Abstract
The spectrum of entities, the therapeutic strategy, and the outcome of mature aggressive B-cell non-Hodgkin lymphomas (maB-NHLs) differs between children and adolescents on the one hand and adult patients on the other. Whereas adult maB-NHLs have been studied in detail, data on molecular profiling of pediatric maB-NHLs are hitherto lacking. We analyzed 65 cases of maB-NHL from patients up to 18 years of age by gene expression profiling, matrix comparative genomic hybridization (CGH), fluorescent in situ hybridization (FISH), and immunohistochemistry. The majority of the analyzed pediatric patients were treated within prospective trials (n = 49). We compared this group to a series of 182 previously published cases of adult maB-NHL. Gene expression profiling reclassified 31% of morphologically defined diffuse large B-cell lymphomas as molecular Burkitt lymphoma (mBL). The subgroups obtained by molecular reclassification did not show any difference in outcome in children treated with the NHL-Berlin-Frankfurt-Muenster (BFM) protocols. No differences were detectable between pediatric and adult mBL with regard to gene expression or chromosomal imbalances. This is the first report on molecular profiling of pediatric B-NHL showing mBL to be much more prominent in children than suggested by morphologic assessment. Based on molecular profiling mBL is a molecularly homogeneous disease across children and adults.
Introduction
Lymphomas are the third most common group of cancers in children and adolescents. Non-Hodgkin lymphomas (NHL), which account for approximately 60% of all lymphomas, represent 6% of all malignancies in children up to 14 years of age (German Childhood Cancer Registry, GCCR, http://info.imsd.uni-mainz.de/K_Krebsregister/english/). The spectrum of NHL occurring in children and adolescents differs strikingly from adults. Whereas indolent lymphomas are frequent in adults, the vast majority of lymphomas in children and adolescents are aggressive lymphomas, mainly mature aggressive B-cell lymphomas (maB-NHL) including particularly Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).1
With currently available combination chemotherapy for both BL and DLBCL an overall survival (OS) rate of 90% and more can be reached in children.2,3 In most pediatric study groups BL and DLBCL, although recognized by the World Health Organization (WHO) classification as distinct lymphoma entities, are currently treated according to the same treatment protocols in children.2-4 The stratification of treatment intensity is based on clinical risk factors like stage and lactate dehydrogenase (LDH), but not on the histopathologic diagnosis. In adults, only BL is treated with protocols derived from those used in children.5,6 However, for DLBCL CHOP-like regiments are the standard for adult patients.2,3,7,8
Gene expression profiling can be used to classify maB-NHL into BL and DLBCL more precisely than it is possible with the criteria of the WHO classification, which are based on morphology, immunophenotype, and genetics.9,10 Using this molecular approach, we and other groups were able to recognize a subgroup of lymphomas described as “molecular BL” (mBL) and to separate these from non-mBL, a group mainly composed of DLBCL, leaving a set of yet unclassifiable (intermediate) lymphomas.9,10 In adult DLBCL, gene expression and genomic profiling indicated the existence of several biologic subgroups which might explain the clinical heterogeneity of the disease.11,12 Using a immunohistochemical classifier, we showed recently that pediatric DLBCL are predominantly lymphomas of the germinal center subtype (GCB) which, in contrast to their adult counterpart, are virtually devoid of the translocation t(14;18). These findings indicate that pediatric DLBCL might be a more homogeneous disease than adult DLBCL.13
Based on our previous results, we hypothesised that mBL occur in pediatric and adult patients but subtle molecular differences between pediatric and adult lymphomas might explain clinical differences such as the gender distribution. To gain further insights into the molecular characteristics of pediatric B-NHL, we performed molecular profiling, including gene expression analysis, matrix-CGH, interphase fluorescence in situ hybridization (FISH) and immunohistochemistry on a series of 65 maB-NHL from patients diagnosed at an age of 18 years or younger, including 54 patients 14 years or younger. Of the latter, 49 were treated within prospective clinical trials of the German NHL-BFM study group. We compared the pediatric cohort to 182 maB-NHL in adults. Our data provide new insights into the biology of pediatric maB-NHL and might help to design future therapeutic strategies.
Methods
Patients
We analyzed a total of 65 maB-cell lymphomas from patients 18 years or younger, 29 of whom had been already included in a previously published series but have not been analyzed under the aspects presented. A total of 36 lymphomas were newly analyzed herein (Figures S1 and S2, available on the Blood website; see the Supplemental Materials link at the top of the online article.).9 Because BL are much rarer than DLBCL, the efforts to collect BL were stronger than to collect non-BL/DLBCL in our previous study, which focused of the differences between both entities independent of the patient's age.9 Therefore, the percentage of BL selected for molecular analysis was higher in our previous study9 compared with the cohort of patients newly analyzed herein (Figures S1,S2). A panel review of all cases was performed by expert hematopathologists who applied the criteria of the WHO classification without knowledge of the age of the patient.14 Lymphomas for which no consensus for a classification according to WHO was found, were designated as B-NHL high-grade. The reasons for lacking consensus among the specialists were ambiguous morphologic characteristics of the lymphoma or insufficient quality for a morphologic evaluation.9 The pathology review was done on hematoxylin and eosin (H&E)– or Giemsa-stained slides with the use of immunohistochemical stainings which included CD20, BCL2, and Ki-67. At the time of the review sessions the pathologists were neither aware of the age of the patient nor of any information concerning genetic alterations. The review was done on several sessions in which adult and pediatric lymphomas were mixed. All specimens analyzed in our published series9 and the newly analyzed series herein had a tumor cell content of more than 70%.
Of the 65 patients, 54 were 14 years or younger at diagnosis. Of those, 49 (91%) were treated within controlled clinical trials of the Berlin-Frankfurt-Muenster groups (BFM; Figure S2). A previously published series of 182 aggressive maB-NHL in adult patients served as a control group.9 The study was performed as part of the “Molecular Mechanisms in Malignant Lymphomas” Network Project of the Deutsche Krebshilfe for which central and local ethics approval was obtained. Informed consent was obtained in accordance with the Declaration of Helsinki.
Immunohistochemistry and fluorescence in situ hybridization
Immunohistochemistry and fluorescence in situ hybridization (FISH) were performed and evaluated using standard protocols. Antibodies and FISH probes applied have been described in detail in Hummel et al.9
Gene expression profiling
Gene expression profiling using Affymetrix (Santa Clara, CA) GeneChip U133A was performed as described recently.9 The gene expression raw data were normalized by the variance-stabilizing procedure and summarization of probes within probe sets.15,16 To rank differentially expressed genes between pediatric and adult mBL, microarray samples were measured by a 2-sample t score regularized by adding a constant fudge factor to the denominator. Statistical significance was assessed by calculating p-values based on 1000 random permutations of the class labels and subsequently estimating false discovery rates (FDR) based on the distribution of these P values.17 The data discussed are available from the Gene Expression Omnibus18 of the National Center for Biotechnology Information through GEO accession numbers GSE10172 (36 newly analyzed cases herein) and GSE4475 (cases described in Hummel et al9 ).
Array comparative genomic hybridization (array CGH)
DNA was extracted from frozen sections and CGH performed as described recently.9 Each of the approximately 2800 BAC clones on a CGH-array was classified as a genomic gain, loss, normal copy number, or a missing value.19 A chi-square test statistic was computed for each clone to test for a disproportionate number of genomic aberrations in a given subgroup of cases. Missing values were removed before computing each statistic. Multiple testing was performed with the step-down minP method implemented in the R package multitest (http://www.r-project.org/).
Statistical analyses
There exists no clear-cut age border between pediatric and adult lymphoma.20 Thus, all analyses were run in parallel with a cutoff of 14 years of age or less and 18 years of age or less for the definition of “pediatric” patients. The contrast group was defined as more than 14 or more than 18 years, respectively. No significant different results were obtained when applying these 2 cutoffs (data not shown). For better readability, the data presented here base on defining “pediatric” as 14 years of age (n = 54) and “adult” as more than 18 years. “Old pediatric” patients aged 15-18 years (n = 11) will only be displayed but not included in the statistical tests. Because the subsequent treatment protocols NHL-BFM 83, 86, 90, 95 and B-NHL BFM 04 have a common backbone of chemotherapeutic treatment regimen and outcome over the past 20 years within these trials was comparable2,21,22 the patients treated in the different NHL-BFM studies were lumped together with regard to clinical analyses.
Chi-square, Fisher exact and log-rank tests were applied to test for group differences. Survival was calculated from the day of diagnosis until death or until the date of last follow-up.
Results
Patient characteristics
To characterize the molecular spectrum of pediatric maB-NHL we performed molecular profiling in a series of 65 lymphomas from patients 18 years of age or younger. Of the 54 patients 14 years of age or younger, 49 (91%) were treated within controlled clinical trials of the Berlin-Frankfurt-Muenster group (BFM), namely NHL-BFM 81 (n = 1), BFM 83 (n = 2), 86 (n = 7), 90 (n = 14), 95 (n = 24), and B-NHL BFM 04 (n = 1). These trials include more than 90% of children diagnosed with NHL in Germany. There were no significant differences with regard to epidemiologic, histologic or clinical features between the subset analyzed herein and the overall population of 1325 patients included in the named BFM-trials (Table S1). Thus, the present series can be regarded as representative for maB-NHL in the German pediatric population.
Molecular profiling of a novel series of 36 pediatric maB-NHL
A total of 36 pediatric lymphomas which were not part of our previous study9 were newly profiled as part of this study. The lymphomas were molecularly classified into mBL and non-mBL based on the mBL index.9 We did not detect any significant differences in gene expression and matrix CGH profiles comparing the cases from our old series to the cases newly analyzed herein (data not shown). Of the newly analyzed, 13 were classified as mBL, 14 as non-mBL leaving 9 unclassifiable intermediates (Table S2). Of the 13 mBL all were IG-MYC positive and 11 were classified as MYC-simple, meaning that in addition to IG-MYC fusion no or only little additional genetic abnormalities were detectable.9 On the other hand, only 1 of the 14 non-mBL carried the IG-MYC translocation. In this independent dataset these findings confirm the good agreement between the mBL index and the pattern of genetic aberrations, and thus the validity of our previously described mBL signature to distinguish BL from other maB-NHL.9
Molecular spectrum of maB-NHL in children
Together, we molecularly profiled maB-NHL from a total of 65 patients no older than 18 years of age in the present as well as our previous study.9 The clinical and molecular characteristics of these lymphomas are shown in Tables 1 and 2, respectively.
Clinical characteristics according to age groups
| . | 14 y or younger, n = 54 . | 15 to 18 y, n = 11 . | Older than 18 y, n = 182 . | P* . | |||
|---|---|---|---|---|---|---|---|
| no. . | % . | no. . | % . | no. . | % . | ||
| Age | |||||||
| Less than 60 y | 54 | 100 | 11 | 100 | 67 | 37 | |
| 60 y or older | 0 | 0 | 0 | 0 | 115 | 63 | |
| Ann Arbor stage† | |||||||
| I or II | 49 | 42 | |||||
| III or IV | 69 | 58 | |||||
| Sex | |||||||
| Female | 16 | 30 | 3 | 27 | 80 | 44 | |
| Male | 38 | 70 | 8 | 73 | 100 | 56 | .06 |
| Lesions† | |||||||
| Extranodal only | 14 | 13 | |||||
| Nodal only | 59 | 54 | |||||
| Nodal/extranodal | 37 | 34 | |||||
| B symptoms | |||||||
| No | 26 | 67 | 3 | 43 | 62 | 60 | |
| Yes | 13 | 33 | 4 | 57 | 41 | 40 | .56 |
| Chemotherapy | |||||||
| NHL-BFM backbone like | 50 | 98 | 6 | 75 | 10 | 8 | |
| CHOP/COPBLAM like | 0 | 0 | 2 | 25 | 83 | 69 | |
| Other | 1 | 2 | 0 | 0 | 27 | 23 | < .001 |
| Radiotherapy | |||||||
| No | 51 | 100 | 8 | 100 | 80 | 71 | |
| Yes | 0 | 0 | 0 | 0 | 32 | 29 | < .001 |
| Treatment within NHL-BFM trial | |||||||
| Yes | 49 | 91 | 5 | 45 | 0 | 0 | |
| No | 5 | 9 | 6 | 55 | 182 | 100 | |
| . | 14 y or younger, n = 54 . | 15 to 18 y, n = 11 . | Older than 18 y, n = 182 . | P* . | |||
|---|---|---|---|---|---|---|---|
| no. . | % . | no. . | % . | no. . | % . | ||
| Age | |||||||
| Less than 60 y | 54 | 100 | 11 | 100 | 67 | 37 | |
| 60 y or older | 0 | 0 | 0 | 0 | 115 | 63 | |
| Ann Arbor stage† | |||||||
| I or II | 49 | 42 | |||||
| III or IV | 69 | 58 | |||||
| Sex | |||||||
| Female | 16 | 30 | 3 | 27 | 80 | 44 | |
| Male | 38 | 70 | 8 | 73 | 100 | 56 | .06 |
| Lesions† | |||||||
| Extranodal only | 14 | 13 | |||||
| Nodal only | 59 | 54 | |||||
| Nodal/extranodal | 37 | 34 | |||||
| B symptoms | |||||||
| No | 26 | 67 | 3 | 43 | 62 | 60 | |
| Yes | 13 | 33 | 4 | 57 | 41 | 40 | .56 |
| Chemotherapy | |||||||
| NHL-BFM backbone like | 50 | 98 | 6 | 75 | 10 | 8 | |
| CHOP/COPBLAM like | 0 | 0 | 2 | 25 | 83 | 69 | |
| Other | 1 | 2 | 0 | 0 | 27 | 23 | < .001 |
| Radiotherapy | |||||||
| No | 51 | 100 | 8 | 100 | 80 | 71 | |
| Yes | 0 | 0 | 0 | 0 | 32 | 29 | < .001 |
| Treatment within NHL-BFM trial | |||||||
| Yes | 49 | 91 | 5 | 45 | 0 | 0 | |
| No | 5 | 9 | 6 | 55 | 182 | 100 | |
Percentages were calculated on the basis of the number of cases that could be evaluated; data were not available for all cases. Percentages may not total 100 because of rounding.
P values were calculated with the use of Fisher exact test or the chi-square test and refer to differences between the cases 14 years or younger and older than 18 years.
Parameters not assessed in patients 18 years or younger.
Morphologic, immunohistochemical, genetic, and molecular characteristics of patients according to age groups
| . | 14 y or younger, n = 54 . | 15 to 18 y, n = 11 . | Older than 18 y, n = 182 . | P* . | |||
|---|---|---|---|---|---|---|---|
| no. . | % . | no. . | % . | no. . | % . | ||
| Morphology | |||||||
| Burkitt lymphoma | 16 | 30 | 1 | 9 | 7 | 4 | |
| Atypical Burkitt lymphoma | 10 | 19 | 1 | 9 | 13 | 7 | |
| DLBCL | 16 | 30 | 8 | 73 | 149 | 82 | |
| Follicular lymphoma III° | 2 | 4 | 1 | 9 | 1 | 1 | |
| B-NHL high grade | 10 | 19 | 0 | 0 | 12 | 7 | < .001 |
| CD10-IHC | |||||||
| Neg. | 8 | 15 | 7 | 70 | 106 | 62 | |
| Pos. | 44 | 85 | 3 | 30 | 66 | 38 | < .001 |
| BCL-2 IHC | |||||||
| Neg. | 33 | 65 | 4 | 40 | 40 | 23 | |
| Pos. | 18 | 35 | 6 | 60 | 137 | 77 | < .001 |
| BCL-6 IHC | |||||||
| Neg. | 3 | 6 | 2 | 18 | 32 | 20 | |
| Pos. | 45 | 94 | 9 | 82 | 132 | 80 | .028 |
| Ki67-index | |||||||
| Less than 95% | 25 | 46 | 7 | 64 | 143 | 81 | |
| 95% or more | 29 | 54 | 4 | 36 | 34 | 19 | < .001 |
| MYC-partner | |||||||
| IG-MYC | 38 | 70 | 3 | 27 | 33 | 19 | |
| Non-IG-MYC | 0 | 0 | 0 | 0 | 15 | 8 | |
| MYC-negative | 16 | 30 | 8 | 7 | 129 | 73 | < .001 |
| Cell of origin | |||||||
| ABC | 3 | 6 | 0 | 0 | 57 | 31 | |
| GCB | 47 | 87 | 8 | 73 | 86 | 47 | |
| Unclassified | 4 | 7 | 3 | 27 | 39 | 21 | < .001 |
| Molecular diagnosis | |||||||
| mBL | 34 | 63 | 2 | 18 | 20 | 11 | |
| Intermediate | 11 | 20 | 2 | 18 | 40 | 22 | |
| Non-mBL | 9 | 17 | 7 | 64 | 122 | 67 | < .001 |
| Major genetic groups | |||||||
| MYC-simple | 29 | 54 | 2 | 18 | 17 | 9 | |
| MYC-complex | 9 | 17 | 1 | 9 | 35 | 19 | |
| MYC-neg. | 16 | 30 | 8 | 73 | 129 | 71 | < .001 |
| . | 14 y or younger, n = 54 . | 15 to 18 y, n = 11 . | Older than 18 y, n = 182 . | P* . | |||
|---|---|---|---|---|---|---|---|
| no. . | % . | no. . | % . | no. . | % . | ||
| Morphology | |||||||
| Burkitt lymphoma | 16 | 30 | 1 | 9 | 7 | 4 | |
| Atypical Burkitt lymphoma | 10 | 19 | 1 | 9 | 13 | 7 | |
| DLBCL | 16 | 30 | 8 | 73 | 149 | 82 | |
| Follicular lymphoma III° | 2 | 4 | 1 | 9 | 1 | 1 | |
| B-NHL high grade | 10 | 19 | 0 | 0 | 12 | 7 | < .001 |
| CD10-IHC | |||||||
| Neg. | 8 | 15 | 7 | 70 | 106 | 62 | |
| Pos. | 44 | 85 | 3 | 30 | 66 | 38 | < .001 |
| BCL-2 IHC | |||||||
| Neg. | 33 | 65 | 4 | 40 | 40 | 23 | |
| Pos. | 18 | 35 | 6 | 60 | 137 | 77 | < .001 |
| BCL-6 IHC | |||||||
| Neg. | 3 | 6 | 2 | 18 | 32 | 20 | |
| Pos. | 45 | 94 | 9 | 82 | 132 | 80 | .028 |
| Ki67-index | |||||||
| Less than 95% | 25 | 46 | 7 | 64 | 143 | 81 | |
| 95% or more | 29 | 54 | 4 | 36 | 34 | 19 | < .001 |
| MYC-partner | |||||||
| IG-MYC | 38 | 70 | 3 | 27 | 33 | 19 | |
| Non-IG-MYC | 0 | 0 | 0 | 0 | 15 | 8 | |
| MYC-negative | 16 | 30 | 8 | 7 | 129 | 73 | < .001 |
| Cell of origin | |||||||
| ABC | 3 | 6 | 0 | 0 | 57 | 31 | |
| GCB | 47 | 87 | 8 | 73 | 86 | 47 | |
| Unclassified | 4 | 7 | 3 | 27 | 39 | 21 | < .001 |
| Molecular diagnosis | |||||||
| mBL | 34 | 63 | 2 | 18 | 20 | 11 | |
| Intermediate | 11 | 20 | 2 | 18 | 40 | 22 | |
| Non-mBL | 9 | 17 | 7 | 64 | 122 | 67 | < .001 |
| Major genetic groups | |||||||
| MYC-simple | 29 | 54 | 2 | 18 | 17 | 9 | |
| MYC-complex | 9 | 17 | 1 | 9 | 35 | 19 | |
| MYC-neg. | 16 | 30 | 8 | 73 | 129 | 71 | < .001 |
Percentages were calculated on the basis of the number of cases that could be evaluated; data were not available for all cases. Percentages may not total 100 because of rounding.
IHC indicates immunohistochemistry.
P values were calculated with the use of Fisher exact test or the chi-square test and refer to differences between the cases 14 years or younger and older than 18 years. The group of patients 15 to 18 years were not included in the analysis and are displayed for completeness only.
Among the 54 patients who were 14 years old or younger at presentation, the morphologic diagnoses were BL (n = 16, 30%), atypical BL (BL-like, n = 10, 19%), DLBCL (n = 16, 30%), follicular lymphoma (FL grade 3, n = 2, 4%) and high-grade B-NHL not further classified (n = 10, 19%, Table 1). Gene expression profiling revealed 34 mBL (63%), 11 intermediates (20%) and 9 (17%) non-mBL lymphomas in the group of pediatric patients (Table 2). Morphologic BL/atypical BL as well as mBL defined by gene expression were more frequent in children than adults (49% vs 11% and 63% vs 11%, respectively). Vice versa, DLBCL and non-mBL were less frequent in children than in adults (30% vs 82% and 17% vs 67%, respectively). The percentages of molecularly unclassifiable cases that were called “intermediates”9 were similar in the 2 age groups (20% in children vs 22% in adults, Table 2).
Two lymphomas were diagnosed morphologically as follicular lymphoma grade 3. These 2 lymphomas were included in the study because the initial diagnosis before the review was maB-NHL. One of the 2 lymphomas was molecularly defined as mBL. Reanalysis of the histologic slides suggest that this lymphoma might present a rare example of a follicular growing BL whereas the other lymphoma, a non-mBL presents a follicular lymphoma with transformation to a DLBCL.
Remarkably, of the 16 morphologically defined DLBCL in children, 5 (31%) were reclassified as mBL, with 3 of them being IG-MYC positive suggesting that they are indeed biologic BL (Table S4). In contrast to pediatric lymphomas, only 4 of 149 morphologically defined DLBCL in adults were classified as mBL. Thus, gene expression profiling leads to a reclassification of morphologically diagnosed DLBCL significantly more frequent in children (31%) than in adults (2.7%; P < .001). On the other hand, the morphologic diagnosis of BL/atypical BL correlated well with the molecular diagnosis mBL in children and only 2/26 morphologically defined BL (1 BL and 1 atypical BL, together 7.7%) could not be classified as mBL by gene expression profiling but were assigned to the intermediate group.
Prognostic impact of molecular profiling in pediatric maB-NHL
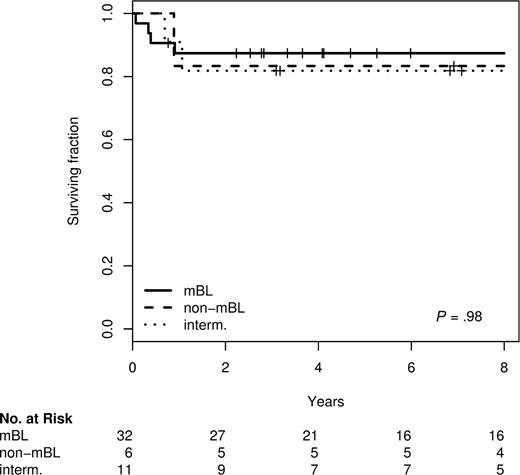
Within the NHL-BFM trials the prognosis does not differ between morphologically defined DLBCL and BL.2,21,22 Given the frequent molecular reclassification of morphologic DLBCL into mBL in children described above, we wondered whether molecular classification might be superior to morphology in distinguishing prognostic groups in pediatric patients. Nevertheless, we did not find any difference in survival between molecularly defined mBL, intermediate and non-mBL lymphomas in children 14 or 18 years of age or younger. This holds true for both (1) all pediatric patients included in these series as well as (2) for the subset treated homogeneously within the prospective NHL-BFM trials (Figure 1). The 5-year survival rates of the pediatric patients with mBL, intermediate, and non-mBL lymphomas treated within the BFM-NHL trials were 84%, 82%, and 83%, respectively. A comparison of survival differences between molecular defined groups in adult patients is not feasible because therapy differs strikingly between mBL and non-mBL with most non-mBL being treated with CHOP-like and most mBL with NHL-BFM like therapies.9,10 The clinical characteristics of all BFM-patients analyzed are shown in Table S3.
Kaplan-Meier plot for overall survival of 49 patients 14 years old or younger treated in NHL-BFM-trials according to the molecular diagnosis.
Kaplan-Meier plot for overall survival of 49 patients 14 years old or younger treated in NHL-BFM-trials according to the molecular diagnosis.
Compared with pediatric patients, a much stronger heterogeneity of the adult lymphoma with regard to biologic features, clinical parameters and treatment modalities can be assumed. Nevertheless, we aimed at an initial molecular comparison between both mBL and non-mBL between children and adults. Lymphomas classified as intermediate were not compared in detail between children and adults as they do not constitute a separate group but merely represent unclassifiable cases.
Comparison of pediatric and adult mBL
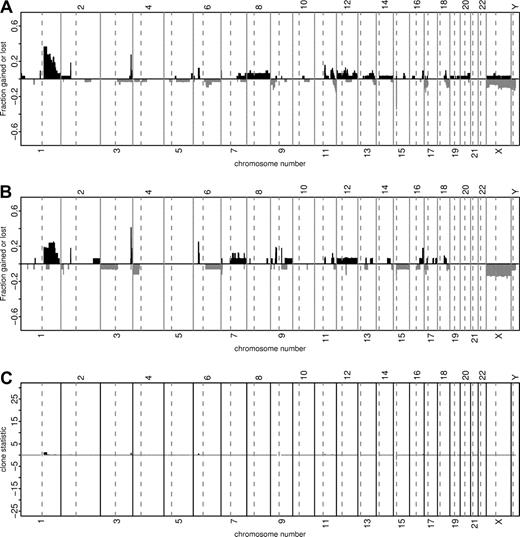
We compared a total of 34 pediatric (≤14 years) and 20 adult (>18 years) mBL. There were no significant differences between adults and children with respect to the expression of CD10, BCL-2, BCL-6 or Ki-67 detected by immunohistochemistry (Table S4). Similarly, the genomic imbalances detected by array CGH were remarkably similar in pediatric (n = 33) and adult (n = 20) mBL (Figure 2).
Comparison of chromosomal imbalances detected by matrix-CGH in pediatric (≤14 years, n = 33) and adult (>18 years, n = 17) mBL. Every vertical bar on the plots represents a CGH clone in its genomic position. The green and red bars show relative frequencies of gains and losses, respectively. The heights of the red bars are given a negative value for clarity. Panel A shows the relative frequency of genomic imbalances in pediatric mBL (≤14 years). Panel B refers to the population of adult mBL (>18 years). Panel C illustrates the discrepancies in the frequency of genomic aberrations between the patient groups from Panel A and B with the corresponding chi-square statistics. The chi-square statistics for losses are shown with a negative sign. Clones with more than 10% missing values or having 5 or fewer aberrations overall are not shown.
Comparison of chromosomal imbalances detected by matrix-CGH in pediatric (≤14 years, n = 33) and adult (>18 years, n = 17) mBL. Every vertical bar on the plots represents a CGH clone in its genomic position. The green and red bars show relative frequencies of gains and losses, respectively. The heights of the red bars are given a negative value for clarity. Panel A shows the relative frequency of genomic imbalances in pediatric mBL (≤14 years). Panel B refers to the population of adult mBL (>18 years). Panel C illustrates the discrepancies in the frequency of genomic aberrations between the patient groups from Panel A and B with the corresponding chi-square statistics. The chi-square statistics for losses are shown with a negative sign. Clones with more than 10% missing values or having 5 or fewer aberrations overall are not shown.
The mBL-signature genes (n = 74 features) were selected with pediatric and adult patients as input. Hence, the mBL-index is not expected to differ between children and adults. However, we considered the whole transcriptome (>20 000 features) while comparing children and adult mBLs. For this purpose the gene expression profiles of pediatric and adult mBL were compared directly using regularized t-scores and empirical P values computed by random permutations of the class labels. For this purpose the gene expression profiles of pediatric and adult mBL were compared directly using regularized t scores and empirical P values computed by random permutations of the class labels. We were unable to find any evidence of significant differences in gene expression between the 2 groups (data not shown). Finally, we did not observe a significant difference in survival between pediatric and adult mBL treated with BFM or comparable B-ALL–like protocols (5-year survival: 87% vs 70%, P = .67) or any other significant differences in clinical characteristics except a trend for lower frequency of B-symptoms (Table S5). Thus, with the exception of the well known marked male predominance characteristic for pediatric BL that is not present in adult BL (79% vs 55%, P = .07), mBL seems to be a rather homogenous disease in children and adults considering the molecular and clinical features analyzed herein.
As reported by us and others, some mBL display features that are considered uncommon for BL like BCL2 protein expression, Ki-67 index of less than 95% or a high genetic complexity.9,10 Figure 3 demonstrates that these “uncommon” features of mBL also occur in pediatric patients. However, if mBL with at least one “uncommon” feature (n = 17) were compared with mBL without “uncommon” features (n = 17), no statistically significant differences were found of clinical features including overall survival (data not shown).
Molecular features of pediatric maB-NHL classified by gene expression profiling using the mBL index.9 Genomic complexity of the cases is shown as bar plots on top of the panels, with complexity increasing with height. Below, the plot with the orange background shows the mBL-index on the y-axes indicating the similarity to the core-BLs {Hummel, Bentink, et al 2006 1205 /id} (gray box on the left to the mBL-index plot). The vertical lines delineate the 3 groups of lymphomas (mBL on the left, intermediate in the middle, and non-mBL on the reight) and the dashed horizontal lines indicate the index-score cutoffs defining the mBL group (0.95) and the non-mBL group (0.05) according to the mBL-index.9 Below, the heat map shows the gene-expression levels of the 58 mBL-signature genes, with 1 gene shown per row. Bright blue indicates a low level of expression (3 SD below the average of all cases), bright yellow indicates a high level of expression (3 SD above the average), and black the average level of expression across all samples. The cases are ordered from left to right on the basis of decreasing mBL-signature index score, given again below the heat map. Green represents a high index score (mBL), and red a low index score (non-mBL). The color gradient of the intermediate cases highlights the continuous transition of the index score between the mBL and non-mBL cases. The myc translocation partners are shown according MYC break (bright green) and MYC-breakpoint absent (red). The histologic diagnosis is shown in the panel below. Bright green indicates core-Burkitt lymphoma; dark green, Burkitt lymphoma; red, diffuse large-B-cell lymphoma; blue, follicular lymphoma and gray, unclassifiable mature aggressive B-cell lymphoma. The proliferation-index is coded with green for Ki-67 greater than 95% and red for Ki-67 less than 95%. Finally, BCL2 protein expression is indicated in green for negativity and red for positivity. White bars indicate not assessable.
Molecular features of pediatric maB-NHL classified by gene expression profiling using the mBL index.9 Genomic complexity of the cases is shown as bar plots on top of the panels, with complexity increasing with height. Below, the plot with the orange background shows the mBL-index on the y-axes indicating the similarity to the core-BLs {Hummel, Bentink, et al 2006 1205 /id} (gray box on the left to the mBL-index plot). The vertical lines delineate the 3 groups of lymphomas (mBL on the left, intermediate in the middle, and non-mBL on the reight) and the dashed horizontal lines indicate the index-score cutoffs defining the mBL group (0.95) and the non-mBL group (0.05) according to the mBL-index.9 Below, the heat map shows the gene-expression levels of the 58 mBL-signature genes, with 1 gene shown per row. Bright blue indicates a low level of expression (3 SD below the average of all cases), bright yellow indicates a high level of expression (3 SD above the average), and black the average level of expression across all samples. The cases are ordered from left to right on the basis of decreasing mBL-signature index score, given again below the heat map. Green represents a high index score (mBL), and red a low index score (non-mBL). The color gradient of the intermediate cases highlights the continuous transition of the index score between the mBL and non-mBL cases. The myc translocation partners are shown according MYC break (bright green) and MYC-breakpoint absent (red). The histologic diagnosis is shown in the panel below. Bright green indicates core-Burkitt lymphoma; dark green, Burkitt lymphoma; red, diffuse large-B-cell lymphoma; blue, follicular lymphoma and gray, unclassifiable mature aggressive B-cell lymphoma. The proliferation-index is coded with green for Ki-67 greater than 95% and red for Ki-67 less than 95%. Finally, BCL2 protein expression is indicated in green for negativity and red for positivity. White bars indicate not assessable.
Comparison of pediatric and adult non-mBL
Due to differences in therapy between children and adults with non-mBL, a direct comparison of survival is not feasible with the currently available data.9,10 Similarly, a direct comparison of gene expression or matrix-CGH profiles between subgroups of pediatric and adult non-mBL seemed not to be feasible because of the small pediatric sample size. Four of the non-mBL were assigned to the GCB type (44%) and 3 to the ABC type (33%) of DLBCL, leaving 2 unclassified cases (22%). This distribution did not significantly differ from that in adult cases. Analogous, we failed to detect any significant differences with regard to antigen expression between pediatric and adult non-mBL, though the power of these comparisons was of course limited by the low number of pediatric cases.
Discussion
This is the first report of a comprehensive molecular and genetic profiling approach to pediatric maB-NHL. We combined gene expression profiling and matrix-CGH with a thorough histopathologic characterization based on morphology and immunohistochemistry. A unique characteristic of our series of 65 pediatric lymphomas is the large number of patients treated within controlled clinical trials, building the basis for reliable clinical evaluations. The major goal of our study was to gain insights into the molecular and genetic characteristics of pediatric lymphomas and to delineate similarities and differences between pediatric and adult DLBCL and BL.
A major finding of this study is that the group of molecularly defined Burkitt lymphomas (mBL) does not differ between children and adults with respect to gene expression and genetic aberrations. This observation represents a biologic rationale for the recent harmonization of treatment protocols between children and adult BL patients who resulted in an improvement in clinical outcome in the adult age group.5,6 The reason for the high frequency of B-symptoms and the strong male predominance in children but not in adults remains elusive.23
Morphologic DLBCL are less frequent in children than in adults.1 Nevertheless, our data indicate that non-mBL (the molecular counterpart of morphologic/histopathologic DLBCL) exists in pediatric patients. However, gene expression profiling revealed that non-mBL is even rarer in the pediatric age group than anticipated by morphology and immunohistochemistry alone. Within the group of pediatric patients one third of the morphologically diagnosed DLBCL were classified as non-mBL by gene expression profiling and the others as unclassifiable (intermediates) or mBL. These data might explain the high frequency of MYC breaks reported for pediatric patients,24 because the group of morphologically defined DLBCL in children seems to contain a higher rate of “contamination” with lymphomas with a mBL expression profile than their adult counterpart. Although adequate studies are lacking, initial reports suggest that morphologically defined DLBCL with an mBL gene expression signature might benefit from BL therapy protocols.9,10,25 Because the therapeutic strategies in BL and DLBCL are the same in children, the problem of assigning a patient with a maB-NHL to an insufficient therapy based on the morphologic/histopathologic diagnosis is clinically less relevant in children than in adults at the current stage. However, future targeted therapeutic strategies might need to be based on precise distinction of mBL from non-mBL. It should be taken into consideration that the morphologic/histopathologic diagnosis in our study was generated without knowledge of the age of the patient, which would otherwise influence morphologic diagnostic decisions toward BL. Thus, these results challenge the morphologic criteria for distinguishing BL and DLBCL in pediatric patients.
The GCB subtype of non-mBL shows a favorable outcome compared with the ABC subtype in adult patients.11 Recent data suggested that morphologically defined DLBCL might differ between children and adults in their composition of molecular subtypes with a higher proportion of the GCB subtype of DLBCL (based on assignment by immunohistochemistry) in the younger age group.13,26 In the light of our molecular profiling data, these differences might at least partially be explained by the high contamination of morphologically defined DLBCL by mBL in pediatric patients. Indeed, if gene expression profiling is applied, mBL are classified as the GCB subtype of DLBCL if this algorithm for DLBCL is used for mBL.9 Using gene expression as the gold standard of DLBCL classification into ABC and GCB subtypes, we found that pediatric non-mBL showed only a moderate predominance of the GCB over the ABC type compared with adults. We have to stress that the number of non-mBL in our cohort is rather small. However, the sub-classification of non-mBL using the molecular categories GCB, ABC or unclassifiable, although successful in adults,11 might not provide a sufficient molecular explanation for the favorable outcome of pediatric patients. Nevertheless, as new molecular classification strategies are developing,27 future studies will provide more insight into similarities and differences between pediatric and adult non-mBL.
In summary, our data presented here suggest that mBL share a molecular profile in children and adults. Nevertheless, the reason for biologic differences between both age groups, such as sex distribution, remains elusive. Molecular reclassification of pediatric lymphoma does not yield prognostic relevant groups if the patients are treated with the current NHL-BFM therapy protocols.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Olivera Batic, Edda Sevecke-Wessel, Michael Weiss, Monika Hauberg, Christiane Stange, Hedwig Lammert, Erika Berg, Claudia Becher, Dorit Schuster, and Reina Zühlke-Jenisch for their excellent technical support.
This work was carried out within the framework of the research network “Molecular Mechanisms in Malignant Lymphoma” supported by the Deutsche Krebshilfe (Bonn, Germany; 70-3173-Tr3). W.K. and R.S. are supported by the Kinderkrebs Initative (Buchholz, Holm-Seppensen, Germany).
Authorship
Contributions: P.M., M.L.H., H.-W.B., G.O., H.S., and W.K. provided samples and performed the pathology review; M.S., C.S., S.W., R.S., and M.H. performed the molecular analysis; H.B., M.R., S.B., R.S., and M.L. performed the statistical analysis; B.B., M.Z., L.T., and A.R. provided clinical data and administrative support; and R.S. and W.K. designed the project, analyzed the data, and wrote the manuscript.
A complete list of the members of the “Molecular Mechanisms in Malignant Lymphomas” network is listed in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Klapper, Institute of Pathology, Department of Hematopathology and Lymph Node Registry University-Hospital Schleswig-Holstein, Campus Kiel/Christian-Albrechts-University Kiel Michaelisstraβe 11 24105 Kiel, Germany; e-mail: wklapper@path.uni-kiel.de.
References
Author notes
*M.S., B.B., H.B., and M.R. contributed equally to this work.