To the editor:
Recently Zeiser et al reported the results of their study on the effects of atorvastatin on acute graft-versus-host disease (aGVHD) and graft-versus-leukemia (GVL) activity.1 Using murine models of aGVHD they showed that treatment with atorvastatin of both the donor and the recipient has a protective effect against aGVHD without diminishing the GVL activity. Atorvastatin affected aGVHD severity by inducing a Th-2 polarization of the alloreactive donor T-cell response. Treatment of the recipient led to an atorvastatin-mediated down-regulation of the major histocompatibility complex class II and the costimulatory molecules CD80 and CD86 on antigen-presenting cells (APC).
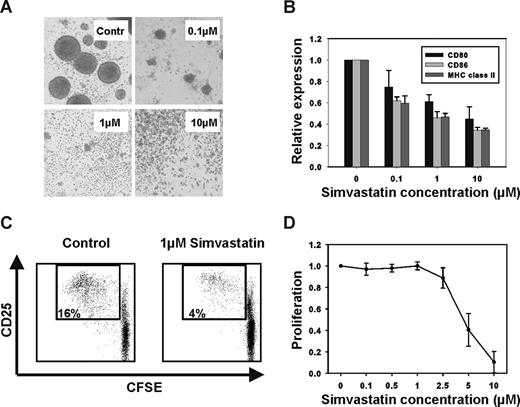
We observed similar effects of statins on human APC. CD40-activated B cells are potent antigen-presenting cells.2 Gene expression analysis of B cells using microarrays showed that the enzymes of the mevalonate pathway were up-regulated after activation via CD40 (data not shown), implying that this pathway could be important for APC function. Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase, the key enzyme of the mevalonate pathway, and in addition have been shown to interfere with the interaction of leukocyte function-associated antigen-1 (LFA-1) with its ligands, which is important for activation and adhesion of APCs.3 We therefore studied the effects of the HMG-CoA reductase inhibitor simvastatin on antigen presentation by CD40-activated B cells. We stimulated purified B cells by coculturing them with CD40 ligand-transfected NIH3T3 cells as described previously.2 Upon activation through CD40 B cells usually up-regulate the expression of adhesion molecules and form round clusters through homotypic adhesion. Addition of simvastatin to the cultures inhibited the formation of these cluster at concentrations as low as 0.1 μM (Figure 1A). We further found that treatment with simvastatin reduced the expression of costimulatory molecules and major histocompatibility complex (MHC) class II (Figure 1B). Consequently, the proliferation of alloreactive T cells in response to simvastatin-treated CD40-activated B cells was reduced significantly (Figure 1C). We (data not shown) and others4 observed similar effects of statins on the antigen presenting function of dendritic cells. Interestingly, APCs seem to be especially sensitive to statins. Inhibition of T-cell proliferation by CD3/CD28-coated beads required a 1 log higher concentration of simvastatin (Figure 1D). This suggests that inhibition of antigen presentation is an important mechanism of immunomodulation by statins in the in vivo setting. In addition, given the importance of APCs, including B cells, in the pathogenesis of acute and chronic GVHD5,6 our results suggest a potential mechanism by which statins inhibit the development of both acute and chronic GVHD.7-9
Statin treatment inhibits the antigen-presenting function of CD40-activated B cells. (A) Purified B cells were activated through CD40 in the presence of simvastatin (0.1, 1, or 10 μM) or vehicle as a control (Contr). Addition of simvastatin to the culture medium prevented activation and homotypic adhesion. Photographs were taken at 100× magnification using a TELEVAL 31 microscope (Carl Zeiss, Jena, Germany fitted with a Canon EOS 350D digital camera. (B) The surface expression of MHC class II and the costimulatory molecules CD80 and CD86 was reduced in a dose-dependent fashion. Expression levels are expressed relative to vehicle-treated controls (n = 4). (C) The immunostimulatory function of CD40-activated B cells was assessed in a mixed lymphocyte reaction (MLR) with carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled CD4+ T cells as responders and CD40-activated B cells, which have been treated with vehicle or 1 μM simvastatin, as stimulators. FACS plots representative of 4 separate experiments are shown. (D) CFSE-labeled CD4+ T cells (n = 4) were stimulated with CD3/CD28-coated beads at a ratio of 1:1. Addition of simvastatin at the indicated concentrations interfered with the T cell proliferation. Error bars in panels B and D represent SD.
Statin treatment inhibits the antigen-presenting function of CD40-activated B cells. (A) Purified B cells were activated through CD40 in the presence of simvastatin (0.1, 1, or 10 μM) or vehicle as a control (Contr). Addition of simvastatin to the culture medium prevented activation and homotypic adhesion. Photographs were taken at 100× magnification using a TELEVAL 31 microscope (Carl Zeiss, Jena, Germany fitted with a Canon EOS 350D digital camera. (B) The surface expression of MHC class II and the costimulatory molecules CD80 and CD86 was reduced in a dose-dependent fashion. Expression levels are expressed relative to vehicle-treated controls (n = 4). (C) The immunostimulatory function of CD40-activated B cells was assessed in a mixed lymphocyte reaction (MLR) with carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled CD4+ T cells as responders and CD40-activated B cells, which have been treated with vehicle or 1 μM simvastatin, as stimulators. FACS plots representative of 4 separate experiments are shown. (D) CFSE-labeled CD4+ T cells (n = 4) were stimulated with CD3/CD28-coated beads at a ratio of 1:1. Addition of simvastatin at the indicated concentrations interfered with the T cell proliferation. Error bars in panels B and D represent SD.
Furthermore, a recent study showed that, unlike in mice, treatment with atorvastatin resulted in an increase of CD4+CD25+Foxp3+ regulatory T cells in humans.10 Even though Zeiser et al1 did not observe an increase in regulatory T cells in their model, atorvastatin-mediated increases in regulatory T cells might therefore still play a role in suppressing GVHD in humans.
Because there are already many approved statins on the market and the toxicity profiles of these agents have been studied extensively, these results should be readily translatable to the clinical setting. The pleiotropic effects of statins on the diverse immune cells, including APCs, involved in the pathogenesis of GVHD hold the promise of reducing the need for other immunosuppressants with a more unfavourable toxicity profile.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Shimabukuro-Vornhagen, Max Eder Junior Research Group and Stem Cell Transplantation Program, Department I of Internal Medicine, University Hospital of Cologne, 50924 Cologne, Germany; e-mail: alexander.shimabukuro-vornhagen@uk-koeln.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal