In this issue of Blood, Giblin and colleagues report that the continuous spontaneous release of VWF from endothelial cells is largely due to secretion of a stored form of the mature protein and not to its continuous transit out of the cell by classic constitutive secretion.
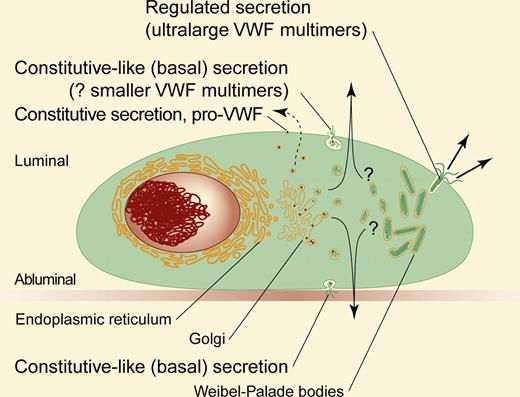
von Willebrand Factor (VWF) is secreted by 2 pathways, one continuous and requiring no cellular stimulation, and the other regulated and responsive to secretagogues. The distinction between regulated and constitutive protein secretion pathways at first blush appears obvious. Regulated secretion requires a stimulus to provoke protein release from storage granules, whereas constitutive secretion is continuous, requires no stimulus, and is thought to occur by uninterrupted synthesis and exocytosis of proteins after their processing in the Golgi. However, a third pathway, which the authors call basal secretion (also called constitutive-like secretion), involves elements of both, with proteins targeted to storage granules after Golgi processing (as in regulated secretion) but continuously secreted from this storage pool without provocation (similar to constitutive secretion).
The continuous unstimulated release of VWF has been termed “constitutive” secretion. Its mechanism is unknown, but it differs from regulated secretion in human umbilical vein endothelial cells (HUVECs) in that it produces smaller VWF multimers on average, is sensitive to inhibitors of protein synthesis, does not require microtubules, and releases VWF to both the luminal and abluminal cell surfaces (regulated secretion is only toward the luminal surface).1
Giblin and colleagues bring a fresh perspective to VWF secretion by integrating older studies of VWF trafficking with new evidence in HUVECs to conclude that continuously secreted VWF derives primarily from post-Golgi secretory organelles and not from uninterrupted protein passage from the Golgi to the plasma membrane (see figure). They show that most VWF is held in the cell before release, and that spontaneously secreted VWF originates from a storage pool. Unstimulated HUVECs also continuously secrete a small amount of pro-VWF (VWF containing the propeptide), which has the hallmarks of being secreted by the conventional constitutive pathway. One of the defining characteristics of constitutive VWF release is that it can be inhibited by blocking protein synthesis.2 In the article by Giblin and coauthors, an experiment with carefully timed blockade of protein synthesis showed that VWF that would otherwise be spontaneously secreted is retained in the cell, consistent with inhibition of release from secretory granules.
Depicted are the various pathways and modes of VWF secretion from endothelial cells. See text. Professional illustration by Paulette Dennis.
Depicted are the various pathways and modes of VWF secretion from endothelial cells. See text. Professional illustration by Paulette Dennis.
This work raises many interesting questions. Previous observations have suggested that VWF multimer size depends on the route of secretion, with larger, more hemostatically active multimers secreted after stimulation.1 If Weibel-Palade bodies are the main secretory organelles for both continuous release and regulated secretion, then they may be heterogeneous based on the multimeric composition of their cargo. Some Weibel-Palade bodies have been shown to move continuously but stochastically in unstimulated HUVECs3 ; could these be the source of continuous VWF secretion? Understanding these pathways has clinical implications. If endothelial cells in vivo secrete VWF by the constitutive-like route, this could account for a significant portion of circulating plasma VWF. Disruption of VWF secretion has been implicated in Type 2A von Willebrand disease, while targeted disruption of one (or both) pathways could be useful in some prothrombotic states, such as thrombotic thrombocytopenic purpura. Whether continuous VWF secretion is called constitutive, constitutive-like, or basal, the insightful work of Giblin and colleagues raises questions that warrant further investigation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal