Abstract
Epigenetic changes have been implicated in silencing several B-cell genes in Hodgkin and Reed-Sternberg cells (HRS) of Hodgkin lymphoma (HL), and this mechanism has been proposed to promote HRS survival and escape from immunosurveillance. However, the molecular and functional consequences of histone deacetylase (HDAC) inhibition in HL have not been previously described. In this study, we report that the HDAC inhibitor vorinostat induced p21 expression and decreased Bcl-xL levels causing cell-cycle arrest and apoptosis. Furthermore, vorinostat inhibited STAT6 phosphorylation and decreased its mRNA levels in a dose- and time-dependent manner, which was associated with a decrease in the expression and secretion of Thymus and Activation-Regulated Chemokine (TARC/CCL17) and interleukin (IL)–5 and an increase in IP-10 levels. Moreover, vorino-stat inhibited TARC secretion by dendritic cells that were activated by the thymic stromal lymphopoietin (TSLP). Collectively, these data suggest that pharmacologic HDAC inhibition in HL may induce favorable antitumor activity by a direct antiproliferative effect on HRS cells, and possibly by an immune mediated effect by altering cytokine and chemokines secretion in the microenvironment.
Introduction
The malignant Hodgkin and Reed-Sternberg (HRS) cells of Hodgkin lymphoma (HL) reside among an overwhelming number of CD4+ TH2 lymphocytes and secrete high levels of cytokines and chemokines, including interleukin (IL)–5, -6, -7, -9, -10, -13, and thymus and activation-regulated chemokine (TARC/CCL17).1,2 These cytokines bind to their surface receptors and activate Janus kinases (JAK), which recruit and phosphorylate a family of signal transducer and activator of transcription (STAT) proteins. STAT phosphorylation on tyrosine and serine sites leads to their dimerization and translocation from the cytoplasm to the nucleus where they induce gene transcription. To date, 7 STAT proteins have been identified that can be activated by a variety of cytokines. In HL, activated STAT3 and STAT6 are frequently observed in cultured and primary HRS cells and are reported to promote their survival.3-5 STAT3 activation can be induced by a variety of cytokines, including IL-2, -6, -7, -9, -10, and -15. In contrast, STAT6 activation is predominantly induced by IL-4 and IL-13.6 In fact, an autocrine IL-13 loop has recently been shown to be responsible for STAT6 activation and promotion of HL cell survival.7
During immune responses, antigen-stimulated naive CD4+ T cells can differentiate into at least 2 functionally distinct subsets of T helper (TH) cells. TH1 cells secrete interferon (IFN)-γ and lymphotoxin and are important in cell-mediated immunity, whereas TH2 cells secrete IL-4, IL-5, and IL-13 and are involved in humoral immunity and allergic responses. The polarization of naive CD4+ T cells toward TH1 and TH2 subsets is primarily regulated by signals through the T-cell receptor and cytokine receptors: IL-12 promotes TH1 while and IL-4 promotes TH2 development. IL-4 binds to its receptor on naive CD4+ T cells and activates JAK1 and JAK3, which then recruit and phos-phorylate the transcription factor STAT6. Consequently, phosphorylated STAT6 (pSTAT6) translocates to the nucleus and induces the expression of STAT6 target genes, including TARC.8,9 Importantly, HRS cells secrete an abundant amount of TARC, contributing to the attraction and homing of activated TH2 T lymphocytes to areas surrounding HRS cells.10 Collectively, these data suggest that STAT6 activation not only play a central role in promoting HRS cell survival, but may also play a critical role in promoting TH2 differentiation and attraction by mediating IL-13 signaling and TARC production.
In addition to STAT phosphorylation, lysine acetylation was recently shown to be involved in regulating STAT3 dimerization and function, suggesting that HDAC inhibitors may also have a role in regulating STAT function.11 Moreover, epigenetic changes have been implicated in silencing several B-cell genes in HRS cells, and this mechanism has been proposed to promote HRS survival and escape from immunosurveillance.12,13 However, the molecular and functional consequences of HDAC inhibition in HL, especially their effect on STAT3 and STAT6 status, have not been previously described. In this study, we report that the HDAC inhibitor vorinostat induced p21 expression and decreased Bcl-xL levels causing cell cycle arrest and caspase-mediate apoptosis. Furthermore, vorinostat inhibited STAT6 and STAT6-mediated TH2 cytokine and chemokine production, including TARC. Our data suggest that in addition to a direct antitumor activity, HDAC inhibition may alter the composition and function of the reactive CD4+ T cells in the HL microenvironment by inhibiting STAT6 and TARC.
Methods
Cell lines and cell culture
The human HRS-derived cell lines L-428 and KM-H2 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures, Braunschweig. The phenotypes and genotypes of these cell lines have been previously published.14 All cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL, Gaithersburg, MD), 1% l-glutamine, and penicillin/streptomycin in a humid environment of 5% CO2 at 37°C.
Reagents, antibodies, and recombinant proteins
The HDAC inhibitor suberoylanilide hydroxamic (vorinostat, SAHA) was purchased from Biovision (Mountain View, CA). Antibodies to histone 3; acetylated histone 3; AKT; phospho-AKT; caspase 3, 8, and 9; cleaved poly ADP-ribose polymerase; STAT3; phospho-STAT3; and STAT6 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies to p21, Bcl-xL, and phospho-STAT6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) Antibody to β-actin was from Sigma-Aldrich (St Louis, MO).
In vitro proliferation assay
Cells were cultured in 6-, 12-, and 24-well plates at a concentration of 0.5 × 106 cells/mL. Cell viability was assessed with the nonradioactive cell proliferation MTS assay using CellTiter96AQueous One Solution Reagent (Promega, Madison, WI), as previously published.15 Briefly, 80 μL cell suspension with 20 μL CellTiter96AQueous One Solution Reagent were incubated in 96-well plates for 1 hour at 37°C, 5% CO2, and formazan absorbance was measured at 490 nm on a μQuant plate reader equipped with KC4 software (Biotek Instruments, Winooski, VT). Each measurement was made in triplicate and the mean value was determined.
Dendritic cell purification
CD11c+ dendritic cells (DCs) were purified from the buffy coats of donated blood from healthy volunteers obtained from the Gulf Coast Regional Blood Center, Houston, Texas, following an Institutional Review Board–approved protocol for human research at M. D. Anderson Cancer Center as previously described.16 In brief, the CD11c+ DC was enriched from peripheral blood mononuclear cells (PBMCs) by Ficoll centrifugation and a negative depletion of cells that were expressed CD3, CD14, CD16, and glycophorin A, using goat anti–mouse IgG coated magnetic beads (Invitrogen, Carlsbad, CA and Miltenyi Biotec, Bergisch Gladbach, Germany). Enriched cells were further stained with FITC-labeled mAbs against lineage markers, CD3, CD14, CD16, CD19, CD56, allophycocyanin (APC)–labeled anti–CD11c mAb, and APC-Cy7–labeled anti–CD4 mAb. The CD11c+ CD4+ lineage− cells were isolated by a FACS Aria (BD Biosciences, San Jose, CA) with more than 99% purity.
Cytokine and chemokine production and ELISA
CD11c+ DCs were cultured immediately after being sorted in IMDM containing 2% human AB serum. Cells were seeded at a density of 0.7 × 105 cells/mL or 0.9 × 105 cells/mL in flat-bottomed half area 96-well plates with 100 ng/mL of human thymic stromal lymphopoietin (TSLP) or culture medium alone in the presence or absence of 2 μM or 0.2 μM of vorinostat. DMSO was used as control of Vorinostat. Supernatants from cultured DCs was collected at 24 hours and analyzed for TARC levels by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Similarly, the effect of vorinostat on cytokine and chemokine production by HL cell lines was examined by ELISA. For these experiments, HL cell lines were incubated with or without 2 to 5 μM of vorinostat for 24 hours, before supernatants were collected and examined for TARC, IL-4, IL-5, interferon-γ, IP-10, and IL-13 by ELISA.
Flow cytometry
Apoptosis was determined by annexin-V–FLUOS and propidium iodide double staining (Roche Molecular Biochemicals, Indianapolis, IN), according to the manufacturer's instructions and as we have previously published.15,17 Cell-cycle fractions were determined by propidium iodide nuclear staining. Briefly, cells were harvested, washed in PBS, fixed with 70% ETOH, and incubated with propidium iodide for 30 minutes at 37°C. Data were collected and analyzed on a FACSCalibur flow cytometer using CellQuestPro software (BD Biosciences), as described previously.15,17 For cell-cycle analysis, cell-cycle fragments were calculated by applying a model for diploid cells, with ModFit LT software (Verity Software House, Topsham, ME).15,17
Western blot analysis
Total cellular proteins were extracted by sonication and incubation in lysis buffer (Cell Signaling Technology) for 30 minutes on ice and then centrifuged to remove cellular debris. The protein in the resulting supernatant was quantified by the BCA method (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. Then, protein was diluted 1:2 in protein SDS loading buffer (Cell Signaling Technology), and heated to 95°C for 5 minutes. A total of 30 μg of protein was loaded onto 12% Tris-HCl SDS polyacrylamide electrophoresis Ready Gels (Bio-Rad, Hercules, CA), transferred to a nitrocellulose transfer membrane (Bio-Rad), and detected using SuperSignalWest Dura Extended Duration Substrate (Pierce Chemical), as previously described.15,17
Real-time PCR
Total RNA was extracted with the Qiagen (Valencia, CA) RNeasy mini protocol and was converted to cDNA using oligo-dT, random hexamers, and iScript (Bio-Rad). After diluting cDNA in dH20 1:20, and real-time polymerase chain reaction (PCR) was performed using a sequence detector (model ABI PRISM 7700; PerkinElmer). Real-time PCR was performed on duplicate 1-μL cDNA samples using an ABI Prism sequence detector (Applied Biosystems, Foster City, CA) and target mixes (TaqMan Gene Expression Assays; Applied Biosystems) with the following thermal cycling profile: 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for 60 seconds. The assays used were: STAT6 (Hs00598618_m1), CCL17/TARC (Hs001710741_m1), and GAPDH (Hs99999905_m1). The expression of GAPDH was used as an internal standard in calculating relative gene expression. Results are represented as the mean plus or minus SEM of 3 independent experiments.
Selective inhibition of STAT6 expression by short interfering RNA
Short interfering RNA (SiRNA) oligonucleotides used to block STAT6 expression (5′-GGGAGAAGAUGUGUGAAACUCUGAA-3′) and (5′-GAAUCCGGGAUCUUGCUCAGCUCAA-3) and nonspecific control siRNA were purchased from Invitrogen. Hodgkin lymphoma cell line (L428) was plated at a 106/ mL concentration in 12-well plates. Double-stranded siRNAs were transfected at time 0 hours using Nucleofection Kit (Amaxa, Gaithersburg, MD). The final concentration of siRNA was 40 nmol/L. Cells and supernatants were harvested after 12, 24, 48, and 72 hours and were subjected to Western blot analysis and ELISA assay. Down-regulation of the target gene (STAT6) by specific siRNA but not negative controls was confirmed by Western blot. This protocol gave a transfection efficiency of between 40 and 50%.
[3H] thymidine incorporation assay
Cells were plated as triplicate wells in 200 μL RPMI, 10% FCS, with indicated antibody reagents in a 96-well culture plate and incubated in 5% CO2/air at 37°C. After 12, 24, 48, and 72 hours, each well was pulsed with 1 μCi [3H]thymidine (GE Healthcare, Little Chalfont, United Kingdom) and incubated for 8 hours; then, cells were harvested and counted in a Beckman LS3800 liquid scintillation counter (Hialeah, FL).
Statistical methods, isobologram, and combination index calculation
The effectiveness of the drugs used in the present study, and their combination, were analyzed using the Calcusyn Software (Biosoft, Ferguson, MO). The combination index and isobologram plot were calculated according to the Chou-Talalay method.15,17 A combination index value of 1 indicates an additive effect between 2 drugs. Combination index values less than 1 indicate synergy, and the lower the value, the stronger the synergy. In contrast, combination index values more than 1 indicate antagonism. Effects of certain conditions on cell proliferation, apoptosis, and cytokine production were performed in 3 independent experiments in triplicate. The 2-tailed Student t test was used to estimate statistical significance of the differences in results from the 3 experiments. The level of significance was set to .05.
Results
Effect of vorinostat on HL proliferation
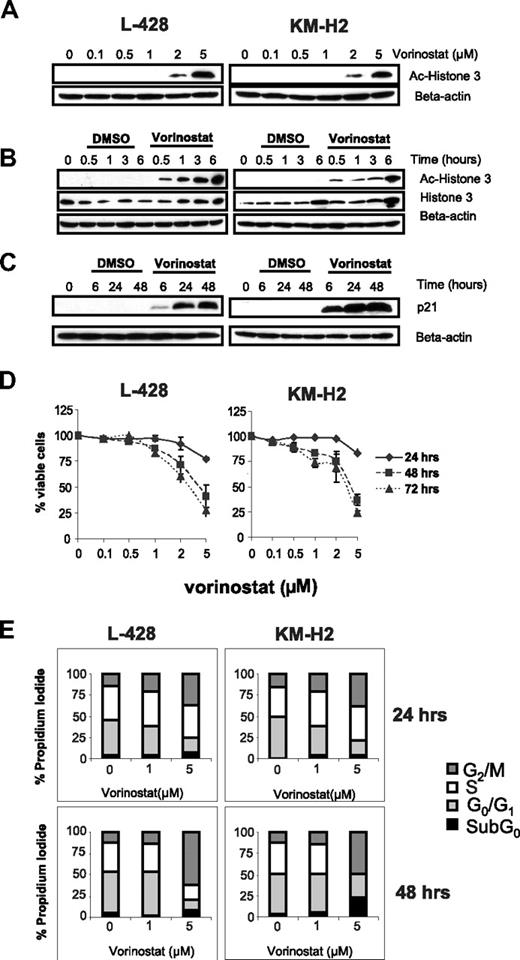
A common feature of HDAC inhibitors is their ability to acetylate histones, resulting in the restoration of the expression of tumor suppressor genes, such as p21. 18 Therefore, we first examined the effect of vorinostat on histone 3 acetylation and on p21 expression. When HL cells were incubated with increasing concentrations of vorinostat (0.1-5 μM) for 6 hours, histone 3 acetylation was first observed with 2 μM and was further increased with 5 μM vorinostat (Figure 1A). When HL cells were incubated with 5 μM vorinostat, histone acetylation was observed as early as 30 minutes of incubation (Figure 1B). Histone acetylation was associated with p21 induction, which lasted for up to 48 hours (Figure 1C). These molecular changes were associated with significant effect on cell growth that became evident after 48 hours of incubation with an IC50 between 2 μM and 5 μM (Figure 1D). There was no significant antiproliferative effect within the first 24 hours even with higher concentrations (5 μM) of vorinostat (Figure 1D). When these cells were analyzed for changes in the cell-cycle fractions by FACS analysis, vorinostat preferentially induced cell-cycle arrest at the G2/M phase, which was more evident after 48 hours of incubation and when using higher concentrations of vorinostat (Figure 1E).
Effect of HDAC inhibitor vorinostat in Hodgkin lymphoma (HL) cell lines. (A) Cells were incubated with 0.1 to 5 μM of vorinostat for 6 hours and whole cell lysates were examined for histone 3 acetylation (Ac-histone 3) using Western blot analysis. During this time frame, histone acetylation was observed using concentrations of 2 μM or higher. (B) Cells were incubated with 5 μM of vorinostat for 0.5 to 6 hours demonstrated that histone acetylation is achieved as early as 0.5 hours of incubation and lasted for at least 6 hours. (C) Vorinostat (5 μM) induced p21 expression as early as 6 hours and lasted for up to 48 hours. (D) Vorinostat induced antiproliferative effects in HL cell lines as determined by the MTS cell proliferation assay. This effect was prominent after 48 hours in culture and was dose dependent. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). (E) The antiproliferative effect of vorinostat was associated with an increase in the G2/M cell-cycle fraction, especially when higher concentrations were used. The G2M fraction was higher after 48 hours of incubation (bottom panel) compared with 24 hours of incubation (top panel). Results are the means of 3 independent experiments.
Effect of HDAC inhibitor vorinostat in Hodgkin lymphoma (HL) cell lines. (A) Cells were incubated with 0.1 to 5 μM of vorinostat for 6 hours and whole cell lysates were examined for histone 3 acetylation (Ac-histone 3) using Western blot analysis. During this time frame, histone acetylation was observed using concentrations of 2 μM or higher. (B) Cells were incubated with 5 μM of vorinostat for 0.5 to 6 hours demonstrated that histone acetylation is achieved as early as 0.5 hours of incubation and lasted for at least 6 hours. (C) Vorinostat (5 μM) induced p21 expression as early as 6 hours and lasted for up to 48 hours. (D) Vorinostat induced antiproliferative effects in HL cell lines as determined by the MTS cell proliferation assay. This effect was prominent after 48 hours in culture and was dose dependent. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). (E) The antiproliferative effect of vorinostat was associated with an increase in the G2/M cell-cycle fraction, especially when higher concentrations were used. The G2M fraction was higher after 48 hours of incubation (bottom panel) compared with 24 hours of incubation (top panel). Results are the means of 3 independent experiments.
Vorinostat activates the caspase pathway and induces apoptosis in HL cells
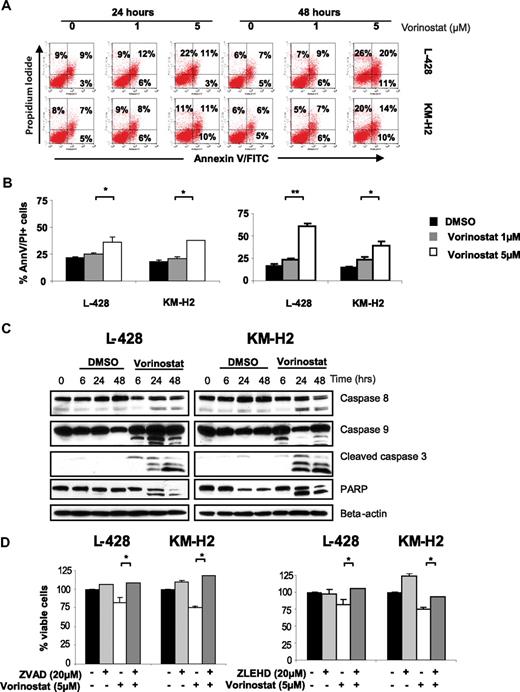
Previous work indicated that the antiproliferative effects of HDAC inhibitors are induced by several mechanisms, including induction of cell-cycle arrest and induction of caspase-dependent and caspase-independent cell death.19-21 Using annexin V and propidium iodide dual staining and FACS analysis, we found that vorinostat induces cell death in HL cells in a dose- and time-dependent manner (Figure 2A,B). When higher concentration of vorinostat was used (5 μM), induction of cell death was associated with caspase 8, 9, and 3 activation. However, caspase 9 activation was observed as early as after 6 hours, whereas caspase 8 and 3 activation was only observed after 24 hours of incubation (Figure 2C). Caspase activation was not observed with lower concentration of vorinostat (1 μM). The contribution of caspase activation to vorinostat-induced cell death was further examined using the pan caspase inhibitor Z-VAD-FMK and the caspase 9 inhibitor Z-LEHD-FMK (Figure 2D). In short-term culture conditions (24 hours), both the pan-caspase and caspase 9 inhibitors showed similar effects and rescued HL cell lines from vorinostat-induced cell death, demonstrating the important role of caspase 9 activation in mediating vorinostat-induced apoptosis. However, this inhibitory effect was lost when the cells were kept in culture for 48 to 72 hours (data not shown), suggesting that vorinostat-induced cell death was in an advanced stage and is no longer a reversible process. Alternatively, caspase-independent mechanisms may have contributed to the delayed cell death, including autophagy.19,22
Vorinostat induces apoptosis in HL cell lines. (A) A representative experiment demonstrating the effect of 2 different concentrations of vorinostat (1 and 5 μM) on apoptosis as determined by the annexin-V binding assay. HL cell lines were incubated with medium or vorinostat (1-5 μM) for 24 or 48 hours before the percentage of dead cells was determined using dual staining with annexin-V and propidium iodide. Vorinostat induces apoptosis in both HL cell lines especially when higher concentrations were used for 48 hours. Percentages of dead cells are shown in each quadrant. (B) Summary results of vorinostst-induced cell death (PI and annexin-V positive cells) from 3 independent experiments. Results after incubations for 24 hours (left panel) and 48 hours (right panel) are shown. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). * denotes a P value of less than .05, and ** denotes a P value of less than .005. (C) HL cell lines were incubated with medium or 5 μM of vorinostat for 6 to 48 hours. Whole cell lysates were examined by Western blot for changes in intracellular proteins. Using this high concentration of vorinostat (5 μM) apoptosis was associated with activation of caspases 8, 9, 3 and PARP cleavage. Consistent with data presented in Figure 2A and B, lower concentrations of vorinostat (1 μM) did not induce caspase activation during the same time frame (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). (D) Vorinostat-induced cell death of HL cell lines was either partially or completely blocked by the pan-caspase inhibitor Z-VAD-FMK (left panel) or by the caspase 9 inhibitor Z-LEHD-FMK (right panel). HL cell lines were incubated with medium or Z-VAD-FMK (20 μM), Z-LEHD-FMK (20 μM), vorinostat (5 μM), or a combination of vorinostat and Z-VAD-FMK or vorinostat and Z-LEHD-FMK. After 24 hours, the viable cells were counted using a 3-(4,5dimethyltioazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTS) assay. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). * denotes a P value of less than .05.
Vorinostat induces apoptosis in HL cell lines. (A) A representative experiment demonstrating the effect of 2 different concentrations of vorinostat (1 and 5 μM) on apoptosis as determined by the annexin-V binding assay. HL cell lines were incubated with medium or vorinostat (1-5 μM) for 24 or 48 hours before the percentage of dead cells was determined using dual staining with annexin-V and propidium iodide. Vorinostat induces apoptosis in both HL cell lines especially when higher concentrations were used for 48 hours. Percentages of dead cells are shown in each quadrant. (B) Summary results of vorinostst-induced cell death (PI and annexin-V positive cells) from 3 independent experiments. Results after incubations for 24 hours (left panel) and 48 hours (right panel) are shown. Each value is the mean of 3 independent experiments performed in triplicate (± SEM). * denotes a P value of less than .05, and ** denotes a P value of less than .005. (C) HL cell lines were incubated with medium or 5 μM of vorinostat for 6 to 48 hours. Whole cell lysates were examined by Western blot for changes in intracellular proteins. Using this high concentration of vorinostat (5 μM) apoptosis was associated with activation of caspases 8, 9, 3 and PARP cleavage. Consistent with data presented in Figure 2A and B, lower concentrations of vorinostat (1 μM) did not induce caspase activation during the same time frame (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). (D) Vorinostat-induced cell death of HL cell lines was either partially or completely blocked by the pan-caspase inhibitor Z-VAD-FMK (left panel) or by the caspase 9 inhibitor Z-LEHD-FMK (right panel). HL cell lines were incubated with medium or Z-VAD-FMK (20 μM), Z-LEHD-FMK (20 μM), vorinostat (5 μM), or a combination of vorinostat and Z-VAD-FMK or vorinostat and Z-LEHD-FMK. After 24 hours, the viable cells were counted using a 3-(4,5dimethyltioazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTS) assay. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). * denotes a P value of less than .05.
Vorinostat inhibits Akt and STAT6 survival signals
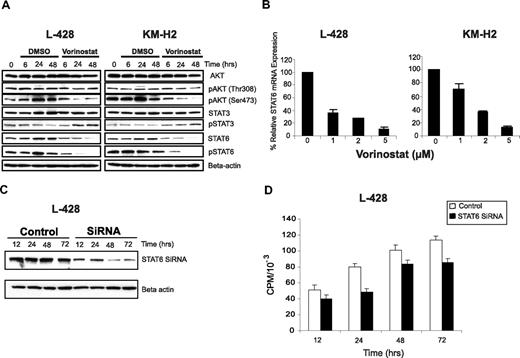
Several studies have demonstrated that HRS cells aberrantly express activated Akt, STAT3, and STAT6 pathways that promote their survival.5,23-25 Thus, we examined the effect of vorinostat on these signaling pathways. Vorinostat had no effect on total Akt cellular level, but decreased the level of active phosphorylated Akt (Ser473) in a time-dependent manner (Figure 3A). In contrast, vorinostat had no significant effect on Akt phosphorylation at Thr308. These data suggest that vorinostat selectively inhibits Akt phosphorylation at Ser473 by regulating upstream kinases or protein phosphatases. Vorinostat selectively inhibited STAT6 phosphorylation and decreased STAT6 cellular contents. In contrast, vorinostat had no significant effect on STAT3, and modestly increased STAT3 phosphorylation, especially after 24 hours of incubation.
Effect of vorinostat inhibits STAT6 in HL cell lines. (A) L-428 and KM-H2 cell lines were incubated with 5 μM of vorinostat for 6 to 48 hours. Whole cell lysates were examined by Western blotting. As shown, vorinostat induced down-regulation of pAkt (Ser473) without inducing changes in total Akt or pAkt (Thr308), and decreased STAT6 and pSTAT6 levels without a significant effect on STAT3. (B) Vorinostat down-regulated STAT6 mRNA expression in a dose-dependent manner. Cells were cultured with medium and vorinostat (1-5 μM) for 24 hours, and the effects on STAT6 mRNA expression was determined by the TaqMan PCR assay. Results were normalized to GAPDH and represent the mean of 3 independent experiments (± SEM). (C) L428 cell line was transfected with STAT6 siRNA and the efficacy of this method was confirmed by measuring STAT6 protein levels by Western blot. Cells that were incubated with STAT6 siRNA demonstrated a 50% reduction in STAT6 protein after 48 hours compared with those incubated with control siRNA. This is a representative experiment of 3 independent experiments demonstrating similar results. (D) Effect of STAT6 knock down by siRNA on cell proliferation. L-428 cells that were transfected with STAT6 siRNA demonstrated lower proliferative rate and thymidine incorporation compared with those transfected with control siRNA.
Effect of vorinostat inhibits STAT6 in HL cell lines. (A) L-428 and KM-H2 cell lines were incubated with 5 μM of vorinostat for 6 to 48 hours. Whole cell lysates were examined by Western blotting. As shown, vorinostat induced down-regulation of pAkt (Ser473) without inducing changes in total Akt or pAkt (Thr308), and decreased STAT6 and pSTAT6 levels without a significant effect on STAT3. (B) Vorinostat down-regulated STAT6 mRNA expression in a dose-dependent manner. Cells were cultured with medium and vorinostat (1-5 μM) for 24 hours, and the effects on STAT6 mRNA expression was determined by the TaqMan PCR assay. Results were normalized to GAPDH and represent the mean of 3 independent experiments (± SEM). (C) L428 cell line was transfected with STAT6 siRNA and the efficacy of this method was confirmed by measuring STAT6 protein levels by Western blot. Cells that were incubated with STAT6 siRNA demonstrated a 50% reduction in STAT6 protein after 48 hours compared with those incubated with control siRNA. This is a representative experiment of 3 independent experiments demonstrating similar results. (D) Effect of STAT6 knock down by siRNA on cell proliferation. L-428 cells that were transfected with STAT6 siRNA demonstrated lower proliferative rate and thymidine incorporation compared with those transfected with control siRNA.
To further clarify the mechanism by which vorinostat regulate STATs expression, we examined its effect on STAT6 and STAT3 mRNA expression by PCR. As shown in Figure 3B, vorinostat decreased STAT6 mRNA levels in both HL cell lines in a dose-dependent manner. To confirm the contribution of STAT6 inhibition to the antiproliferative effect of vorinostat, we selectively knocked down STAT6 mRNA using siRNA in the L-428 cells. The efficacy of this assay was verified by Western blot analysis of STAT6 protein, which was down-regulated by 50% within 48 hours (Figure 3C). The down-regulation of STAT6 protein was associated with a decrease in cell proliferation as determined by [3H] thymidine incorporation (Figure 3D).
Vorinostat inhibits STAT6 mediated TARC transcription and secretion
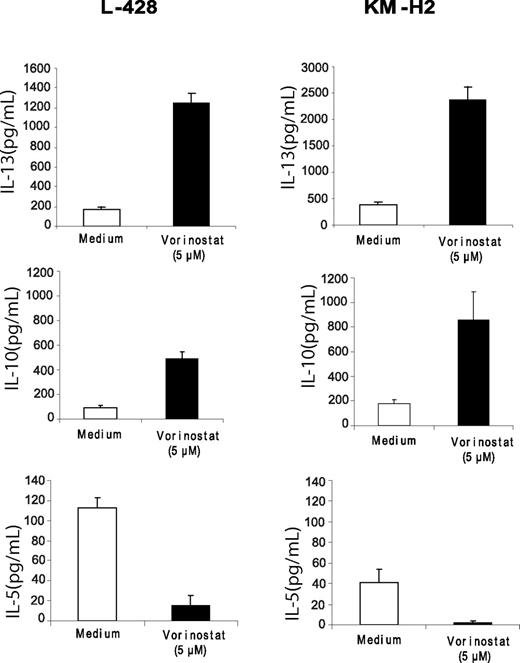
STAT6 activation has been shown to be critical in promoting TH2 type CD4+ T-cell differentiation. Thus, we hypothesized that down-regulation of STAT6 by vorinostat may alter the balance toward a TH1 type response. HL cell lines were incubated with medium or vorinostat, and the supernatants were collected and examined for the level of TH1/TH2 type cytokines by ELISA. As stated above these cell lines did not secrete IL-4, but they are known to secrete IL-13.26,27 Secretion of IL-13 was increased in both cell lines in response to vorinostat, perhaps by a feedback loop, whereas IL-5 levels were significantly decreased (Figure 4). In contrast, IP-10 levels were significantly increased, which was not associated with an increase in interferon-γ secretion (data not shown). Collectively, these data suggest that in short-term culture, the balance between TH1 and TH2 type cytokines is disturbed, favoring a TH1-type response.
Effect of vorinostat on TH1/TH2 cytokine secretion in HL cell lines. Cells were incubated with medium or vorinostat (5 μM) for 24 hours and supernatants were examined for cytokine levels by ELISA. Each value represents a mean of 3 independent experiments done in triplicate (± SEM).
Effect of vorinostat on TH1/TH2 cytokine secretion in HL cell lines. Cells were incubated with medium or vorinostat (5 μM) for 24 hours and supernatants were examined for cytokine levels by ELISA. Each value represents a mean of 3 independent experiments done in triplicate (± SEM).
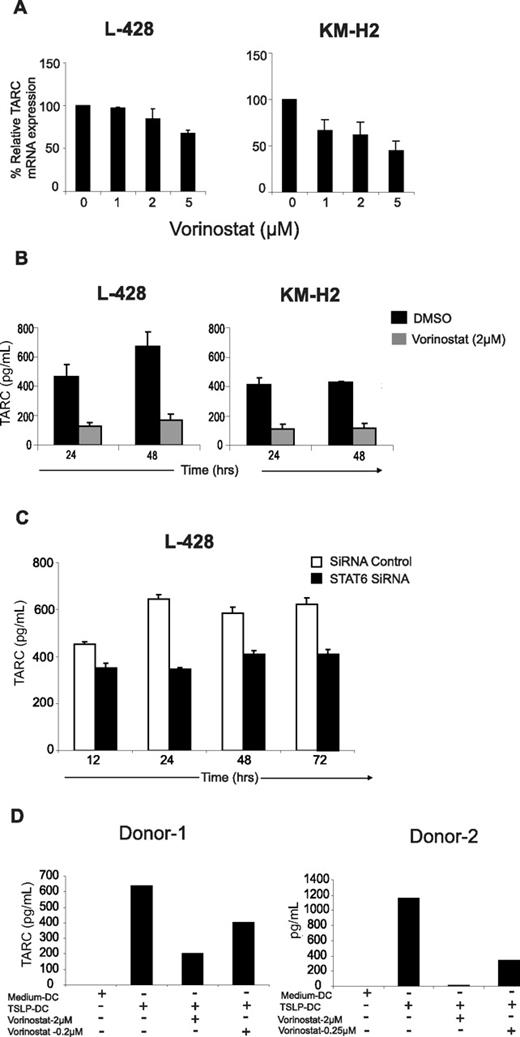
Lymph nodes of patients with HL are characterized by an increased number of TH2-type CD4+ T cells. Recent studies have suggested that HRS cells secrete abundant amounts of TARC, thereby attracting such cells to the HL microenvironment. Furthermore, STAT6, which is frequently activated in HRS cells, was recently implicated in regulating TARC expression. Therefore, we examined the effect of vorinostat on TARC in HL cell lines. As shown in Figure 5A, vorinostat decreased TARC mRNA expression in a dose-dependent manner, an effect that was more prominent in the KM-H2 cell line compared with L-428 cells. The inhibition of TARC mRNA was associated with a profound decrease in TARC secretion in the supernatants of these cell lines as determined by ELISA. After 24 and 48 hours of incubation, nonlethal concentrations of vorinostat (2 μM) decreased TARC secretion by more than 50% in both cell lines (Figure 5B). To further investigate the role of STAT6 in regulating TARC secretion, we examined the effect of STAT6 knock down by siRNA on TRAC secretion in the L-428 cells. Cells that were treated with STAT6 siRNA produced lower concentrations of TARC in the culture supernatant compared with those that were treated with control siRNA (Figure 5C).
Regulation of TARC by vorinostat. (A) Vorinostat inhibits TARC mRNA expression in HL cell lines. Cells were incubated with increasing concentrations of vorinostat for 24 hours before TARC mRNA levels were determined by RT-PCR as detailed in Methods section. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (B) Vorinostat decreased TARC secretion in L-428 and KM-H2. Cells were incubated with vorinostat 2 μM for 24 to 48 hours before TARC levels were determined in the supernatants by ELISA. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (C) Silencing STAT6 by siRNA decreases TARC secretion in the L-428 cells. Cells were transfected with STAT6 siRNA or control siRNA for 12 to 72 hours. At each time point, culture supernatants were collected and TARC levels were determined by ELISA. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (D) Vorinostat inhibits TARC secretion from DCs collected from 2 healthy donors and activated with TSLP (100 ng/mL). TARC levels were determined in DC supernatants after 24 hours in culture by ELISA. The right and left panels represent 2 independent experiments using DCs from 2 healthy donors.
Regulation of TARC by vorinostat. (A) Vorinostat inhibits TARC mRNA expression in HL cell lines. Cells were incubated with increasing concentrations of vorinostat for 24 hours before TARC mRNA levels were determined by RT-PCR as detailed in Methods section. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (B) Vorinostat decreased TARC secretion in L-428 and KM-H2. Cells were incubated with vorinostat 2 μM for 24 to 48 hours before TARC levels were determined in the supernatants by ELISA. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (C) Silencing STAT6 by siRNA decreases TARC secretion in the L-428 cells. Cells were transfected with STAT6 siRNA or control siRNA for 12 to 72 hours. At each time point, culture supernatants were collected and TARC levels were determined by ELISA. Each value represents a mean of 3 independent experiments done in triplicate (± SEM). (D) Vorinostat inhibits TARC secretion from DCs collected from 2 healthy donors and activated with TSLP (100 ng/mL). TARC levels were determined in DC supernatants after 24 hours in culture by ELISA. The right and left panels represent 2 independent experiments using DCs from 2 healthy donors.
Vorinostat inhibits TARC secretion by TSLP-activated dendritic cells
Thymic stromal lymphopoietin (TSLP) is a cytokine that is produced by barrier epithelial cells and is a potent activator of myeloid DCs (mDCs).28 TSLP-activated mDCs express OX40 ligand (OX40L) and TARC that play important role in DC polarization of naive CD4+ T helper cells to produce TH2 cytokines and of their chemotaxis.29,30 Because HL microenvironment contains abundant number of DCs, we investigated whether vorinostat can inhibit TARC secretion by TSLP-activated mDCs. Using this experimental system, TSLP profoundly increased TARC secretion in mDC supernatants, without influencing their survival (Figure 5D). This effect was inhibited by vorinostat in a dose-dependent manner. Submicromolar concentrations of vorinostat (0.2 μM) decreased TARC secretion by 50% to 60% within 24 hours of incubation, while 2 μM of vorinostat reduced TARC secretion by 60% to 100% (Figure 5D). Collectively, these data demonstrate that vorinostat inhibits TARC secretion in both malignant HRS cells and from mDCs.
Vorinostat decreases Bcl-xL levels and synergizes with chemotherapy
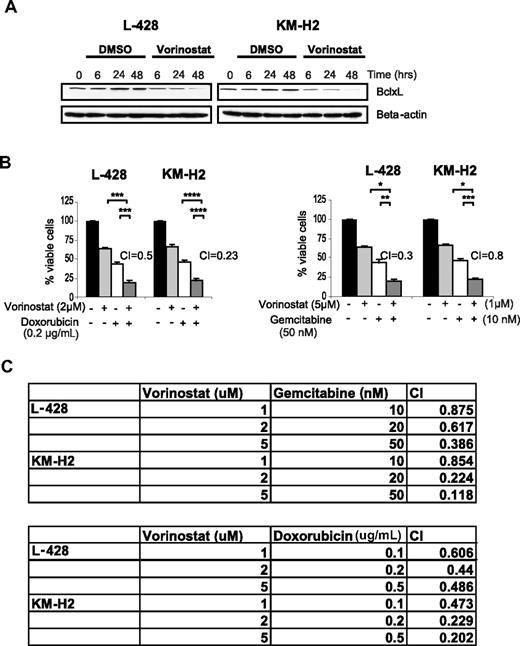
Because Bcl-xL is known to be a STAT6 target gene, we examined the effect of vorinostat on Bcl-xL expression.31 Vorinostat decreased Bcl-xL expression in both HL cell lines in a time-dependent manner (Figure 6A), which was associated with a synergistic activity between vorinostat and doxorubicin and gemcitabine chemotherapy (Figure 6B). This synergy was observed using vorinostat doses ranging between 1 and 5 μM, with variable doses of doxorubicin and gemcitabine (Figure 6C).
Vorinostat decresases Bcl-xL protein levelsand enhances the effect of doxorubicin and gemcitabine chemotherapy in HL. (A) Vorinostat (5 μM) decreases the level of the antiapoptotic protein Bcl-xL in HL cell lines in a time dependent manner as determined by Western blot. (B) Representative experiments demonstrating synergistic effects between vorinostat and doxorubicin (left panel) and gemcitabine (right panel). Cells were incubated with each drug alone or in combination for 48 hours and cell viability was determined by the MTS assay. Synergy was determined by calculating the combination index (CI) analyzed by Calcusyn software. Each value represents a mean of 3 independent experiments performed in triplicate (± SEM). (C) Synergy between Vorinostat and gemcitabine (top table) or doxorubicin (bottom table) using a range of drug concentrations. Synergy was determined by calculating the CI using the Calcusyn software. A CI less than 1 indicates synergy.
Vorinostat decresases Bcl-xL protein levelsand enhances the effect of doxorubicin and gemcitabine chemotherapy in HL. (A) Vorinostat (5 μM) decreases the level of the antiapoptotic protein Bcl-xL in HL cell lines in a time dependent manner as determined by Western blot. (B) Representative experiments demonstrating synergistic effects between vorinostat and doxorubicin (left panel) and gemcitabine (right panel). Cells were incubated with each drug alone or in combination for 48 hours and cell viability was determined by the MTS assay. Synergy was determined by calculating the combination index (CI) analyzed by Calcusyn software. Each value represents a mean of 3 independent experiments performed in triplicate (± SEM). (C) Synergy between Vorinostat and gemcitabine (top table) or doxorubicin (bottom table) using a range of drug concentrations. Synergy was determined by calculating the CI using the Calcusyn software. A CI less than 1 indicates synergy.
Discussion
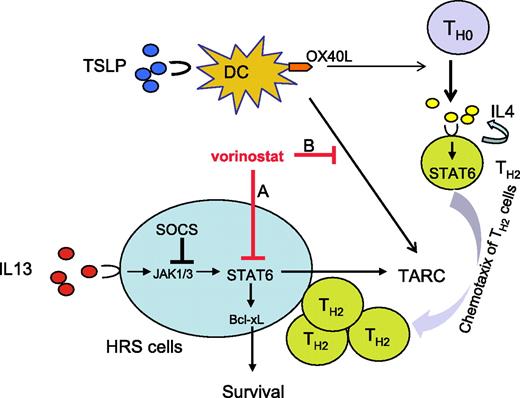
In this study, we have examined the biologic activity and molecular mechanisms of the antiproliferative activity of vorinostat in HL-derived cell lines. Our results demonstrate that HDAC inhibition by vorinostat may have a dual effect: (1) a direct antiproliferative effect on the malignant HRS cells and (2) an indirect immune modulatory effect caused by inhibiting STAT6-mediated TH2 cytokine and chemokine secretion in the tumor microenvironment (Figure 7).
A model for vorinostat activity in HL. HL is characterized by the presence of abundant numbers of dendritic cells (DCs) and Th2-type CD4 cells in the microenvironment. HRS cells aberrantly express activated STAT6 by an autocrine IL-13 loop. Both DC and HRS cells secrete TARC that attracts TH2 type cells. In this model, vorinostat has a dual effect on HL. (A) A direct effect on HRS cells by modulating a variety of growth and survival proteins, including p21 and Akt, in addition to inhibiting STAT6 transcription. Inhibition of STAT6 decreases Bcl-xL protein levels causing apoptosis, and decreases TARC secretion causing inhibition of Th2-type CD4+ cell chemotaxis. (B) An indirect effect on DCs in the microenvironment. Thymic stromal lymphopoietin (TSLP) activates myeloid DCs (mDCs) to express OX40 ligand (OX40L) and to secrete TARC, favoring polarization and attraction of TH2-type CD4+ cells. Vorinostat inhibits TRAC secretion by DCs, thus modulating the function and component of the reactive cells in HL microenvironment.
A model for vorinostat activity in HL. HL is characterized by the presence of abundant numbers of dendritic cells (DCs) and Th2-type CD4 cells in the microenvironment. HRS cells aberrantly express activated STAT6 by an autocrine IL-13 loop. Both DC and HRS cells secrete TARC that attracts TH2 type cells. In this model, vorinostat has a dual effect on HL. (A) A direct effect on HRS cells by modulating a variety of growth and survival proteins, including p21 and Akt, in addition to inhibiting STAT6 transcription. Inhibition of STAT6 decreases Bcl-xL protein levels causing apoptosis, and decreases TARC secretion causing inhibition of Th2-type CD4+ cell chemotaxis. (B) An indirect effect on DCs in the microenvironment. Thymic stromal lymphopoietin (TSLP) activates myeloid DCs (mDCs) to express OX40 ligand (OX40L) and to secrete TARC, favoring polarization and attraction of TH2-type CD4+ cells. Vorinostat inhibits TRAC secretion by DCs, thus modulating the function and component of the reactive cells in HL microenvironment.
The direct antiproliferative effect was produced by regulating several survival and cell-cycle regulatory proteins. First, as previously reported in other cancer types, vorinostat up-regulated p21 protein expression and induced cell-cycle arrest. Furthermore, vorinostat decreased Akt phosphorylation (pAkt) on Ser473, which has been reported to induce cell-cycle arrest and apoptosis in HL cells.23 The ability of vorinostat to inhibit Akt phosphorylation suggests that vorinostat may have altered a key protein phosphatase expression or function, or phosphatidylinositol 3-kinase (PI3K) activity.
In this study, we also have shown that vorinostat had a differential effect on STAT3 and STAT6 expression in HL cell lines. The decrease in STAT6 mRNA could be related to inhibition of its transcription or due to increased instability in STAT6 mRNA, for example by regulating microRNAs. HDACs have been reported to posttranscriptionally regulate STAT3 protein acetylation status and function.11 Thus, although, our data demonstrate that vorinostat decreased STAT6 mRNA and resulting in a decrease in TARC secretion and cell proliferation, the antiproliferative effect of vorinostat may have been attenuated by the presence of active phosphorylated STAT3. This suggests that combining vorinostat with STAT3 inhibitors may achieve a better therapeutic index.3,25 Furthermore, future studies should examine the effect of vorinostat on other components of the Jak/STAT pathway, including suppressors of cytokine signaling (SOCS) proteins that are known to be epigenetically regulated.3,32 The antiproliferative effect of vorino-stat is therefore complex and multifactorial caused by regulating several survival pathways that are known to induce cell-cycle arrest and apoptosis by caspase-dependent and caspase-independent mechanisms. For example, we have previously reported that inhibition of Akt phosphorylation causes caspase 9 activation and apoptosis.23 Thus, vorinostat-induced caspase 9 activation (Figure 2C) may have been caused by inhibition of Akt phosphorylation (Figure 3A).
Vorinostat inhibition of STAT6 altered the balance of TH1/TH2-type cytokines and chemokines that are secreted by HL cell lines (Figure 4), as it increased IP10 and decreased IL-5 secretion. However, vorinostat paradoxically increased IL-13 levels, suggesting a feedback mechanism. This data also demonstrate that the inhibition of STAT6 phosphorylation was not induced by a reduction in IL-13 production, but rather by a direct effect on STAT6 transcription (Figure 3B). The effect of vorinostat on TH1/TH2-type cytokines and chemokines provides an additional rationale for use of HDAC inhibitors in the treatment of HL aimed at creating an unfavorable microenvironment for tumor growth. These cytokines and chemokines preferentially attract TH2-type CD4+ cells, which may not only protect HRS cells from immunosurveillance but may also support their growth and survival. This hypothesis should be evaluated in vivo in patients with HL receiving HDAC inhibitors. In fact, early results from an ongoing clinical trial using the isotype-selective HDAC inhibitor MGCD0103 have demonstrated significant clinical responses that were associated with a decrease in serum TARC levels.33 The ability of vorinostat to inhibit TARC secretion by mDCs may also play a role in shifting the balance toward Th1 type cytokine in HL patients in vivo, and may possibly have wide therapeutic implications in cancer immunotherapy and supports further examination of this effect in novel treatment strategies aimed at eliciting host antitumor response.
Vorinostat demonstrated a synergistic effect with 2 widely used chemotherapeutic drugs in the treatment of HL, doxorubicin and gemcitabine. This synergistic effect is likely related to the ability of vorinostat to lower the apoptotic threshold by inhibiting STAT6, Bcl-xL, and pAkt. Collectively, our results provide the proof-of-principle that supports investigating HDAC inhibitors either alone or in combination with chemotherapy, for the treatment of HL. Furthermore, our data suggest that such clinical trials should evaluate the in vivo effects of HDAC inhibitors on the TH1/TH2 balance in the HL microenvironment and correlate the results with clinical responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by The Gilder Foundation and the Jack Stotsky Memorial Fund.
Authorship
Contribution: D.B. performed experiments, interpreted data, and wrote the manuscript; G.V.G. performed experiments; S.H. performed experiments and interpreted data; K.A. and N.M.K. performed experiments; Y.-J.L. designed experiments and reviewed the manuscript; and A.Y. designed experiments, interpreted results, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anas Younes, MD, Department of Lymphoma and Myeloma, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: ayounes@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal