In this issue of Blood, Tam and coworkers, led by M. Keating, report mature results with FCR in previously untreated patients with CLL. The overall response rate was 95%, with an impressive CR rate of 72%. Six-year overall and failure-free survivals were 77% and 51%, respectively, and median time to progression was 80 months. Patients who achieved response had a much better outlook than those who did not respond.
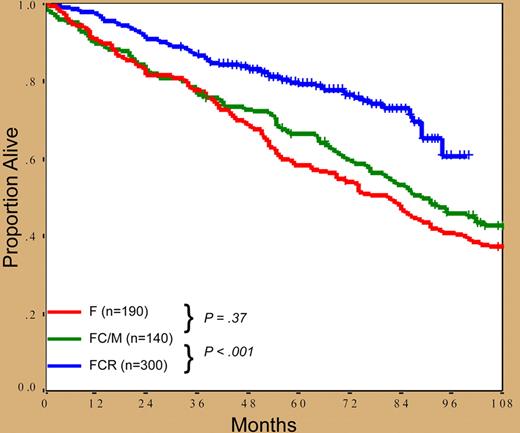
What are the central messages from this study? In short, fludarabine, cyclophosphamide, and rituximab (FCR) combination therapy produces the largest proportion of complete responses (CRs) ever reported in CLL and, even more importantly, patients treated with this regimen have better outcomes, based on historical comparisons, than similar patients treated with fludarabine or fludarabine and cyclophosphamide or mitoxantrone (see figure). In addition, the relationship between the quality of the response and clinical outcome is confirmed. Furthermore, patients achieving CR with no detectable minimal residual disease (MRD)—albeit not studied by the technique currently considered preferable1 —do much better than the rest, thus confirming that, whenever possible, obtaining MRD-negative status is a desirable treatment end-point in CLL.2 Notably, FCR abrogates the poor prognostic significance of classic variables, which indicates that it actually changes the natural history of CLL, the best that can be said for any new therapy for neoplastic disorders. On the downside, there are manageable toxicities, the poor response of patients with chromosome 17 abnormalities, the risk of secondary myelodysplasia, and the fact that all patients are eventually projected to relapse.
Based on this report, should FCR be considered the new gold standard for CLL therapy? It could be reasonably argued that the remarkable results of this study are not derived from a randomized phase 3 trial and that, consequently, the relative superiority of the FCR regimen needs validation in other series and, above all, in randomized studies. If this is the concern, we need only await the shortly due and eagerly expected results of the German CLL Group clinical trial comparing FCR to FC, recently closed because the main end-point of the study has been reached.
As happens with all good studies, the work of Tam et al not only offers important answers, but also raises important questions and inspires future research. Among these: Is FCR necessary for all patients? Should FCR be given as up-front therapy or could it be part of a more conservative, sequential therapy? Can FCR toxicity be reduced? What is a patient's fate once progression occurs? Is retreatment safe? Given that all patients eventually relapse, should some kind of maintenance therapy be considered? How should lessons from this study be applied to the predominantly elderly or physically unfit population of patients with CLL?
All in all, however, it is easy to predict that FCR will become an important new gold standard for CLL therapy. Treatment of patients with CLL is rapidly evolving, and we can surely expect dramatic improvements in the management of this common form of leukemia founded on its biologic and clinical diversity, most likely not with a single, unique gold-standard therapy, but different and individualized treatment approaches.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal