Abstract
Src family kinases (SFKs) were described to be overexpressed in chronic lymphocytic leukemia (CLL). We wished to examine the effects of the Src and Abl kinase inhibitor dasatinib on the intracellular signaling and survival of CLL cells. Dasa-tinib showed a dose- and time-dependent reduction of global tyrosine phosphorylation and of activating phosphotyrosine levels of SFKs. Treatment with 100 nM dasatinib led to decreased levels of the activated, phosphorylated forms of Akt, Erk1/2, and p38, and induced PARP cleavage through caspase activity. In Mec1 and JVM-3 cell lines, dasatinib increased p53 protein levels and inhibited proliferation. In freshly isolated CLL cells, dasatinib reduced the expression of Mcl-1 and Bcl-xL. Combination of 5 μM dasatinib and fludarabine increased the apoptosis induction of each by approximately 50%. In 15 primary CLL samples, cells with unmutated immunoglobulin variable heavy chain (IgVH) genes were more sensitive to dasatinib than those with mutated IgVH genes (P = .002). In summary, dasatinib shows potent inhibitory effects on the survival of CLL cells in vitro, most prominently in samples obtained from patients with unfavorable prognostic features.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by the progressive accumulation of CD5-positive monoclonal B cells, which escape apoptosis.1 According to the hypermutation status of immunoglobulin heavy chain variable (IgVH) regions,2 ZAP-70 expression,3-5 and BCR responsiveness,6 2 subgroups with differential prognosis can be distinguished. Because the B-cell receptor (BCR) and many of its coreceptors as well as cytokine receptors are devoid of intrinsic kinase activity, non-receptor kinases such as Src family kinases (SFKs) are expected to play crucial roles for the intracellular transduction of survival signals. Lyn, a SFK that binds to the signal-transducing subunits of the BCR, is aberrantly expressed in CLL cells, which correlates with their apoptosis defects.7
A role for SFKs in the pathogenesis of Bcr-Abl-positive leukemias was demonstrated by combination of experimentally induced disease models and triple-knockout mice deficient in the SFKs Lyn, Hck, and Fgr for B-cell acute lymphocytic leukemia (B-ALL), but not for chronic myeloid leukemia (CML).8 The treatment of CML has been revolutionized by the availability of the ATP-competitive Abl inhibitor imatinib mesylate, which targets the constitutive kinase activity of the Bcr-Abl fusion protein. To overcome imatinib resistance, different strategies were followed to develop second-generation Abl inhibitors.9,10 Whereas nilotinib (AMN-107, Tasigna; Novartis, Basel, Switzerland) resulted from modification of the imatinib scaffold,11 dasatinib (BMS-354825, Sprycel; Bristol-Myers Squibb, New York, NY) is derived from an aminothiazole scaffold, which was identified and developed as an inhibitor of the SFK Lck.12,13 Structural studies show that dasatinib binds to the ATP-binding site in Abl14-16 in contrast to imatinib without preference for the closed inactive kinase conformation.17 The better accessibility of the hydrophobic inhibitor binding pocket for dasatinib as compared with imatinib leads to a wider spectrum of inhibited kinases, comprising SFKs and the receptor tyrosine kinases EGFR and c-kit.18
The suggested role of the SFK Lyn in the survival signaling of CLL cells7 and the success of dasatinib in the treatment of CML19 prompted us to investigate the effects on protein tyrosine phosphorylation and apoptosis induction in CLL patient cells and in prolymphocytic cell lines. SFKs as primary dasatinib targets were probed for tyrosine phosphorylation at their autophosphorylation site, which indicates activation.20 Moreover we investigated the phosphorylation status of the downstream kinases Akt, Erk and p38. The apoptosis induction assessed as annexin V–binding was confirmed by cleavage of poly (ADP ribose) polymerase (PARP) by caspases and altered expression levels of bcl-2 family members. Because kinase inhibition and DNA damage response constitute completely different mechanistic principles of apoptosis induction, we investigated, whether dasatinib sensitizes CLL cell lines for the cytotoxic drug fludarabine. Subsets of samples from patients with unfavorable prognoses due to unmutated IgVH genes or ZAP-70 expression corresponded to a high proapoptotic in vitro response to dasatinib. Therefore, our results suggest dasatinib as a potential treatment option for CLL, particularly for the subgroup with a more aggressive disease course.
Methods
Patient samples
Peripheral blood samples were obtained from patients who were previously diagnosed for CLL according to standard criteria.21 The studies were performed in accordance with the local ethics committee regulation and after informed written consent had been obtained from the patients. The clinical and biochemical characteristics of samples from 28 patients (75% male, median age 64 years) are summarized in Table 1. ZAP-70 and CD38 expression status were determined by fluorescence-activated cell sorting analysis using fluorescence-labeled antibodies as described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).22,23 The determination of IgVH mutation status by PCR and sequencing24 and of cytogenetic aberrations was performed at Münchner Leukämie Labor (MLL; Munich, Germany). Informed consent for the use of patient samples was obtained in accordance with the Declaration of Helsinki and with the approval of the internal review board of the University Hospital Cologne.
Characteristics of study patient samples
| ID no. . | Age, y/sex . | Stage (Binet) . | Prior treatment . | Cytogenetic aberrations . | IgVH2 . | ZAP-70* . | CD38* . | % viable relative to control after treatment with 5 μM dasatinib . |
|---|---|---|---|---|---|---|---|---|
| 1 | 73/F | A | yes | none | M | n.d. | n.d. | n.d. |
| 2 | 65/M | A | no | n.d. | M | − | − | 65.5 |
| 3 | 58/M | A | no | trisomy 12 | M | − | + | 72.4 |
| 4 | 83/M | C | yes | n.d. | M | − | n.d. | n.d. |
| 6 | 69/M | C | yes | n.d. | um | + | + | 36.1 |
| 7 | 58/F | B | yes | n.d. | n.d. | n.d. | n.d. | 77.4 |
| 8 | 67/M | B | no | Δ13q14 | M | − | − | 80.5 |
| 9 | 63/F | A | no | n.d. | n.d. | − | − | 78.3 |
| 10 | 60/F | C | no | n.d. | n.d. | − | − | 82.4 |
| 11 | 49/M | A | no | none | M | + | − | 73.3 |
| 12 | 59/M | C | no | n.d. | M | − | − | 70.4 |
| 16 | 54/M | B | no | Δ13q14 | um | + | − | 17.4 |
| 17 | 62/M | B | no | Δ11q (ATM) | um | + | + | 39.1 |
| 18 | 66/M | C | no | n.d. | n.d. | + | − | n.d. |
| 19 | 75/F | n.d. | no | Δ13q14 | um | − | + | 41.8 |
| 22 | 64/M | C | no | n.d. | n.d. | − | − | 67.5 |
| 23 | 64/M | B | n.d. | Δ11q (ATM) | M | − | − | 53.2 |
| 25 | 59/M | n.d. | no | n.d. | n.d. | + | − | 50.7 |
| 26 | 48/M | n.d. | no | Δ6q21, Δ17p (p53) | um | + | + | 38.6 |
| 27 | 57/M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 70.3 |
| 28 | 49/M | A | no | Δ13q14 | M | − | − | 79.3 |
| 29 | 58/M | A | no | n.d. | n.d. | + | − | 83.1 |
| 30 | 65/M | C | yes | n.d. | n.d. | n.d. | n.d. | 54.2 |
| 31 | 76/M | B | yes | n.d. | n.d. | + | + | 37.4 |
| 32 | 72/M | B | yes | n.d. | M | − | − | 88.0 |
| 33 | 66/M | B | yes | n.d. | n.d. | − | − | 74.1 |
| 34 | 42/M | A | no | Δ11q (ATM) | um | n.d. | n.d. | 79.7 |
| 35 | 88/F | B | yes | n.d. | M | − | n.d. | 71.2 |
| ID no. . | Age, y/sex . | Stage (Binet) . | Prior treatment . | Cytogenetic aberrations . | IgVH2 . | ZAP-70* . | CD38* . | % viable relative to control after treatment with 5 μM dasatinib . |
|---|---|---|---|---|---|---|---|---|
| 1 | 73/F | A | yes | none | M | n.d. | n.d. | n.d. |
| 2 | 65/M | A | no | n.d. | M | − | − | 65.5 |
| 3 | 58/M | A | no | trisomy 12 | M | − | + | 72.4 |
| 4 | 83/M | C | yes | n.d. | M | − | n.d. | n.d. |
| 6 | 69/M | C | yes | n.d. | um | + | + | 36.1 |
| 7 | 58/F | B | yes | n.d. | n.d. | n.d. | n.d. | 77.4 |
| 8 | 67/M | B | no | Δ13q14 | M | − | − | 80.5 |
| 9 | 63/F | A | no | n.d. | n.d. | − | − | 78.3 |
| 10 | 60/F | C | no | n.d. | n.d. | − | − | 82.4 |
| 11 | 49/M | A | no | none | M | + | − | 73.3 |
| 12 | 59/M | C | no | n.d. | M | − | − | 70.4 |
| 16 | 54/M | B | no | Δ13q14 | um | + | − | 17.4 |
| 17 | 62/M | B | no | Δ11q (ATM) | um | + | + | 39.1 |
| 18 | 66/M | C | no | n.d. | n.d. | + | − | n.d. |
| 19 | 75/F | n.d. | no | Δ13q14 | um | − | + | 41.8 |
| 22 | 64/M | C | no | n.d. | n.d. | − | − | 67.5 |
| 23 | 64/M | B | n.d. | Δ11q (ATM) | M | − | − | 53.2 |
| 25 | 59/M | n.d. | no | n.d. | n.d. | + | − | 50.7 |
| 26 | 48/M | n.d. | no | Δ6q21, Δ17p (p53) | um | + | + | 38.6 |
| 27 | 57/M | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 70.3 |
| 28 | 49/M | A | no | Δ13q14 | M | − | − | 79.3 |
| 29 | 58/M | A | no | n.d. | n.d. | + | − | 83.1 |
| 30 | 65/M | C | yes | n.d. | n.d. | n.d. | n.d. | 54.2 |
| 31 | 76/M | B | yes | n.d. | n.d. | + | + | 37.4 |
| 32 | 72/M | B | yes | n.d. | M | − | − | 88.0 |
| 33 | 66/M | B | yes | n.d. | n.d. | − | − | 74.1 |
| 34 | 42/M | A | no | Δ11q (ATM) | um | n.d. | n.d. | 79.7 |
| 35 | 88/F | B | yes | n.d. | M | − | n.d. | 71.2 |
n.d. indicates not determined; um, unmutated (<2% sequence divergence with the closest germline gene); M, mutated (>2% sequence divergence with the closest germline gene).
+ indicates that more than 20% of cells and − indicates that less than 20% percentage of cells showed higher fluorescence after specific antibody staining than after isotype antibody staining.
Inhibitors
Dasatinib tablets were kindly provided by Bristol Myers Squibb. Nilotinib capsules and pure substance vials of imatinib and CGP76030 were kind gifts of Novartis. Fludarabine monophosphate was purchased from Schering (Berlin, Germany), and PP1 from Sigma-Aldrich (Taufkirchen, Germany). All inhibitors were dissolved in DMSO and stored at −20°C. Equal DMSO concentrations were maintained at all inhibitor doses and the solvent control.
Cell isolation and culture
Sources for the cell lines used are mentioned in Document S1. Peripheral blood mononuclear cells were isolated from heparinized blood samples by Ficoll-Paque Plus sedimentation (GE Healthcare, Freiburg, Germany). To obtain pure CLL cells, the Ficoll gradient was preceded by incubation of whole blood with the Rosette Sep B-cell purification antibody cocktail (StemCell Technologies, Vancouver, BC) to aggregate unwanted cells with erythrocytes. The purity of CLL cell populations was determined by flow cytometry using FITC-labeled anti-CD5 and PE-labeled anti-CD19 antibodies (BD Biosciences, Heidelberg, Germany). Freshly prepared CLL cells and all cell lines were cultured in RPMI medium supplemented with 10% heat-inactivated fetal calf serum (FCS) and antibiotics and antimycotics at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Proliferation and apoptosis assays
Performance of the XTT assay of cellular metabolism according to the instructions of the supplier (Roche, Mannheim, Germany) is detailed in Document S1.
For apoptosis assays, 5 × 105 cells were collected and washed once before the determination of annexin V–binding or mitochondrial membrane potential. The cells were either stained with FITC-labeled annexin V and 7-amino-actinomycin (7AAD; BD Biosciences) or with 50 nM 3,3′-dihexyloxacarbocyanine (DiOC6; Invitrogen, Karlsruhe, Germany), and analyzed using a FACS-Canto flow cytometer (BD Biosciences). The percentages of nonapoptotic cells relative to control were calculated from the percentages of viable (ie, annexin V–negative) dasatinib-treated and control cells.
Error bars indicate standard deviations of the means from experiments done in triplicate. Significance thresholds of apoptosis induction and growth inhibition were determined by 2-tailed Student t test.
Western blot analysis and immunoprecipitation
The preparation of cell lysates, performance of immunoblots, and detection using the Odyssey imager (Licor, Bad Homburg, Germany) are described in Document S1. The following antibodies were used: antiphosphotyrosine (pY) 4G10, anti-Lck (Millipore, Schwalbach, Germany), antiphosphotyrosine (PY99), anti-Lyn (H-6 and 44) anti-Bax (2D2), anti Mcl-1 (S19), anti-p38, anti-p53, antitubulin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-pSrc (Y416), anti-pErk1/2 (T202/Y204), anti-Akt, anti-pAkt (S473), anti-p44/42 MAP kinase, antiphospho-p38, anti-PARP, anti-BclxL, anti-caspase 3, 7, and 9 (Cell Signaling Technology, Beverly, MA) and anti-hIL/XIAP, anti-Bcl2, anti-Bid, and anti-caspase 8 (BD Biosciences).
For IP, cell lysates with a total protein content of 300 μg were incubated overnight at 4°C with 1 μg of anti-Lyn (44) or anti-Lck antibody followed by 2 hours of incubation with 30 μL of swollen protein A/G-agarose bead suspension (Roche). Protein A/G-immune complexes were pelleted by centrifugation at 1000g at 4°C for 15 minutes and washed 3 times with ice-cold lysis buffer containing 0.1% NP-40. After being boiled in 2× sample buffer, the agarose beads were pelleted and the eluates were analyzed by Western blotting.
Results
SFK inhibition in primary CLL patient cells
Aberrant expression and activity of the SFK Lyn have been linked to enhanced survival of CLL cells7 and the second generation dual-specific Abl/SFK inhibitor dasatinib showed high potency for SFKs with dissociation constants below 1 μM for all tested family members.18 In agreement with the observation that SFK inhibition, particularly of Lyn, induces apoptosis in primary CLL cells,7 we observed more than 10% decrease of viability by 5 μM dasatinib in all 25 investigated CLL patient samples (Table 1). Therefore we compared the tyrosine phosphorylation in lysates of freshly isolated CLL patient cells, that had been cultivated either untreated or in the presence of tyrosine kinase inhibitors including dasatinib (Figure 1). In these experiments the patterns of size-fractionated tyrosine phosphorylated proteins were detected on Western blots by means of phosphotyrosine-specific antibodies. In addition, phosphorylation of the positively regulatory tyrosine residue in SFKs was probed by a pan-phospho-Src antibody and controlled by the determination of the amounts of Lyn, which is the predominantly expressed SFK in B-cells. In freshly prepared PBMCs from a CLL patient, dasatinib reduced global tyrosine phosphorylation and SFK activation, more strongly than the other investigated inhibitors, although these were used at higher concentrations. Reduction by tyrosine kinase inhibitors of the band intensities corresponding to the molecular weight of SFKs in global phosphotyrosine profiles corresponded to that of activating SFK phosphorylation. The observed inhibition increased in the following order: 5 μM nilotinib, 10 μM imatinib mesylate, 10 μM PP1, 10 μM CGP76030, 0.1 μM dasatinib (Figure 1). Among the investigated Abl inhibitors without reported direct inhibition of SFKs, 10 μM imatinib mesylate slightly reduced global tyrosine phosphorylation and SFK activation, whereas these markers were unchanged after incubation with 5 μM nilotinib. Tyrosine phophorylation was reduced by dasatinib in all investigated samples, although there were differences between individual tyrosine phosphorylation profiles (Figure 1).
Effect of different kinase inhibitors on global tyrosine phosphorylation and the phosphorylation status of the positively regulating tyrosine residue in SFKs in CLL cells. Fresh PBMCs from 2 CLL patients or the cell line JVM-3 were cultivated for 2 hours and treated with either solvent control or the indicated concentrations of kinase inhibitors. Western blots were developed with primary antibodies directed against phosphotyrosine, pTyr416 of Src, Lyn, or tubulin. The inhibitor effects on patient cells are representative for 3 patient samples.  indicate the position of SFK bands.
indicate the position of SFK bands.
Effect of different kinase inhibitors on global tyrosine phosphorylation and the phosphorylation status of the positively regulating tyrosine residue in SFKs in CLL cells. Fresh PBMCs from 2 CLL patients or the cell line JVM-3 were cultivated for 2 hours and treated with either solvent control or the indicated concentrations of kinase inhibitors. Western blots were developed with primary antibodies directed against phosphotyrosine, pTyr416 of Src, Lyn, or tubulin. The inhibitor effects on patient cells are representative for 3 patient samples.  indicate the position of SFK bands.
indicate the position of SFK bands.
For assessment of the dose and time dependency of dasatinib action on phosphotyrosine levels, incubation times ranged from 0.5 hours to 16 hours and dasatinib concentrations ranged from 20 nM to 300 nM (Figure 2). Using 100 nM dasatinib, maximal inhibition of global and SFK activating tyrosine phosphorylation was observed after 2 hours in CLL patient cells (Figure 2A). Reduction of the phosphotyrosine levels to approximately 20% of those in untreated cells was obtained after 2 and 16 hours of treatment with 100 nM dasatinib (Figure 2B). Therefore, treatment with 100 nM dasatinib for 2 hours was chosen for further experiments. To assess which specific phosphotyrosine residues in specific SFK members were affected by dasatinib, immunoprecipitated Lyn and Lck from patient samples and CLL cell lines was analyzed using antibodies exhibiting general antiphosphotyrosine specificity or specifically directed against the phosphotyrosine of the activation loop in SFKs (Figure 2C). As Lyn is reported as the most abundantly expressed SFK in CLL cells, we used Lyn expression as the reference for activating SFK phosphorylation. Lyn expression levels in JVM-3 cells were considerably lower than in CLL patient cells but exceeded those in Mec1 cells, which is reflected by the Lyn amounts detected after immunoprecipitation. Lyn was targeted by dasatinib in CLL cells, because a similar reduction of tyrosine phosphorylation was observed after dasatinib treatment in lysates and immunoprecipitated Lyn kinase. Reduction of phosphotyrosine signals is detected with antibodies with and without specificity for an additional sequence context, demonstrating that the activating phosphotyrosine of the activation loop in SFK is involved in dasatinib action. Lck is expressed at much lower levels in CLL cells than Lyn, but its tyrosine phosphorylation is equally affected by dasatinib.
Dose and time dependency and specificity of SFK inhibition by dasatinib in CLL cells. Lysates of CLL cells and of prolymphocytic cell lines were analyzed for general tyrosine phosphorylation (pTyr) or specifically for the positive regulatory phosphotyrosine in SFKs (p-Src). Before phosphorylation analysis, freshly prepared purified CLL cells were cultivated either for various times in the presence of 100 nM dasatinib (A) or for 2 or 16 hours in the presence of different concentrations of dasatinib (B). After 2 hours incubation without (−) and with (+) 100 nM dasatinib, cells were analyzed for the activation of specific SFKs by probing immunoprecipitated Lyn or Lck protein for tyrosine phosphorylation (C).
Dose and time dependency and specificity of SFK inhibition by dasatinib in CLL cells. Lysates of CLL cells and of prolymphocytic cell lines were analyzed for general tyrosine phosphorylation (pTyr) or specifically for the positive regulatory phosphotyrosine in SFKs (p-Src). Before phosphorylation analysis, freshly prepared purified CLL cells were cultivated either for various times in the presence of 100 nM dasatinib (A) or for 2 or 16 hours in the presence of different concentrations of dasatinib (B). After 2 hours incubation without (−) and with (+) 100 nM dasatinib, cells were analyzed for the activation of specific SFKs by probing immunoprecipitated Lyn or Lck protein for tyrosine phosphorylation (C).
Inhibition of SFKs and downstream signaling by dasatinib in CLL cells
To complement our findings about SFK inhibition by dasatinib in CLL cells, we studied downstream kinase signaling (Figure 3). Because the MAPK signaling cascade is involved in BCR-mediated cell fate decisions25 and survival signaling in CLL cells,26 we included phosphorylation analysis of Erk1/2 and p38. Similarly PKC and PI3K-pathways, of which Akt is a downstream effector, promote survival of CLL cells.27,28 Therefore we also monitored the phosphorylation status of the serine/threonine kinase PKB/Akt at serine-473.
Dasatinib effects on tyrosine phosphorylation and downstream signaling in leukemia cells. The effects of treatment with 100 nM dasatinib for 2 hours were compared in the Bcr-Abl positive cell line K562 and the prolymphocytic cell lines JVM-3 and Mec1 (A). The phosphorylation status of the downstream mediators Akt, Erk1/2 and p38 with and without dasatinib treatment was probed in the cell lines JVM-3 and Mec1 and in freshly isolated CLL cells using phosphospecific antibodies and controlled by detection of the expression levels of the respective proteins (B).
Dasatinib effects on tyrosine phosphorylation and downstream signaling in leukemia cells. The effects of treatment with 100 nM dasatinib for 2 hours were compared in the Bcr-Abl positive cell line K562 and the prolymphocytic cell lines JVM-3 and Mec1 (A). The phosphorylation status of the downstream mediators Akt, Erk1/2 and p38 with and without dasatinib treatment was probed in the cell lines JVM-3 and Mec1 and in freshly isolated CLL cells using phosphospecific antibodies and controlled by detection of the expression levels of the respective proteins (B).
Because dasatinib was originally developed to overcome resistance of CML blasts against imatinib,19 we took the Bcr-Abl positive cell line K562 as a reference for investigating dasatinib effects on protein phosphorylation in 2 prolymphocytic cell lines, JVM-329 and Mec130 (Figure 3A). As expected, phosphorylated p210 is seen only in K562 cells. For all 3 cell lines, activating SFK phosphorylation and tyrosine phosphorylation at the molecular weight of SFKs were significantly reduced by dasatinib. Akt phosphorylation was inhibited in the prolymphocytic cell lines, but not in K562 cells, indicating different signal transduction pathways in Bcr-Abl-positive and prolymphocytic B cell lines. Dasatinib also caused changes in the intensities of pospho-Erk, whereas the levels of total Erk1/2 remained constant.
To confirm the observations made in cell line models, we assessed dasatinib effects on the phosphorylation and activation status of downstream kinases. Erk 1/2 phosphorylation levels in CLL patient samples and in prolymphocytic cell lines were reduced by 20% to 50% after dasatinib treatment. The phosphorylation signal for the MAP kinase p38 was lower in Mec1 than in JVM-3 cells and clearly reduced by treatment with dasatinib in all investigated CLL cell samples (Figure 3B).
Proapoptotic and antiproliferative effects of dasatinib on CLL cells
In line with our observation that dasatinib inhibited survival signaling cascades involving Akt and MAP kinases, inhibition of SFK activity has been linked to apoptosis induction in primary CLL cells.7 Therefore cultures of CLL cells (Figure 4A) and prolymphocytic cell lines (Figure 4B) were treated with dasatinib doses up to 20 μM for 48 hours and monitored for the percentages of annexin V–binding cells. Here, annexin V–stained cells were considered as apoptotic irrespective of 7AAD staining, but the results were similar taking into account only 7AAD-negative cells. In 2 CLL patient samples representing strong (CLL-6) and weak (CLL-2) response to dasatinib and in prolymphocytic cell lines, the percentages of annexin V–binding cells were increased already by submicromolar concentrations of dasatinib, but not substantially elevated beyond the level reached at 5 μM. Because apoptosis induction only occasionally was more than 50, the response to dasatinib was expressed as the effect elicited by a given dose, namely 5 μM, rather than as concentration that inhibits 50% (IC50). Smaller percentages of apoptotic cells in Mec1 than in JVM-3 cells corresponded to higher metabolic activity as determined in the XTT assay (Figure 4C). The IC50 values obtained for dasatinib in XTT assays were approximately 10 μM and approximately 20 μM for JVM-3 and Mec1 cells, respectively. As compared with freshly isolated CLL patient cells, the percentages of annexin V–stained cells in populations of CLL cell lines were lower and less efficiently increased by dasatinib treatment (Figure 4B). In turn, metabolic activity was more efficiently reduced in immortalized prolymphocytic cell lines (Figure 4C) than in a representative primary culture of a freshly prepared CLL patient cell sample (Figure 4D). To demonstrate a differential effect of dasatinib on normal and tumor lymphocytes, un-stimulated PBMCs from healthy donors and a CLL patient were treated with several concentrations of dasatinib and analyzed by XTT assay (Figure 4D). Whereas reduction of the tetrazolium salt by patient cells was dose-dependently affected by dasatinib starting at 50 nM, PBMCs from healthy donors maintained their metabolic activity up to 5 μM dasatinib showing a small decline only at 50 μM.
Biological effects of dasatinib and combination with fludarabine treatment. Freshly isolated CLL cells (A) and the cell lines JVM-3 and Mec1 (B) were treated with the indicated doses of dasatinib or fludarabine or a combination of the indicated fludarabine concentrations and 5 μM dasatinib and analyzed for annexin V-staining after 48 hours. In addition to apoptosis induction, the influence of dasatinib on the metabolic activity of prolymphocytic cell lines was assessed by the XTT assay (C). Dose-dependent dasatinib effects on cellular respiration in PBMCs from healthy donors and a CLL patient were compared (D). Apoptosis induction by 5 μM dasatinib was assessed in 12 CLL patient samples by flow cytometric analysis after annexin V and DiOC6 staining. In 9 CLL cell samples the investigation was extended to treatment with 5 μM fludarabine and the combination of dasatinib and fludarabine (E). Error bars represent SD.
Biological effects of dasatinib and combination with fludarabine treatment. Freshly isolated CLL cells (A) and the cell lines JVM-3 and Mec1 (B) were treated with the indicated doses of dasatinib or fludarabine or a combination of the indicated fludarabine concentrations and 5 μM dasatinib and analyzed for annexin V-staining after 48 hours. In addition to apoptosis induction, the influence of dasatinib on the metabolic activity of prolymphocytic cell lines was assessed by the XTT assay (C). Dose-dependent dasatinib effects on cellular respiration in PBMCs from healthy donors and a CLL patient were compared (D). Apoptosis induction by 5 μM dasatinib was assessed in 12 CLL patient samples by flow cytometric analysis after annexin V and DiOC6 staining. In 9 CLL cell samples the investigation was extended to treatment with 5 μM fludarabine and the combination of dasatinib and fludarabine (E). Error bars represent SD.
Mechanism of apoptosis induction by dasatinib in CLL cells
Annexin V binding reflects changes in cytoplasma membrane assymetry, which make phosphatidylserine accessible at the cell surface, and indicate early phases of apoptosis. To complement the apoptosis assessment by annexin V binding, we simultaneously determined changes in the mitochondrial membrane potential using the fluorescent dye DioC6 by 5 μM dasatinib (Figure 4E). The percentages of viable cells obtained by the 2 methods were very similar. In a set of 12 freshly prepared CLL patient samples apoptosis induction by 5 μM dasatinib was highly significant according to Student t test using either apoptosis assay (P = 3.3 × 10−5 and P = 1.1 × 10−4, respectively). To confirm apoptosis induction in CLL cells at further mechanistically different levels, PARP cleavage was demonstrated in CLL patient samples and prolymphocytic cell lines (Figure 5A). In all cases examined, dasatinib treatment decreased the amount of uncleaved PARP at 116 kDa and increased the amount of cleavage product at 89 kDa.
Mechanism of apoptosis induction by dasatinib in CLL cells. Apoptosis induction by 5 μM dasatinib was confirmed in patient samples and prolymphocytic cell lines by cleavage of the caspase substrate PARP demonstrated by Western blot analysis (A). The amounts of uncleaved (uc) and cleaved (c) caspase forms, and expression levels of the antiapoptotic bcl-2 family members Mcl-1 and Bcl-xL, and of p53 in the absence (−) or presence (+) of 5 μM dasatinib were recorded in primary CLL cells (B) and the prolymphocytic cell lines JVM-3 and Mec1 (C). In these cell lines, dasatinib effects on the expression levels of proapoptotic bcl-2 family proteins and on cytoplasmic cytochrome c levels were monitored (D).
Mechanism of apoptosis induction by dasatinib in CLL cells. Apoptosis induction by 5 μM dasatinib was confirmed in patient samples and prolymphocytic cell lines by cleavage of the caspase substrate PARP demonstrated by Western blot analysis (A). The amounts of uncleaved (uc) and cleaved (c) caspase forms, and expression levels of the antiapoptotic bcl-2 family members Mcl-1 and Bcl-xL, and of p53 in the absence (−) or presence (+) of 5 μM dasatinib were recorded in primary CLL cells (B) and the prolymphocytic cell lines JVM-3 and Mec1 (C). In these cell lines, dasatinib effects on the expression levels of proapoptotic bcl-2 family proteins and on cytoplasmic cytochrome c levels were monitored (D).
To refine the mechanistic investigation of apoptosis induction by dasatinib, we probed different steps of the caspase cascade and examined the expression levels of bcl-2 family members and of p53 in CLL cells (Figure 5B) and model cell lines (Figure 5C). Dasatinib treatment decreased the uncleaved forms or increased the activated cleaved fragments of the initiator caspases-8 and -9 and of the downstream effector caspases-3 and -7. Caspase 9 forms the apoptosome together with Apaf-1 and cytochrome c released from damaged mitochondria,31 which was detected at increased levels in the cytoplasm after dasatinib treatment (Figure 5D).
In accordance with apoptosis induction, expression of the antiapoptotic bcl-2 family members Mcl-1 and BclxL was clearly decreased upon dasatinib treatment in primary CLL cells (Figure 5B), whereas expression levels of the latter were unchanged or increased in JVM-3 and Mec1 cells, respectively (Figure 5C). In the prolymphocytic cell lines, expression of the proapoptotic Bcl-2 proteins Bid and Bax was slightly increased by dasatinib treatment (Figure 5D).
Although the p53 protein levels detected in immunoblots were not influenced by dasatinib treatment in primary CLL cells (Figure 5B), they were increased in prolymphocytic cell lines (Figure 5C), which resembled the induction pattern of p53-insensitive CLL cells in an irradiation-based assay of p53 function.32,33 This model classifies the DNA damage response according to basal and induced p53 expression levels. In contrast to most examined CLL clones, eg CLL-23, a patient sample with chromosome 17p deletion (CLL-26) (Figure 5B) and the prolymphocytic cell lines, JVM-3 and Mec1, showed high basal p53 expression, in Mec1 cells at a smaller protein size due to deletion or nonsense/frameshift mutation (Figure 5C). Patient sample CLL-26 with chromosome 17p deletion showed high p53 expression, suggesting mutation of the second allele, and high apoptosis induction by dasatinib (Figure 6A). Thus, deficiency and functional loss of p53 does not seem to preclude dasatinib action.
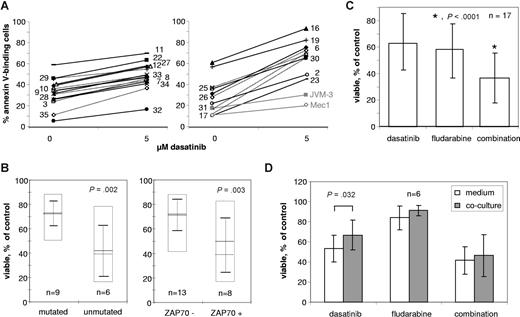
Characterization of the dasatinib response of primary CLL cells. CLL patient cells were incubated in growth medium without or with 5 μM dasatinib for 48 hours and subsequently analyzed for annexin V–binding by flow cytometry. The dasatinib-induced decreases in the percentages of annexin V–negative, viable cells relative to untreated controls are below or above 26% in the left or right diagrams, respectively. For reference the cell lines JVM-3 and Mec1 are indicated in gray in the right diagram. Numbers to the left of the graphs belong to dotted lines, those to the right to solid lines, and refer to the patient information in Table 1 (A). Correlation of IgVH mutation status (left) and ZAP-70 expression (right) with apoptosis induction by 5 μM dasatinib in primary CLL patient cells. Boxes with solid lines indicate the range from minimum to maximum responses; dashed and solid horizontal lines the medians and means, respectively, and error bars the standard deviations of the means (B). Apoptosis induction in primary CLL cells from 17 patients by 5 μM dasatinib, 5 μM fludarabine and a combination of both (C). Influence of coculture with the bone marrow stroma cell line HS5 on drug sensitivity (D). Error bars represent SD.
Characterization of the dasatinib response of primary CLL cells. CLL patient cells were incubated in growth medium without or with 5 μM dasatinib for 48 hours and subsequently analyzed for annexin V–binding by flow cytometry. The dasatinib-induced decreases in the percentages of annexin V–negative, viable cells relative to untreated controls are below or above 26% in the left or right diagrams, respectively. For reference the cell lines JVM-3 and Mec1 are indicated in gray in the right diagram. Numbers to the left of the graphs belong to dotted lines, those to the right to solid lines, and refer to the patient information in Table 1 (A). Correlation of IgVH mutation status (left) and ZAP-70 expression (right) with apoptosis induction by 5 μM dasatinib in primary CLL patient cells. Boxes with solid lines indicate the range from minimum to maximum responses; dashed and solid horizontal lines the medians and means, respectively, and error bars the standard deviations of the means (B). Apoptosis induction in primary CLL cells from 17 patients by 5 μM dasatinib, 5 μM fludarabine and a combination of both (C). Influence of coculture with the bone marrow stroma cell line HS5 on drug sensitivity (D). Error bars represent SD.
Apoptosis induction by dasatinib in freshly isolated CLL cells
Having shown that annexin V binding induced by dasatinib correlated with other indicators of apoptosis, we were interested in the variability of apoptosis induction by dasatinib among CLL cells from different patients. Therefore, we selected a dasatinib concentration of 5 μM from the dose response curves in Figure 4 and cultured 25 primary CLL cell samples for 48 hours with or without 5 μM dasatinib. From the observed percentages of annexin V–stained cells the elicited relative change in viability was calculated (Table 1). According to the difference in the percentages of annexin V–stained cells between treated and untreated cultures or to the relative dasatinib-induced decrease of nonapoptotic cells, using cutoff values of 26% or 33%, respectively, the samples were divided into 15 with weak and 10 with strong response to dasatinib (Figure 6A).
Correlation of the dasatinib sensitivity of primary CLL cells with prognostic groups
Two subgroups of CLL with different prognosis are distinguished on the basis of IgVH mutation status2 and ZAP-70 expression as a surrogate marker.3 Therefore, we tested 15 samples for which information about mutation status and dasatinib treatment effects was available for correlation between these features (Figure 6B). After dasatinib treatment samples with somatic hypermutation of the rearranged IgVH genes showed > 66% viability, whereas those with unmutated IgVH genes, a feature linked to worse prognosis, showed <42% viability, with the exception of patient no. 34 (Table 1, Figure 6B). Thus, unmutated IgVH genes were significantly correlated with high apoptosis induction by dasatinib (P = .002). Because information about ZAP-70 expression was available for 21 primary CLL samples with known dasatinib treatment effects, a similar classification was performed for this bigger group. A significant correlation between ZAP-70 expression and sensitivity to dasatinib was found (P = .003; Figure 6B). Two CLL patient samples typical of weak and strong apoptosis induction by dasatinib (Figure 4A) have the corresponding IgVH mutation and ZAP-70 expression status. Similarly, the cell lines JVM-3 and Mec1, showing relatively strong and weak response to dasatinib (Figure 4B,C) were classified as ZAP-70–positive and –negative, respectively.
Effects of combined fludarabine and dasatinib treatment
Because dasatinib induced apoptosis in CLL cells, we wondered whether it could be exploited to enhance the effects of the cytotoxic purine analog fludarabine, which is commonly used for chemotherapy of CLL. The sensitivity of CLL cells toward DNA-damaging agents is determined in part by their DNA repair capacity.34 Inhibition of Abl kinase activity in primary CLL cells by imatinib resulted in a reduction of chlorambucil-induced Rad51 phosphorylation and in a synergistic increase of chlorambucil sensitivity.35 Therefore, we treated CLL cell lines with dasatinib and fludarabine doses in a range up to 20 μM and with a combination of different fludarabine doses and a fixed dasatinib concentration of 5 μM and recorded the percentages of annexin V–positive cells (Figure 4). Apart from the differential apoptosis induction by dasatinib we also found variations in the sensitivity toward fludarabine, but there was not any obvious correlation between sensitivities toward the 2 drugs. Dasatinib sensitized CLL cells for the cytotoxic treatment. The enhancement of treatment effects by the combination was not correlated to the sensitivity to either of the drugs. As to apoptosis induction, dasatinib sensitivity and the enhancement of fludarabine cytotoxicity by dasatinib were greater in JVM-3 than in Mec1 cells (Figure 4B). In 17 patient samples, apoptosis induction by 5 μM of dasatinib, fludarabine, and a combination of both was assessed (Figure 6C). The combination, on average, increased apoptosis induction by each individual substance by approximately 50% and significantly enhanced the sensitivity to the single agents.
Having established in vitro effects of dasatinib treatment on freshly isolated patient cells we wondered whether the drug effects were maintained in the presence of cell surface interactions and soluble factors from the microenvironment. For this purpose 6 CLL patient samples were either grown in medium or cocultivated with the adherently growing human bone marrow stromal cell line HS5 (Figure 6D). Whereas without drug treatment the percentages of annexin V–binding cells were slightly increased by cocultivation with HS5 cells after 48 hours in 5 of 6 samples, the feeder cells reduced the percentage of apoptotic cells after treatment with dasatinib or fludarabine in 4 of 6 samples, respectively. Cocultivation with HS5 cells provided some degree of protection against drug-induced apoptosis. This effect was statistically significant for dasatinib but not for fludarabine.
Discussion
The aim of this study was to assess the influence of the Src/Abl inhibitor dasatinib on signal transduction and survival of CLL cells. In 2 CLL cell lines and a set of freshly isolated CLL cell samples we showed that SFK inhibition by dasatinib is correlated with apoptosis induction. Moreover, we identified the Akt/MAPK signaling cascade as a signaling pathway affected by dasatinib in CLL cells and investigated the combined treatment of CLL cells with dasatinib and the cytostatic drug fludarabine. The sensitivity to dasatinib was significantly higher in the subsets of CLL cells with unmutated IgVH genes or ZAP-70 expression, which are related to unfavorable prognosis.
Treatment of CLL cells with dasatinib efficiently reduced global protein tyrosine phosphorylation (Figures 1,2). In particular, a significant decrease of the autophosphorylated activated form of SFKs upon dasatinib treatment was observed, whereas Lyn protein levels remained unchanged. Phosphorylation analysis of immunoprecipitated Lyn and Lck showed that these SFK members are involved in dasatinib effects in CLL cells (Figure 2C). Despite the lower specificity of dasatinib18 as compared with imatinib, dasatinib concentrations less than 5 μM did not significantly influence the survival of resting lymphocytes from healthy donors (Figure 4D).
Dysregulated Bcr-Abl kinase activity is the target of tyrosine kinase inhibitor therapy also in imatinib-resistant CML. In K562 cells the dual specific Abl/Src inhibitor dasatinib affected STAT5-mediated survival signaling.36 Among the far more complex dasatinib targets in CLL cells, the SFK Lyn shows increased expression levels and basal activity in CLL cells as compared with normal B cells, presumably due to impaired degradation of the kinase protein.7 Although we did not find any indication for the inhibition of STAT5 phosphorylation by dasatinib in CLL cells (not shown), we were able to demonstrate reduced levels of the activated phosphorylated forms of the downstream kinases Akt, Erk1/2, and p38 (Figure 3B).
Using inhibitor binding to kinases as an indicator of their sensitivity,37 loss of phosphorylation at the inhibitory C-terminal phosphotyrosines of SFKs may be due to inhibition of CSK (Kd = 1 μM), but direct inhibition of Erk and p38 appears unlikely, because Kd values of SFKs (Kd for Lck and Lyn: 0.2 and 0.6 nM, respectively) and the mentioned downstream kinases (Kd for p38-α, p38-β and Erk2: 30 nM and 400 nM or no binding) differ more than 2 orders of magnitude.18 Although dasatinib binding to Akt had not been examined, direct inhibition of Akt by dasatinib can be excluded, because the phosphorylation state of Akt in K562 cells is maintained after treatment by 100 nM dasatinib in K562 cells, but reduced in patient samples and model cell lines (Figure 3B).
The mentioned signaling events may be linked to apoptosis induction by, for example, increasing the expression level of the proapoptotic Bcl-2 family member Bad. The observed decrease in Akt and Erk phosphorylation upon dasatinib treatment corresponded to a variety of apoptosis-related effects, such as annexin V–binding, caspase activation, cytochrome c release, and changes in the expression levels of pro- and antiapoptotic bcl-2 family members. For the tyrosine kinase inhibitor adaphostin, apoptosis induction was also shown to proceed through Erk- and Akt-dependent processes.38 Interestingly, in CLL cells dasatinib appears to inhibit a prosurvival signaling cascade involving Akt and p38 that is also interrupted in the B-cell lymphoma cell line 2F7 by engagement of CD20.39
As the guardian of the genome, p53 mediates apoptosis induction in response to DNA damage caused by, for example, gamma irradiation or treatment with cytotoxic drugs. The DNA damage response is commonly accompanied by an increase of p53 protein levels, as was observed upon dasatinib treatment in the cell lines Mec1 and JVM-3, but not in primary CLL cells (Figure 5B,C). The induction pattern of p53 amounts detected in the cell lines without and with prior dasatinib treatment resembles type A p53-insensitive CLL cells upon irradiation.32,33 In contrast to dasatinib treatment (Figure 5B), exposure of Mec1 cells to fludarabine did not increase expression of the smaller p53 isoform detected in this cell line.40 A CLL patient sample with chromosome 17p deletion expressing high p53 protein levels (CLL-26) belonged to the subgroup with high apoptosis induction by dasatinib. This provided first evidence that dasatinib may be effective in p53-deficient CLL. Similarly, dasatinib, but not imatinib, suppresses p53-deficient leukemic cells in B-ALL mice.41
For the comparison of dasatinib effects on apoptotic pathways in primary CLL cells versus cell lines and for the assessment of dasatinib effects on highly proliferative cells, we included the cell lines JVM-329 and Mec1,30 although they do not represent accurate models of CLL in terms of origin or eg CD5 expression. Although apoptosis induction by dasatinib appeared stronger in freshly isolated patient cells than in immortalized cell lines, cellular metabolic activity was more severely inhibited in cell lines than in CLL patient samples that do not strongly proliferate in vitro without stimulation. Correspondingly, the dasatinib-induced reduction of immunodetected protein levels of inactive uncleaved caspases and of the antiapoptotic bcl-2 members BclxL and Mcl-1 was more pronounced in primary cultures than in cell lines (Figure 5B,C). Although apoptosis is induced in prolymphocytic cell lines and freshly isolated patient by submicromolar amounts of dasatinib, the dose-response curves (Figure 4A) level off at concentrations above 5 μM, so that 50% apoptosis induction is achieved only in some patient samples. Therefore, based on IC50 values, dasatinib appears to be a less potent inducer of apoptosis in CLL cells than adaphostin, which may be explained by the induction of oxidative stress as an additional mechanism of adaphostin action.42
The dose of 5 μM dasatinib used for the assessment of dasatinib sensitivity roughly corresponds to the IC50 obtained by XTT assays on prolymphocytic cell lines. As to apoptosis induction by dasatinib treatment of CLL patient cells, we found that 0.5 μM had almost the same effect as 5 μM, as reflected in the dose response diagrams of Figure 4, and as verified for 15 patient samples (Figure S1). Pharmacokinetic studies suggest that plasma levels of 180 nM are clinically achievable by oral administration and that these treatment regimens inhibit SFK activation in PBMCs by more than 50%.43,44 The strong inhibition by 100 nM dasatinib of global tyrosine and SFK autophosphorylation site phosphorylation (Figures 1,Figure 2–3) is in agreement with dasatinib effects at clinically achievable doses.
The protein kinase inhibitors imatinib and NU7026 sensitize CLL cells for treatment with the cytotoxic drug chlorambucil.35,45 Whereas the combination of these kinase inhibitors with chlorambucil resulted in synergistic enhancement of antiproliferative and proapoptotic effects, the enhancement of fludarabine cytotoxicity by dasatinib was approximately additive (Figure 5). Similarly, the combination of genistein with fludarabine increased induction of apoptosis in CLL cells relative to fludarabine alone.46
Because dasatinib comparatively specifically targets Abl and SFKs, the observed effects on cellular survival functions underscore a role of SFKs in the microenvironment interactions of CLL cells with possible implications for pathogenesis. A potential role of SFKs in the pathogenesis of Bcr-Abl–positive leukemias led to the concept of dual-specific Src-Abl inhibitors47 and the efficacy of dasatinib in their treatment was shown to be due to shutting down SFK activity.41 For IL-6 signaling in multiple myeloma, a malignancy of mature B cells, the involvement of SFKs was shown and modulated by a membrane-permeant peptide.48-50 In CLL, SFKs constitute a signaling component of the B-cell receptor (BCR), which determines cell fate. In the subgroup with unmutated IgVH genes and ZAP-70 expression, the response to BCR stimulation is stronger.6 This is in line with our observation of an increased dasatinib sensitivity of patient cells belonging to this CLL subgroup with unfavorable prognosis. Also the sensitivity to the kinase inhibitor genistein was correlated with ZAP-70 in CLL cells.46 Direct inhibition of ZAP-70 by dasatinib is unlikely, because its KD for the related Syk kinase is tenfold higher than for most SFKs.18 Taken together, the potent inhibitory effects of dasatinib on the survival of CLL cells in vitro, particularly in the subgroup with unfavorable prognosis, suggest dasatinib as the first targeted therapy for CLL with unmutated IgVH genes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Reinhild Brinker for strong support in the purification and characterization of CLL cells. Help by Drs Christoph Scheid and Günter Fingerle-Rowson in obtaining dasatinib is gratefully acknowledged. We thank Dr Eisei Kondo and Rajesh Gandhirajan (Department I for Internal Medicine, University of Cologne) for providing K562, JVM-3, and Mec1 cells and Amparo Hausherr for valuable comments on the manuscript.
This work was supported by grants from the German José Carreras Leukemia Foundation (Munich, Germany; DJCLS R04/07) and the CLL Global Research Foundation (Houston, TX; Hallek 2006).
Authorship
Contribution: A.V. designed and performed research and analyzed data. M.P. performed research. S.H., C.P.P., and C.M.W contributed CLL patient cells and their characterization. M.H. designed research and analyzed data. G.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gunter Krause, PhD, Clinic I for Internal Medicine, University Hospital Cologne, Kerpener Str 62, 50931 Cologne, Germany; e-mail: guenter.krause@uk-koeln.de.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal