minus SD of triplicate samples from 2 different donors and are representative of 4 separate donors. Asterisks denote statistically significant data (*P < .05; **P < .01). (B) IM-MDDCs were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Cells (2 × 105) were pulsed with NL4-3balenv (20 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were maintained in culture for 4 days. Finally, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at different time points (ie, 2, 4, and 6 days) and analyzed for p24 contents. Data shown represent the means plus or minus SD of triplicate samples from 2 different donors and are representative of 3 separate donors. Asterisks denote statistically significant data (*P < .05). (C) IM-MDDCs were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Cells (2 × 105) were pulsed with NL4–3balenv (20 ng p24) for 2 hours at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were maintained in complete culture medium supplemented with GM-CSF and IL-4. Cell-free culture supernatants were collected at the indicated time points and analyzed for the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 2 different donors and are representative of 4 separate donors. Asterisks denote statistically significant data (*P < .05; **P < .01).
DCIR affects both trans- and cis-infection pathways. (A) IM-MDDCs (1 × 106 cells) were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Next, cells were either left untreated or treated with EFV. IM-MDDCs (2 × 105 cells) were pulsed with NL4–3balenv (20 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at day 2 following initiation of the coculture and analyzed for the p24 content. Data shown represent the means plus or minus SD of triplicate samples from 2 different donors and are representative of 4 separate donors. Asterisks denote statistically significant data (*P < .05; **P < .01). (B) IM-MDDCs were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Cells (2 × 105) were pulsed with NL4-3balenv (20 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were maintained in culture for 4 days. Finally, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at different time points (ie, 2, 4, and 6 days) and analyzed for p24 contents. Data shown represent the means plus or minus SD of triplicate samples from 2 different donors and are representative of 3 separate donors. Asterisks denote statistically significant data (*P < .05). (C) IM-MDDCs were treated with oligofectamine and then either left untreated (control) or exposed to a nonspecific siRNA and a DCIR-specific siRNA. Cells (2 × 105) were pulsed with NL4–3balenv (20 ng p24) for 2 hours at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were maintained in complete culture medium supplemented with GM-CSF and IL-4. Cell-free culture supernatants were collected at the indicated time points and analyzed for the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 2 different donors and are representative of 4 separate donors. Asterisks denote statistically significant data (*P < .05; **P < .01).
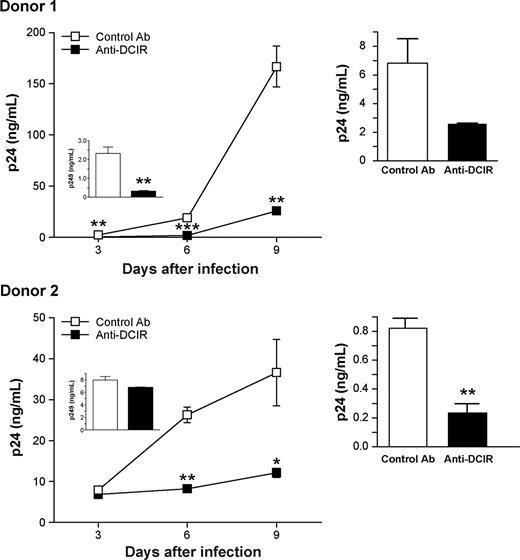
Next, HIV-1 transmission by IM-MDDCs was evaluated in the presence of a polyclonal Ab targeting the single CRD in the extracellular COOH-terminal end of DCIR. The specificity of this Ab was monitored by flow cytometry using 293T cells transiently transfected with mammalian expression vectors coding for DCIR and DC-SIGN (data not shown). A decrease in virus replication was observed when IM-MDDCs were acutely infected with HIV-1 in the presence of polyclonal anti-DCIR (Figure 5 left panels). In addition, pretreatment of IM-MDDCs with anti-DCIR before pulsing with HIV-1 and coculture with autologous CD4+ T cells induced a significant decrease in HIV-1 transfer, compared with transmission in the presence of a control Ab (Figure 5 right panels). These results corroborate those obtained with DCIR-specific siRNAs and indicate that DCIR can act as a receptor for HIV-1 on the surface of IM-MDDCs. Together, these results demonstrate that DCIR is involved in both trans- and cis-infection pathways mediated by DCs.
HIV-1 replication in IM-MDDCs is reduced by anti-DCIR. (Left panels) IM-MDDCs (2 × 105 cells) were initially treated with either a control Ab or a polyclonal anti-DCIR (10 μg per 1.5 × 105 cells). Next, cells were extensively washed to eliminate excess Ab and pulsed with NL4-3balenv (20 ng p24) for 2 hours at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were maintained in complete culture medium supplemented with GM-CSF and IL-4. Cell-free culture supernatants were collected at the indicated time points and analyzed for the p24 content. Results obtained at 3 days after infection are displayed in the small inserts. Data shown represent the means plus or minus SD of triplicate samples from 2 different donors and are representative of 3 separate donors. Asterisks denote statistically significant data (*P < .05; **P < .01; ***P < .001). (Right panels) IM-MDDCs (1 × 105 cells) were first treated with either a control Ab or a polyclonal anti-DCIR (10 μg per 1.5 × 105 cells). Cells were next washed to eliminate excess Ab and pulsed with NL4-3balenv (10 ng p24) for 2 hours at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at day 2 following initiation of the coculture and analyzed for the p24 content. Data shown represent the means plus or minus SD of triplicate samples from 2 distinct donors. Asterisks denote statistically significant data (**P < .01).
HIV-1 replication in IM-MDDCs is reduced by anti-DCIR. (Left panels) IM-MDDCs (2 × 105 cells) were initially treated with either a control Ab or a polyclonal anti-DCIR (10 μg per 1.5 × 105 cells). Next, cells were extensively washed to eliminate excess Ab and pulsed with NL4-3balenv (20 ng p24) for 2 hours at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were maintained in complete culture medium supplemented with GM-CSF and IL-4. Cell-free culture supernatants were collected at the indicated time points and analyzed for the p24 content. Results obtained at 3 days after infection are displayed in the small inserts. Data shown represent the means plus or minus SD of triplicate samples from 2 different donors and are representative of 3 separate donors. Asterisks denote statistically significant data (*P < .05; **P < .01; ***P < .001). (Right panels) IM-MDDCs (1 × 105 cells) were first treated with either a control Ab or a polyclonal anti-DCIR (10 μg per 1.5 × 105 cells). Cells were next washed to eliminate excess Ab and pulsed with NL4-3balenv (10 ng p24) for 2 hours at 37°C. After 3 washes with PBS to eliminate unbound virus, IM-MDDCs were cocultured with autologous CD4+ T cells at a 1:3 ratio. Cell-free culture supernatants were collected at day 2 following initiation of the coculture and analyzed for the p24 content. Data shown represent the means plus or minus SD of triplicate samples from 2 distinct donors. Asterisks denote statistically significant data (**P < .01).
DCIR-mediated virus capture and infection requires the neck domain of DCIR
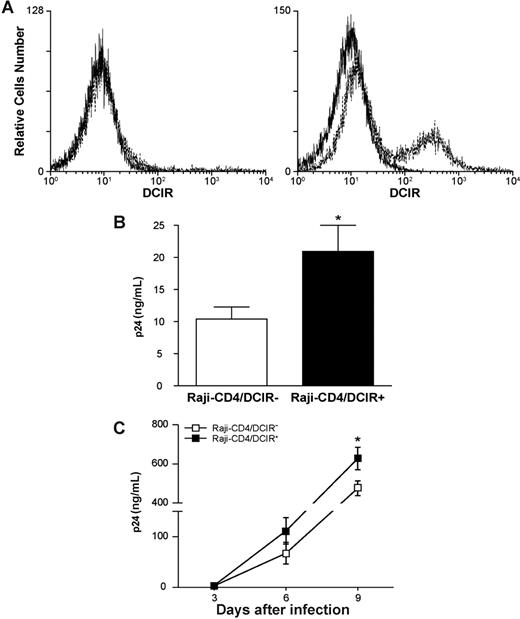
Previous studies have used the Epstein-Barr virus (EBV) genome-carrying B-cell line Raji as a model system to reveal the role of DC-SIGN in HIV-1 capture and transmission.27 To delineate the contribution of DCIR in HIV-1 capture and transfer, our next experiments were carried out using Raji-CD4, a Raji derivative that stably expresses CD4 and is highly susceptible to infection with X4-tropic strains of HIV-1. Transient expression of DCIR was achieved in Raji-CD4 using nucleofection (Figure 6A), an electroporation-based method that is thought to target plasmid DNA directly to the cell nucleus. As illustrated in Figure 6B, binding of HIV-1 was increased in the presence of cell surface DCIR in Raji-CD4. In addition, virus production was enhanced in DCIR-expressing Raji-CD4 compared with the DCIR-negative parental cell line (Figure 6C).
DCIR expression in Raji-CD4 increases HIV-1 binding and virus production. (A) Raji-CD4 cells were nucleofected with either an empty control vector (left panel) or a mammalian expression vector coding for human DCIR (right panel). Five hours later, flow cytometric analysis of DCIR was performed using a combination of PE-labeled anti-DCIR Ab (dotted lines) and a control Ab (continuous lines). Data shown correspond to a single experiment representative of 7 independent experiments. (B) Parental (DCIR-negative) and DCIR-expressing Raji-CD4 (3 × 106 cells) were exposed to NL4–3 (300 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to remove unabsorbed virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 7 combined independent experiments. The asterisk denotes statistically significant data (*P < .05). (C) Parental (DCIR-negative) and DCIR-expressing Raji-CD4 (1.5 × 105 cells) were exposed to NL4–3 (1.5 ng p24) for 2 hours at 37°C. After 3 washes with PBS to remove excess virus, cells were maintained in culture. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 3 combined independent experiments. The asterisk denotes statistically significant data (*P < .05).
DCIR expression in Raji-CD4 increases HIV-1 binding and virus production. (A) Raji-CD4 cells were nucleofected with either an empty control vector (left panel) or a mammalian expression vector coding for human DCIR (right panel). Five hours later, flow cytometric analysis of DCIR was performed using a combination of PE-labeled anti-DCIR Ab (dotted lines) and a control Ab (continuous lines). Data shown correspond to a single experiment representative of 7 independent experiments. (B) Parental (DCIR-negative) and DCIR-expressing Raji-CD4 (3 × 106 cells) were exposed to NL4–3 (300 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to remove unabsorbed virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 7 combined independent experiments. The asterisk denotes statistically significant data (*P < .05). (C) Parental (DCIR-negative) and DCIR-expressing Raji-CD4 (1.5 × 105 cells) were exposed to NL4–3 (1.5 ng p24) for 2 hours at 37°C. After 3 washes with PBS to remove excess virus, cells were maintained in culture. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 3 combined independent experiments. The asterisk denotes statistically significant data (*P < .05).
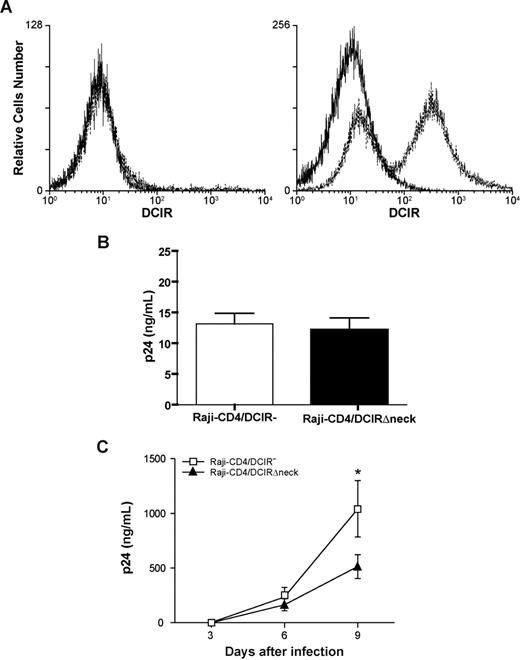
DCIR carries a neck region that is composed of a variable number of 23-amino acid tandem repeats. Previous observations suggest that DC-SIGN, which is closely related to DCIR, forms tetramers stabilized by the neck domain.19,28 The importance of the neck portion in the observed phenomenon was addressed using a neck-deleted DCIR mutant (ie, DCIRΔneck). Nucleofection of Raji-CD4 with an expression vector coding for DCIRΔneck resulted in an efficient surface expression of the mutated DCIR (Figure 7A). Attachment of HIV-1 particles was not modulated following expression of DCIRΔneck on the surface of Raji-CD4 cells (Figure 7B). In contrast to what was seen in cells expressing wild-type DICR (Figure 6C), virus replication was not increased in the presence of DCIRΔneck on the cell surface of Raji-CD4 (Figure 7C). However, expression of the neck-deleted DCIR mutant led to a reduction in virus replication, which might be due to a reduced cell viability (data not shown). Data from these studies indicate that the neck region of DCIR plays a crucial role in its interaction with HIV-1.
The neck domain of DCIR is important for interaction with HIV-1. (A) Raji-CD4 cells were nucleofected with either an empty control vector (left panel) or a mammalian expression vector coding for a neck-deleted DCIR mutant (ie, DCIRΔneck) (right panel). Five hours later, flow cytometric analysis of DCIR was performed using a combination of PE-labeled anti-DCIR monoclonal Ab (dotted lines) and a control Ab (continuous lines). Data shown correspond to a single experiment representative of 5 combined independent experiments. (B) Parental (DCIR-negative) and DCIRΔneck-expressing Raji-CD4 (3 × 106 cells) were exposed to NL4-3 (300 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to remove unabsorbed virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 5 combined independent experiments. (C) Parental (DCIR-negative) and DCIRΔneck-expressing Raji-CD4 (1.5 × 105 cells) were exposed to NL4-3 (1.5 ng p24) for 2 hours at 37°C. After 3 washes with PBS to remove excess virus, cells were maintained in culture. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples and are representative of 5 combined independent experiments. The asterisk denotes statistically significant data (*P < .05).
The neck domain of DCIR is important for interaction with HIV-1. (A) Raji-CD4 cells were nucleofected with either an empty control vector (left panel) or a mammalian expression vector coding for a neck-deleted DCIR mutant (ie, DCIRΔneck) (right panel). Five hours later, flow cytometric analysis of DCIR was performed using a combination of PE-labeled anti-DCIR monoclonal Ab (dotted lines) and a control Ab (continuous lines). Data shown correspond to a single experiment representative of 5 combined independent experiments. (B) Parental (DCIR-negative) and DCIRΔneck-expressing Raji-CD4 (3 × 106 cells) were exposed to NL4-3 (300 ng p24) for 60 minutes at 37°C. After 3 washes with PBS to remove unabsorbed virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples from 5 combined independent experiments. (C) Parental (DCIR-negative) and DCIRΔneck-expressing Raji-CD4 (1.5 × 105 cells) were exposed to NL4-3 (1.5 ng p24) for 2 hours at 37°C. After 3 washes with PBS to remove excess virus, cells were maintained in culture. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. Data shown correspond to the means plus or minus SD of triplicate samples and are representative of 5 combined independent experiments. The asterisk denotes statistically significant data (*P < .05).
Discussion
Attachment of HIV-1 particles to host cells constitutes a critical event in the infection process and is attributed mainly to the interaction of virus-encoded envelope glycoproteins (ie, gp120) with cell surface structures acting as receptors (eg, CD4 and a 7-transmembrane coreceptor). These interactions elicit conformational changes in gp120 that induce fusion of viral and cellular membranes and entry of the viral core within the cell cytoplasm. It has been shown that initial binding of HIV-1 to DCs also involves interactions between gp120 and other cell surface components such as CLRs, which can lead to virus internalization within endosomal compartments. At least 4 distinct CLRs can associate with gp120, namely DC-SIGN, langerin, MR, and a yet to be identified carbohydrate- and calcium-dependent trypsin-resistant CLR (reviewed in Turville et al12,13 ). Interestingly, langerin, as opposed to the 3 other CLRs, was found to reduce HIV-1 transmission, thus suggesting that virus capture is not directly leading to a more efficient transfer process.29 The direct involvement of DC-SIGN in the efficient capture and transmission of HIV-1 has recently been put into question.30,31 Indeed, several studies have demonstrated that DCs can participate in HIV-1 trans-infection of CD4+ T cells via DC-SIGN–independent pathways.32-34 It is now thought that no unique CLR is fully responsible for HIV-1 attachment to all DC subsets and virus dissemination by DCs.2 Therefore, the quest for other receptors on the surface of DCs that can result in virus capture and contribute to HIV-1 dissemination is still a timely subject.
Here we provide evidence that DCIR, a recently described CLR that is considered to be a DC-expressed activating immunoreceptor, can serve as an HIV-1 attachment factor on the surface of DCs. The functional role played by DCIR in HIV-1 trans-infection of CD4+ T lymphocytes by DCs was established through the use of specific siRNAs and a polyclonal Ab. Additional studies indicated that DCIR-mediated virus transfer to CD4+ T cells also involves de novo replication of HIV-1 in DCs (ie, the second phase of the transfer process). We provide evidence that the DCIR-mediated effect on virus propagation is not linked with a diminished capacity of DCs to cause proliferation of CD4+ T cells. Moreover, our studies suggest that DCIR-mediated HIV-1 attachment and subsequent transmission require the neck domain of DCIR. Recently, it has been demonstrated that DC-SIGN blocks HIV-1 budding by inducing internalization of gp120.35 Given that DCIR is promoting HIV-1 capture and transfer, it is thus unlikely that virus budding is affected by this CLR at least under the tested experimental conditions.
Based on observations made in the present work and previous published reports, 3 distinct and not mutually exclusive hypotheses can be proposed to explain how the interaction between HIV-1 and DCIR can contribute to DC-mediated virus capture and dissemination. First, it can be postulated that the binding of HIV-1 particles to cellular DCIR might favor the association between gp120 and a sufficient number of CD4 and coreceptor molecules to trigger formation of a fusion pore. This route of virus entry is known to be the most efficient infection process with the fastest kinetics (reviewed in Clapham and McKnight36 ). This hypothesis is supported by the DCIR-dependent increase in HIV-1 binding and infection when using Raji-CD4 cells as targets. Given that gp120 is one of the most heavily glycosylated proteins in nature (reviewed in Vigerust and Shepherd37 ) and considering that DC-SIGN–dependent HIV-1 capture relies on interactions between the CRD of DC-SIGN and gp120,7,38 we propose that an association between the CRD of DICR and gp120 is most likely responsible for the capacity of DCIR to act as an attachment factor for HIV-1. Studies are currently under way to address this issue using a Fc-gp120 chimera39 along with appropriate anti-gp120 antibodies.5,9,40 However, such studies are compromised by the fact that DCs and Raji B cells do naturally express high surface levels of Fc receptors and blocking agents might affect some basic functions of DCs and, consequently, the interplay with HIV-1. The involvement of the CRD domain of DCIR in virus capture is illustrated by the reduced HIV-1 binding and transfer in the presence of a polyclonal Ab that is specific for the single CRD in the extracellular COOH-terminal end of DCIR. Experiments conducted with a neck-deficient DCIR mutant (ie, DCIRΔneck) suggest that this domain is important to permit the interaction between HIV-1 and DCIR. Based on the previous demonstration that the neck region of CLRs drives multimerization,19,41 it is possible that this domain is required for efficient recognition of multivalent ligands such as gp120-covered HIV-1 particles. The importance of the oligomerization status in ligand binding efficiency is underscored by the asialoglycoprotein receptor,42 another CLR that is closely related to DCIR. The second hypothesis refers to a DCIR-mediated internalization mode where such endocytosed HIV-1 particles would be spared from the rapid and extensive degradation that is normally seen in the endolysosomal apparatus. DCIR bears an intracellular ITIM and is considered to be the first reported DC-expressed, ITIM-bearing receptor of the CLR family.43 It is possible that engagement of DICR by HIV-1 might lead to activation of ITIM and allow recruitment of SHP-1 and SHP-2, which can act as adaptor proteins independently of their catalytic activity, by virtue of their tandem SH2 domains.44 It is of interest to note that poliovirus entry has recently been reported to occur through receptor-induced activation of the protein tyrosine phosphatase SHP-2.45 Assuming that DCIR allows HIV-1 to gain access to nondegradative endosomal organelles, this might allow the virus to persist for a sufficiently long time to be transported from mucosal surfaces to the T-cell compartment in lymphoid tissues, as it has been proposed for DC-SIGN.46,47 Moreover, storage in endosomes could lead to fusion between viral and endosomal membranes and productive infection, as reported in macrophages.48 Finally, it can be proposed that the interaction between HIV-1 and DCIR will initiate intracellular biochemical events that can reverse to some extent the various blocks responsible for the limited virus infection seen in DCs compared with CD4+ T cells (reviewed in Piguet and Steinman49 ). The DCIR-mediated signal transduction events might favor the recruitment of second messengers that might in turn promote reverse transcription or counter the limitation in virus entry, as described recently by Pion et al.50 For example, possible recruitment of SHP-1 or SHP-2 upon engagement of DCIR could release tyrosine kinases such as Lyn and/or Syk, which have been shown to be implicated in the cis-infection pathway in DCs.21
In summary, although no natural ligand of DCIR has yet been described, we demonstrate that DCIR modulates the early and late infection-dependent phases of HIV-1 propagation to CD4+ T cells by interacting with the virus itself. The relative importance of DCIR in HIV-1 pathogenesis is still unknown, but it is expected to be nonnegligible considering that DCIR is expressed on immune cells, and more particularly on APCs (ie, various DC subsets, monocytes, macrophages, and B cells), and can be modulated by some proinflammatory agents.14,15 DCIR could also play a role in mounting a virus-specific immune response. A more complete understanding of the precise contribution of DCIR to the virus life cycle or in directing specific immunity is needed because it might lead to the development of alternative strategies to treat infected individuals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sylvie Méthot for her excellent technical assistance in writing this paper and also Marc Bergeron for statistical analysis. We appreciate the excellent technical contribution of Maurice Dufour, Odette Simard, and Caroline Côté. We express our gratitude to Corinne Barat for critical and constructive comments for this study and Dave Bélanger for his involvement in the construction of DCIR expression vectors.
This work was supported by operating grants to M.J.T. from the Canadian Institutes of Health Research (CIHR) (Ottawa, ON) under the HIV/AIDS research program (MOP-79542 and HET-85519). A.L. holds a Doctoral Award from the CIHR HIV/AIDS research program. C.G was the recipient of a Fellowship Award from the CIHR HIV/AIDS Research Program, and M.J.T. holds the Canada Research Chair (Ottawa, ON) in Human Immuno-Retrovirology (Tier 1 level).
Authorship
Contribution: A.A.L. performed research, analyzed data, and wrote the paper; C.G. and M.J.T. designed research, analyzed data, and wrote the paper; M.R. and A.D.B. contributed vital new reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel J. Tremblay, Laboratoire d'Immuno-Rétrovirologie Humaine, Centre de Recherche en Infectiologie, RC709, Centre Hospitalier de l'Université Laval, 2705 boul, Laurier, Québec (QC), Canada, G1V 4G2; e-mail: michel.j.tremblay@crchul.ulaval.ca.