Abstract
Mast cells have been recognized for well over 100 years. With time, human mast cells have been documented to originate from CD34+ cells, and have been implicated in host responses in both innate and acquired immunity. In clinical immunology, they are recognized for their central role in IgE-mediated degranulation and allergic inflammation by virtue of their expression of the high-affinity receptor for IgE and release of potent proinflammatory mediators. In hematology, the clinical disease of mastocytosis is characterized by a pathologic increase of mast cells in tissues, often associated with mutations in KIT, the receptor for stem cell factor. More recently, and with increased understanding of how human mast cells are activated through receptors including the high-affinity receptor for IgE and KIT, specific tyrosine kinase inhibitors have been identified with the potential to interrupt signaling pathways and thus limit the proliferation of mast cells as well as their activation through immunoglobulin receptors.
Introduction
Mast cells have a rather unique position among cells of the immune response. Their progenitors are bone marrow derived, yet under normal conditions appear in the mature state only within vascularized tissues, where they are long-lived. Mast cells appear historically ancient,1 yet their roles in mammalian biology, including disease pathogeneses and host defense mechanisms, often remain speculative and based on in vitro studies and animal models2-4 with 2 primary exceptions—IgE-mediated immediate hypersensitivity reactions and mastocytosis. Complicating the understanding of the role of mast cells in human biology is that while other normal human immune cell functions often become more obvious in the absence of a specific cell type, such as with agranulocytosis, or in the absence of normal function of a specific pathway, as in autoimmune lympho-proliferative syndrome (ALPS) associated with defective lymphocyte Fas-mediated apoptosis, the single similar situation involving mast cells and human disease characterized to date is mastocytosis, resulting from disturbed control of mast cell proliferation
Mast cell research initially relied upon observation on mast cell appearance and numbers in tissue biopsies, sometimes correlated with tissue histamine levels. With time, methods were developed to obtain and study mast cells ex vivo. The most common protocols relied on obtaining mast cells from the peritoneal cavity of rodents, or enrichment of mast cells from tissue digests. These approaches initiated modern mast cell biology with the first work on histamine, slow-reacting substance of anaphylaxis (SRS-A), and other mast cell–derived mediators including proteases, and the early studies on the mechanisms of mast cell signal transduction. In more recent years, and with the identification of key mast cell growth factors, investigators have discovered how to culture mast cells in vitro from pluripotential precursors (Figure 1). This development has facilitated the further study of human mast cell gene expression, signal transduction, and production of mediators relevant to inflammation.
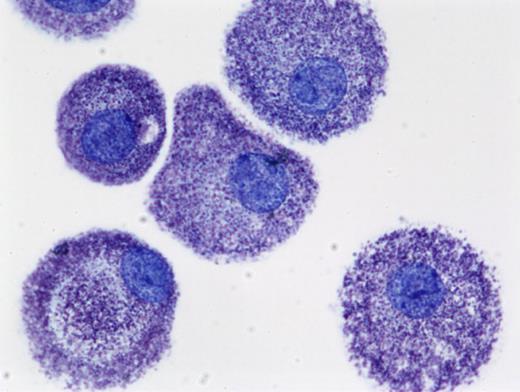
Human mast cells cultured from peripheral blood in SCF and stained with toluidine blue(cytoprep; 400×). The figure is provided courtesy of Madeleine K. Radinger (Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health (LAD/NIAID/NIH). Images were obtained via digital microscopy using a Zeiss Axiophot (Jena, Germany) equipped with a Plan-Apochramat 100×/1.4 numeric aperature (NA) objective. Images were processed using Adobe Photoshop version 3.0 (Adobe Systems, San Jose, CA).
Human mast cells cultured from peripheral blood in SCF and stained with toluidine blue(cytoprep; 400×). The figure is provided courtesy of Madeleine K. Radinger (Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health (LAD/NIAID/NIH). Images were obtained via digital microscopy using a Zeiss Axiophot (Jena, Germany) equipped with a Plan-Apochramat 100×/1.4 numeric aperature (NA) objective. Images were processed using Adobe Photoshop version 3.0 (Adobe Systems, San Jose, CA).
History
The most striking feature of mast cells is that their cytoplasm is filled with dense metachromatic granules that stain red or violet when treated with basic aniline dyes. Using this “metachromasia,” Ehrlich in 1878 first clearly described mast cells or “mastzellen” (maestung—a root of the English word mastication; the active form “measten” is still in use).5 He speculated that these granules were the product of overfeeding (die mast). Ehrlich also noted the tendency of mast cells to be associated with blood vessels, nerves, and glandular ducts. These observations contributed to Ehrlich's Nobel Prize in Medicine in 1908. In 1894, Unna reported that the cutaneous lesions termed urticaria pigmentosa (UP) were associated with increased mast cells below each lesion.6 Thus, by the end of the 19th century, mast cells had been recognized both in the normal state and associated with pathology.
Following a number of reports that mast cells could “explosively” release their granules on contact with irritants, Webb in 1931 reported that the peritoneal cells of the rat undergo degranulation following irritation by such agents as egg white, known to cause a peculiar urticaria-like reaction when injected intraperitoneally into the rat.7 Within a few years, mast cells had been identified as carriers of heparin by Jorpes8 and histamine by Riley.9 These observations and knowledge that histamine and heparin were released simultaneously from dog liver in anaphylactic shock led to the conclusion by the 1950s that mast cells played an important function in anaphylaxis. Also associated with anaphylaxis was SRS-A, which caused smooth muscle contraction and was found to be released from isolated rat peritoneal mast cells exposed to histamine releasing agents.10-13
As information on mast cells increased, it was observed that significant mast cell hyperplasia occurred in association with certain pathologic conditions such as parasitosis with a tissue phase, and then in diseases that would later be classified as variants of mastocytosis. The first description of a disease that was apparently UP is attributed to Nettleship and Tay in 1869,14 later termed UP by Sangster in 1878.15 In 1949, Ellis described an autopsy report of a fatal case of UP in a 1-year-old child. He documented mast cell infiltrations in the skin, liver, spleen, lymph nodes, and bone marrow.16 There followed many descriptions of variants of mastocytosis organized over the years into classification schemes from Degos in 195617 to the contemporary World Health Organization recognized classification18 recently updated.19
Human mast cell mediators
The recognition that mast cells are the source of most tissue histamine, contain heparin, and could generate SRS-A upon activation took place in the mid 20th century. Since then, there have been numerous papers devoted to the identification and characterization of mast cell mediators. A partial list of mediators now associated with the human mast cell is presented in Table 1.20
Major human mast cell–derived mediators
| Class . | Mediators . | Physiological effects . |
|---|---|---|
| Preformed mediators | Histamine, serotonin, heparin, neutral proteases (tryptase and chymase, carboxypeptidase, cathepsin G), major basic protein, acid hydrolases, peroxidase, phospholipases | Vasodilation, vasoconstriction, angiogenesis, mitogenesis, pain, protein processing/degradation, lipid/proteoglycan hydrolysis, arachidonic acid generation, tissue damage and repair, inflammation |
| Lipid mediators | LTB4, LTC4, PGE2, PGD2, PAF | Leukocyte chemotaxis, vasoconstriction, bronchoconstriction, platelet activation, vasodilation |
| Cytokines | TNF-α, TGF-β, IFN-α, IFN-β, IL-1α, IL-1β, IL-5, IL-6, IL-13, IL-16, IL-18 | Inflammation, leukocyte migration/proliferation |
| Chemokines | IL-8 (CXCL8), I-309 (CCL1), MCP-1 (CCL2), MIP-1αS (CCL3), MIP1β (CCL4), MCP-3 (CCL7), RANTES (CCL5), eotaxin (CCL11), MCAF (MCP-1) | Chemoattraction and tissue infiltration of leukocytes |
| Growth factors | SCF, M-CSF, GM-CSF, bFGF, VEGF, NGF, PDGF | Growth of various cell types, vasodilation, neovascularization, angiogenesis |
| Class . | Mediators . | Physiological effects . |
|---|---|---|
| Preformed mediators | Histamine, serotonin, heparin, neutral proteases (tryptase and chymase, carboxypeptidase, cathepsin G), major basic protein, acid hydrolases, peroxidase, phospholipases | Vasodilation, vasoconstriction, angiogenesis, mitogenesis, pain, protein processing/degradation, lipid/proteoglycan hydrolysis, arachidonic acid generation, tissue damage and repair, inflammation |
| Lipid mediators | LTB4, LTC4, PGE2, PGD2, PAF | Leukocyte chemotaxis, vasoconstriction, bronchoconstriction, platelet activation, vasodilation |
| Cytokines | TNF-α, TGF-β, IFN-α, IFN-β, IL-1α, IL-1β, IL-5, IL-6, IL-13, IL-16, IL-18 | Inflammation, leukocyte migration/proliferation |
| Chemokines | IL-8 (CXCL8), I-309 (CCL1), MCP-1 (CCL2), MIP-1αS (CCL3), MIP1β (CCL4), MCP-3 (CCL7), RANTES (CCL5), eotaxin (CCL11), MCAF (MCP-1) | Chemoattraction and tissue infiltration of leukocytes |
| Growth factors | SCF, M-CSF, GM-CSF, bFGF, VEGF, NGF, PDGF | Growth of various cell types, vasodilation, neovascularization, angiogenesis |
The mediators in the table are examples only. In addition, many mediators are identified in human mast cell lines or primary cultures of human mast cells and may not be produced in vivo.
Preformed mediators stored in cytoplasmic granules within the mast cell include proteoglycans, proteases, and histamine. They appear together in structured complexes that allow components such as histamine to be rapidly disassociated upon contact with the extracellular environment. Tissue mast cells account for most histamine found within normal tissues, with the exception of the upper gastrointestinal tract21 and central nervous system. Serotonin is found within rodent mast cells in significant quantities, while human mast cells contain much less serotonin.22
Human mast cells contain variable mixtures of heparin and chondroitin sulfate E/diB glycosaminoglycans. Human mast cells have been estimated to contain 2.4 to 7.8 μg heparin per 106 cells.23 This observation, along with the knowledge that heparin is a negatively charged molecule helps explain why mast cell granules are preferentially stained with cationic dyes. Proteoglycans synthesized by human pulmonary mast cells contain chondroitin sulfate E and heparin chains at 1:1 to 1:2 ratio, while those in the human gastric mucosa have more chondroitin sulfate E,24 contributing to variable staining characteristics among mast cell populations. Heparin macromolecules are known to be resistant to protease degradation, now known to be because this granule constituent has a peptide core with a repeating protease-resistant Ser-Gly sequence25 that is designated “serglycin.”26,27 Heparin is, however, susceptible to oxidative cleavage.28
Tryptases and chymases are the major protein components of mast cell secretory granules. The types and properties of these endopeptidases vary with mammal of origin and subtype. Upon mast cell degranulation, tryptases and chymases are released. Mast cells express tryptases including γ-tryptase, which is membrane anchored, and several soluble tryptases, α-tryptase, β-tryptase, and δ-tryptase, that compartmentalize into inhibitor-resistant oligomers. In the case of β-tryptase, this is a heparin-stabilized tetramer.29 Of these tryptases, β-tryptase, occurring in 3 principal forms (I, II, and III), seems the most biologically important, as it is the only one of the 3 that does not have a catalytic domain defect that reduces activity.30 The α-gene appears to be an allele at the β1 site at the human tryptase gene locus on chromosome 16p13.3, a predictor that some may lack this allele, which is the case in 20% to 25% of the population.
The 2 peptidases with chymotrypsin-like activity made by mast cells are chymases and cathepsin G. Human mast cell subpopulations appear to rather selectively express chymase. The variable expression of these enzymes has led to the recognition of human mast cell subsets. Mast cell subsets expressing tryptase and chymase (MCTC) tend to be abundant in the dermis,31 while mast cells expressing tryptase, but little or no chymase (MCT), tend to be located in the mucosa of organ systems. A third and minor population of mast cells expresses chymase and cathepsin G (MCC).
A number of potential functions have been associated with various mast cell proteases including bronchoconstriction, and the degradation of fibrinogen, extracellular matrix proteins, and endogenous and exogenous peptides. These proteases also hydrolyze chemokines and cytokines and inactivate allergens and neuropeptides. It is likely that additional biologic roles will be identified.
Activated mast cells initiate the de novo synthesis of several lipid-derived substances. Of particular importance are the cyclooxygenase and lipoxygenase metabolites of arachidonic acid, which have potent inflammatory activities and may also play a role in modulating the release process. The major lipoxygenase products derived from mast cells and basophils were originally termed SRS-A, with definitive studies linking mast cells and IgE to SRS-A generation in monkey lung fragments in 1970.32 The identification of SRS-A as a conjugate of a cysteine-containing peptide and a metabolite of arachidonic acid termed leukotriene C4 (LTC4) was reported by Murphy et al in 1979.33 More completely, it is now known that SRS-A is composed of 3 cysteinyl leukotrienes consisting of the biosynthetic intracellular product LTC4 and its extracellular metabolites LTD4 and LTE4. The major cyclooxygenase product of mast cells is prostaglandin D2 (PGD2).
Mast cells isolated from a variety of tissues release both LTC4 and PGD2, whereas peripheral blood basophils release LTC4 but not PGD2. Mast cells also produce LTB4, although in much smaller quantities than PGD2 or LTC4, and some mast cell populations represent a potential source of platelet activating factor (PAF). Th2 cytokines are known to regulate the pathway for cysteinyl leukotriene generation from human mast cells.34 Leukotrienes elicit local dermal erythema and wheal reactions35 and are potent bronchoconstrictors.36
Mast cells are now well documented to synthesize and release cytokines, chemokines, and growth factors following activation. The ability of murine mast cell lines to produce the cytokines including IL-3, IL-4, IL-5, and IL-6 was reported by Plaut et al in 1989.37 TNF was the first cytokine clearly associated with normal mast cells in 1990.38 Endothelins were associated with the mast cell in 1992.39 Some of the TNF released from mouse mast cells upon appropriate stimulation reflects a cytokine that is rapidly released from preformed stores, and even larger amounts of newly synthesized TNF is released over a period of hours after cell activation.38 This observation has been repeated variably with other cytokines, although not all exist preformed in mast cell granules.
Human mast cells are now believed a potential source for a number of cytokines/growth factors and chemokines including IL-5, IL-6, IL-13, IL-16, stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), NGF, bFGF and VEGF, as well as several C-C chemokines.40-43 IL-4 is produced in significant amounts by human basophils, but human mast cells appear to produce minimal IL-4.44 It should be recognized that data implicating some of these mediators were obtained using human mast cell lines and primary mast cell cultures, whereas the mediator patterns expressed by resident tissue mast cells under various conditions remain largely to be determined.
These products may be released when mast cells are activated via IgE- or IgG-dependent mechanisms, and may also be produced under other circumstances such as in response to stimulation by bacterial products through Toll-like receptors (TLRs).45 Production of a specific mast cell–derived cytokine may be related to activation through a specific receptor(s). For example, interferon-α (IFN-α) is produced by human mast cells following exposure to double-stranded DNA through TLR-3 but not after IgE-mediated activation through FcϵRI.45 This production of cytokines and chemokines by human mast cells greatly expands the list of possible mechanisms by which these cells may contribute to the pathophysiology of allergic and immunologic diseases, to host defense, and to homeostasis.
Human mast cell growth factors
Kitamura et al recognized as early as 1977 that mouse mast cells originated from bone marrow by grafting bone marrow cells into irradiated mice.46 This was followed in 1981 by the recognition by Nabel et al that T lymphocytes synthesized a factor that stimulated the proliferation of cloned mast cells.47 In 1983, Ihle et al reported that IL-3 would promote the growth of mast cells from mouse bone marrow.48 However, early studies also found that IL-3 lacked the ability to promote culture of human mast cells, although it did promote the growth of human basophils.49,50 It was known, however, that human mast cells would develop in the presence of fibroblasts, and in 1991 Kirshenbaum et al used this property to determine that human mast cells arose from CD34+ human pluripotent stem cells.51 Following the isolation and characterization in 1990 of stem cell factor (SCF),52-54 which is produced mainly, but not exclusively, by stromal cells, it was reported in 1992 that SCF was the principal growth factor for CD34-derived55 and mononuclear cell–derived56 human mast cells. The human mast cell committed progenitor was later described to be CD34+, KIT+, and CD13+.57
Since then a number of papers have reported that several cytokines inhibit or enhance SCF-dependent human mast cell growth and differentiation. For example, IFN-γ inhibits human mast cell growth and differentiation by inhibiting early progenitor cell division, while IL-5 blocks later cell division.58,59 Cytokine effects on mature cells in culture may vary from the effects of cytokines on human mast cell progenitors during their differentiation. For example, in one study, IL-4 appeared to down-regulate early KIT expression, while when added to mast cells late in culture, it induced some cell division and potentiated FcϵRI-mediated degranulation. Other factors are comitogenic with SCF including lysophosphatidic acid,60 LTD4,61 and thrombopoietin.62
SCF receptor
The receptor for SCF is KIT, a transmembrane protein with intrinsic tyrosine kinase activity. It is expressed on a variety of cell types, including mast cells, hematopoietic progenitor cells, melanocytes, germ cells, and gastrointestinal pacemaker cells. KIT is down-regulated from the surface of progenitor cells as they differentiate into their respective mature forms. Mast cells are one exception to this phenomenon, as they retain surface KIT expression at high levels as mature cells.
KIT is encoded for by c-Kit, the homologue of its viral counterpart, found in the genome of the Hardy-Zuckerman 4 feline sarcoma virus.63 Human c-KIT is localized to chromosome 4 (q11-12) and is structurally related to receptors for platelet-derived growth factor (PDGF) and monocyte colony-stimulating factor.64
Mice deficient in KIT are also deficient in mast cells, and display macrocytic anemia, lack of hair pigmentation, sterility, and reduced numbers of thymic and gastrointestinal pacemaker cells.65 The KIT-SCF interaction is essential for regulation of the proliferation, survival, and migration of melanocytes. Loss-of-function mutations in c-KIT result in piebaldism, an autosomal dominant disorder of pigmentation characterized by patches of white skin and hair. KIT similarly exerts critical functions in both male and female germ cell biology, where it regulates oogenesis, folliculogenesis, and spermatogenesis.
Mast cells in inflammation
Mast cells and IgE have long been associated with the pathogenesis of the acute manifestations of the immediate hypersensitivity reaction, the pathophysiologic hallmark of allergic rhinitis, allergic asthma, and anaphylaxis. The central role of mast cells in these disorders is widely accepted. In 1921, Prausnitz and Kustner demonstrated that serum transferred into the skin of a normal recipient induced a local allergic reaction upon contacting the antigen to which the donor was sensitive. This came to be known as the PK test.66 The sensitizing factor was termed “reagin.” In the late 1960s, reagin was isolated from normal serum by Ishizaka et al67 and from a myeloma by Johansson and Bennich68 and found to be a unique class of immunoglobulin designated IgE.
IgE is now known to bind with high affinity to FcϵRI, and the expression of this receptor is related to the serum IgE concentration.69 High “constitutive” levels of FcϵRI expression are restricted to mast cells and basophils, and this feature helps explain the unique role of mast cells as tissue-based effector cells in allergic inflammation. In humans, low levels of expression are detected in Langerhans cells, peripheral blood dendritic cells, and monocytes. In mast cells and basophils, FcϵRI has a tetrameric structure composed of a single IgE-binding α chain, a single β chain, and 2 identical disulfide-linked γ chains.70 All 3 subunits must be present for efficient cell surface expression in rodents, but human cells can express FcϵRI in the absence of the β chain.71 In humans, the FcϵRI expressed by hematopoietic cells other than mast cells and basophils consists of only the αγ2 form.72 The aggregation of FcϵRI that is occupied by IgE is sufficient for initiating downstream signal transduction events involving tyrosine phosphorylation that activate the mast cells or basophils to degranulate and to secrete lipid mediators and cytokines as shown in Figure 2.73,74 The FcϵRI β chain functions as an amplifier of signaling through FcϵRI. After degranulation, mast cells are believed to survive and regranulate. This may contribute to the increase in mast cells in association with chronic inflammation
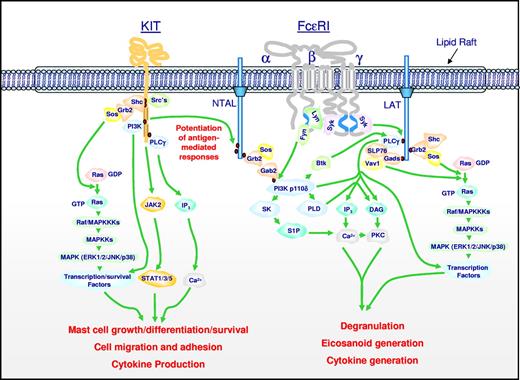
Signal transduction events initiated by Kit and FcϵRI leading to specific mast cell responses, and the integration of these pathways for the synergistic enhancement of mast cell mediator release. Dimerization of Kit, following ligation by SCF, results in activation of its intrinsic tyrosine kinase activity and autophosphorylation of specific tyrosine residues on its cytosolic tail. These phosphorylated residues provide docking sites for critical signaling molecules including Src kinases, Shc, phosphoinositide 3-kinase (PI3K), and phospholipase Cγ (PLCγ). Subsequent activation of the Ras-Raf-Map kinase (MAPK) cascade via the GTP exchanger Sos, PI3K, PLCγ, and JAK2 leads to the enhanced concentrations of Ca2+, and activation of transcription factors and survival pathways necessary for mast cell growth, differentiation, survival, cell migration, adhesion, and cytokine production. The Src kinases Lyn and Fyn may also contribute to some of these responses. Aggregation of FcϵRI, following binding of antigen to antigen-specific IgE molecules occupying the FcϵRI, results in recruitment of the tyrosine kinase Syk to the γ chain-ITAMs following translocation to the lipid raft microdomains and phosphorylation of specific tyrosines within these motifs by Lyn. This results in activation of Syk and the phosphorylation of the transmembrane adaptor molecules LAT and NTAL that coordinate downstream signaling by providing multiple phosphotyrosine-based docking sites for associating molecules such as PLCγ1 and the cytosolic adaptors Grb2, Gads, and Shc. These events regulate the activation of PLCγ1 and PLCγ2, which are required for calcium mobilization and PKC activation, essential signals for mast cell mediator release. A parallel pathway initiated by the tyrosine kinase, Fyn, leads to the activation of PI3K via the phosphorylation of the cytosolic adaptor Gab2. These events are also required for optimal degranulation and cytokine production, likely as a consequence of PI3K-dependent membrane association of the tyrosine kinase kinase, Btk, and activation of transcription factors. PI3K may also regulate the activation of sphingosine kinase (SK) and PLD, which produce the lipid mediators sphingosine-1-phosphate (S-1-P) and diacylglycerol (DAG), which also contribute to the critical calcium signal and PKC activation regulating mast cell mediator release. Concurrent with these events, the GTP exchangers Sos and Vav activate the Ras-Raf-MAPK pathway, which in turn contributes to the activation of specific transcription factors required for cytokine production. In addition, the MAPKs ERK1/2 control the activation of PLA2, which liberates arachidonic acid from membrane lipids for the subsequent generation of eicosanoids. NTAL appears to be a point of integration between the 2 pathways, providing an amplification mechanism also requiring PI3K and BTK, for the potentiation of antigen-dependent mediator release by SCF. Note that, although not depicted as such for clarity, many signaling events occur within specific microdomains within the cytosolic membrane. In addition, for clarity, several of the intermediary steps have been simplified and other pathways that may down-regulate mast cell activation have not been included. The figure and legend are provided courtesy of Alasdair M. Gilfillan (LAD/NIAID/NIH).
Signal transduction events initiated by Kit and FcϵRI leading to specific mast cell responses, and the integration of these pathways for the synergistic enhancement of mast cell mediator release. Dimerization of Kit, following ligation by SCF, results in activation of its intrinsic tyrosine kinase activity and autophosphorylation of specific tyrosine residues on its cytosolic tail. These phosphorylated residues provide docking sites for critical signaling molecules including Src kinases, Shc, phosphoinositide 3-kinase (PI3K), and phospholipase Cγ (PLCγ). Subsequent activation of the Ras-Raf-Map kinase (MAPK) cascade via the GTP exchanger Sos, PI3K, PLCγ, and JAK2 leads to the enhanced concentrations of Ca2+, and activation of transcription factors and survival pathways necessary for mast cell growth, differentiation, survival, cell migration, adhesion, and cytokine production. The Src kinases Lyn and Fyn may also contribute to some of these responses. Aggregation of FcϵRI, following binding of antigen to antigen-specific IgE molecules occupying the FcϵRI, results in recruitment of the tyrosine kinase Syk to the γ chain-ITAMs following translocation to the lipid raft microdomains and phosphorylation of specific tyrosines within these motifs by Lyn. This results in activation of Syk and the phosphorylation of the transmembrane adaptor molecules LAT and NTAL that coordinate downstream signaling by providing multiple phosphotyrosine-based docking sites for associating molecules such as PLCγ1 and the cytosolic adaptors Grb2, Gads, and Shc. These events regulate the activation of PLCγ1 and PLCγ2, which are required for calcium mobilization and PKC activation, essential signals for mast cell mediator release. A parallel pathway initiated by the tyrosine kinase, Fyn, leads to the activation of PI3K via the phosphorylation of the cytosolic adaptor Gab2. These events are also required for optimal degranulation and cytokine production, likely as a consequence of PI3K-dependent membrane association of the tyrosine kinase kinase, Btk, and activation of transcription factors. PI3K may also regulate the activation of sphingosine kinase (SK) and PLD, which produce the lipid mediators sphingosine-1-phosphate (S-1-P) and diacylglycerol (DAG), which also contribute to the critical calcium signal and PKC activation regulating mast cell mediator release. Concurrent with these events, the GTP exchangers Sos and Vav activate the Ras-Raf-MAPK pathway, which in turn contributes to the activation of specific transcription factors required for cytokine production. In addition, the MAPKs ERK1/2 control the activation of PLA2, which liberates arachidonic acid from membrane lipids for the subsequent generation of eicosanoids. NTAL appears to be a point of integration between the 2 pathways, providing an amplification mechanism also requiring PI3K and BTK, for the potentiation of antigen-dependent mediator release by SCF. Note that, although not depicted as such for clarity, many signaling events occur within specific microdomains within the cytosolic membrane. In addition, for clarity, several of the intermediary steps have been simplified and other pathways that may down-regulate mast cell activation have not been included. The figure and legend are provided courtesy of Alasdair M. Gilfillan (LAD/NIAID/NIH).
Two recent observations show that the functions of KIT and FcϵRI in mast cell biology are closely interrelated. The first evidence for this interrelationship is the observation that SCF enhances FcϵRI-mediated mast cell degranulation, and that the phosphorylation of NTAL/LAB/LAT2, a membrane adaptor molecule, was the crucial link between the signaling cascades following KIT activation and FcϵRI aggregation (Figure 2).75 Further, in vitro data from studies with mouse and human mast cells indicates that, under some circumstances, the binding of certain IgE antibodies to FcϵRI can promote proliferation of developing mast cells and mast cell survival.76
In addition to IgE and specific antigen, and in some cases IgG,77 a variety of biologic substances including products of complement activation, neuropeptides, bacterial products,45 cytokines, animal venom components, chemical agents, and physical stimuli may elicit the release of mast cell mediators. Morphine and other narcotics are among the pharmacologic agents that induce human mast cell mediator release, especially from skin mast cells. The responsiveness of specific populations of mast cells to individual stimuli varies, and some stimuli induce a pattern of mediator release that differs from the one associated with IgE-dependent mast cell activation.
The activation of mast cells following phagocytosis of bacteria and following activation by a variety of viral and bacterial products through TLRs had led to a growing recognition that mast cells offer a protective function through their innate immune functions. For example, mast cells perform a critical protective role in a mouse model of acute septic peritonitis involving mast cell–derived TNF.78 SCF enhances this innate immunity through effects on mast cells.79
Diseases of abnormal mast cell proliferation
As might be expected, given the complexity of the control of mast cell numbers, there is the potential for disturbances to arise that lead to a pathologic increase in mast cells within tissues, with attendant consequences both systemically and locally from the excess mast cell burden and the associated mast cell mediators. Such disorders are categorized under the general term “mastocytosis.” Clinical features of diseases of pathologic mast cell proliferation include pruritus, flushing, nausea, vomiting, diarrhea, abdominal pain, and vascular instability. Their most remarkable pathologic features are abnormal accumulations of mast cells in the skin, gastrointestinal (GI) tract, bone marrow, liver, spleen, and lymph nodes, and the frequent association of this increase in mast cell numbers with hematologic disorders. Mastocytosis may occur at any age. The exact prevalence of mastocytosis and its variants is unknown, and familial occurrence is unusual.
Variants of disease are now believed to be in part dependent on specific inherited genetic polymorphisms and acquired somatic mutations, and are categorized under cutaneous and systemic mastocytosis. The complexity of this classification system increases as data accumulate about clinical patterns of disease and associated genetic abnormalities. For example, a consensus conference in 1990 recognized 4 patterns of disease determined on the basis of severity and prognosis.80 A 2000 consensus conference added subclassifications to cutaneous mastocytosis and to systemic mastocytosis and set criteria for diagnosis.18,19
Pathogenesis of mastocytosis
The recognition of SCF as the principal human mast cell growth factor led to early studies to determine whether SCF was elevated in patients with mastocytosis. While there was some early indication that SCF was elevated in skin lesions associated with mastocytosis,81 later studies on SCF levels in skin and blood did not support this hypothesis, at least for adult patients.82 Rather, groups turned their attention to KIT, the SCF receptor.
In 1995, Nagata et al identified a point mutation consisting of a substitution of valine for aspartic acid in the catalytic domain of c-KIT (ASP816VAL or D816V) in the peripheral blood of 4 of 4 patients with mastocytosis with predominately myelodysplastic features.83 The next year, the same mutation was identified in a patient with urticaria pigmentosa and aggressive systemic mastocytosis in both skin and spleen.84 This mutation was one of 2 mutations identified in the HMC-1 cell line cultured from a patient with mast cell leukemia85 and known to be an activating mutation. It is now accepted that the majority of adults with mastocytosis have the D816V mutation if bone marrow mononuclear cells are examined. With time and disease severity, and in a subset of patients, the clone expands sufficiently to be detected in peripheral blood. Some patients within this subset will develop the smoldering systemic mastocytosis variant. These observations support the concept that mastocytosis is in part a result of the presence of “overactive” KIT in adult patients with mastocytosis and in children with more severe patterns of disease, with other secondary or coexisting events giving rise to mastocytosis disease variants. There is no evidence yet that the ASP816VAL (D816V) mutation is passed from generation to generation.
Additional c-KIT mutations have been identified that may play a role in the etiology of mastocytosis. These mutations consist of V560G within the juxtamembrane domain of KIT detected in the human mast cell leukemia (HMC) cell line HMC-1; D816Y, D816F, and D816H; the E839K dominant inactivating mutation in several reported cases of pediatric mastocytosis; and the rare germ-line mutation F522C. Exceedingly rare c-KIT mutations that are reported to be present in less than 1% of patients with mastocytosis include the following: R815K, D820G, V533D, V559A, del419, K509I, and A533D.86,87 It appears that mast cell proliferative disorders associated with mutations in c-KIT may be modified by the genetic composition of the effected individual. For example, a polymorphism in the gene for the IL-4 receptor alpha chain (Q576R) resulting in a gain of function has been shown to be associated with less extensive mast cell involvement, with disease usually localized to the skin.88 Here, the hypothesis is that because addition of IL-4 early to human mast cell cultures decreases the number of mast cells by down-regulating KIT expression,59 a polymorphism in the IL-4 receptor resulting in increased signaling through that receptor might limit the consequences of an “overactive” SCF receptor.
In addition to KIT-dependent pathways, inhibition of mast cell apoptosis through other biologic mechanisms may also lead to mastocytosis. For example, we have recently described an unusual case of a patient presenting with peripheral basophilia and systemic mastocytosis where cytogenetics revealed a t(4;5)(q21.1;q31.3) involving PDGFRβ. Molecular analyses revealed that PDGFRβ, encoding an imatinib-sensitive tyrosine kinase, was fused to PRKG2. Functional studies confirmed that the activity and transforming properties of PRKG2-PDGFRβ were dependent on the disruption of the autoinhibitory juxtamembrane domain.89
As an increase in mast cell numbers is not limited to mastocytosis, confusion may arise in diagnosis and selection of therapy. For example, some patients with hypereosinophilic syndrome who carry the Fip1-like-1–platelet-derived growth factor receptor (FIP1L1-PDGFRα) fusion protein resulting from an approximately 800-kb interstitial deletion of chromosome 4q12 and thus have chronic eosinophilic leukemia (CEL) also have increased mast cells and an increase in serum tryptase levels. The sometimes overlapping clinical manifestations of D816V KIT-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRα-associated CEL, coupled with the increase in activated eosinophils and mast cells seen in both disorders, have led to confusion in the nomenclature. It is of paramount importance, however, to distinguish between these 2 groups of patients because of differences in clinical sequelae, prognoses, and selection of treatment. Such patients can be differentiated by identification of either a mutation in c-KIT or identification of the fusion protein. Clinical criteria are also available to distinguish these disorders.90
Mastocytosis variants
Diseases of pathologic mast cell proliferation are classified into disease variants based on clinical presentation, pathologic findings, and prognosis. The contemporary classification system for mastocytosis is shown in Table 2.18,19 Patients with cutaneous mastocytosis (CM) have the best prognosis, followed by those with indolent systemic mastocytosis (ISM) and 2 subvariants of SM termed bone marrow mastocytosis and smoldering mastocytosis. Patients with systemic mastocytosis with an associated clonal hematologic non–mast cell lineage disease (SM-AHNMD), aggressive systemic mastocytosis (ASM), or mast cell leukemia (MCL) experience more rapid and complex courses. Patients with CM or ISM may experience progressive difficulties, but their condition may be managed for decades with medications that offer largely symptomatic therapy. In SM-AHNMD, examination of the peripheral blood and bone marrow leads to the diagnosis of one of several hematologic disorders; survival of these patients is determined by the course of the hematologic disorder. Patients with ASM or MCL have a poor prognosis, as do those with MCS. Patients with ASM experience a rapid increase in the number of mast cells. Medical management may be difficult, and prognosis is less favorable. MCL is rare, with the most fulminant behavior. In this form of mast cell disease, numerous immature mast cells are found in peripheral blood smears. MCL is distinguished from the other disease types by its clinical and pathologic picture. MCS is exceedingly rare, and a leukemia phase may occur.
WHO systemic mastocytosis variants
| Variant term . | Subvariants . |
|---|---|
| Cutaneous mastocytosis (CM) | Urticaria pigmentosa (UP), maculopapular CM (MPCM), diffuse CM (DCM), mastocytoma of skin |
| Indolent systemic mastocytosis (ISM) | Smoldering SM, isolated bone marrow mastocytosis |
| Systemic mastocytosis with an associated clonal hematologic non–mast cell lineage disease (SM-AHNMD) | SM-AML, SM-MDS, SM-MPD, SM-CMML, SM-NHL |
| Aggressive systemic mastocytosis (ASM) | |
| Mast cell leukemia (MCL) | Aleukemic MCL |
| Mast cell sarcoma | |
| Extracutaneous mastocytoma |
| Variant term . | Subvariants . |
|---|---|
| Cutaneous mastocytosis (CM) | Urticaria pigmentosa (UP), maculopapular CM (MPCM), diffuse CM (DCM), mastocytoma of skin |
| Indolent systemic mastocytosis (ISM) | Smoldering SM, isolated bone marrow mastocytosis |
| Systemic mastocytosis with an associated clonal hematologic non–mast cell lineage disease (SM-AHNMD) | SM-AML, SM-MDS, SM-MPD, SM-CMML, SM-NHL |
| Aggressive systemic mastocytosis (ASM) | |
| Mast cell leukemia (MCL) | Aleukemic MCL |
| Mast cell sarcoma | |
| Extracutaneous mastocytoma |
Hematologic findings
The bone marrow, spleen, liver, and lymph nodes have been recognized as the most common sites of pathologic mast cell infiltrates in systemic mastocytosis. Since the first description of the systemic nature of mastocytosis,16 information accumulated that mastocytosis is often associated with hematologic disorders. And with evidence that human mast cells were derived from a bone marrow hematopoietic progenitor in the late 1980s,49,51 investigators around the same time began to conclude that mastocytosis might represent a myeloproliferative disorder.91 The bone marrow is currently thus recognized as the most useful biopsy site, and is required for establishing the pathologic diagnosis and for staging, and should include inspection of the aspirate. Examination of the bone marrow both reveals diagnostic infiltrates and allows study of the hematopoietic marrow, which provides important prognostic information. Immunohistochemical staining of the bone marrow biopsy with antitryptase used to characterize mast cells into subsets31 is now the method of choice to visualize mast cells in paraffin-embedded decalcified specimens.
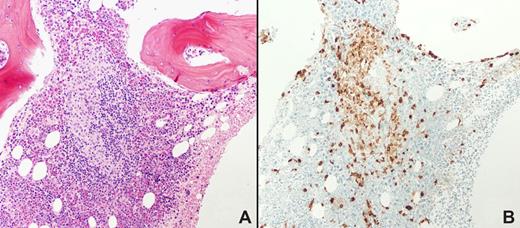
The morphologic appearance of mastocytosis-related bone marrow infiltrates in trephine core biopsy sections is distinctive. The majority of infiltrates are focal (Figure 3), although the lesions may be diffuse. Focal mast cell lesions are most commonly situated paratrabecularly, followed by perivascular and parafollicular distributions. Focal aggregates of spindle-shaped mast cells are often accompanied by lymphocytes and eosinophils. In the case of patients with tryptase-positive round cell infiltrates where the infiltrates comprise greater than 95% round cells and less than 5% spindle-shaped cells, application of additional immunohistochemistry markers to confirm the diagnosis of mastocytosis should be applied (eg, CD34, CD117, and the basophil-specific antibodies 2D7 or BB1), since basophils and sometimes blast cells also express tryptase. Bone marrow lesions are cellular in the early stages of mastocytosis. As the disease progresses, the number of mast cells may decrease and the lesions may become fibrotic. Mastocytosis infiltrates in the bone marrow may be associated with osteosclerotic or osteolytic changes in the bone trabeculae. The report that flow cytometry could be used to determine the coexpression of CD2 and/or CD25 in CD117 (KIT)–positive mast cells by flow cytometry of bone marrow aspirates, and that these markers revealed neoplastic mast cells, further refined the diagnostic options for mastocytosis.92 Such markers can now also be detected using immunohistochemistry.93
Bone marrow biopsy from a patient with systemic mastocytosis. Hematoxylin and eosin staining is shown in panel A, tryptase staining in panel B. Magnification ×100. Note the focal collections of mast cells, major criteria for the diagnosis of systemic mastocytosis. Courtesy of Irina Maric (NIH/Clinical Center, Department of Laboratory Medicine). Images were obtained via digital microscopy using an Olympus BH-2 microscope (Olympus, Melville, NY) equipped with a DPlan 10×/0.65 NA objective. Images were captured using an Olympus DP12 digital camera system and recorded on a 3.3V SmartMedia SSFDC card. Imaging software was Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA).
Bone marrow biopsy from a patient with systemic mastocytosis. Hematoxylin and eosin staining is shown in panel A, tryptase staining in panel B. Magnification ×100. Note the focal collections of mast cells, major criteria for the diagnosis of systemic mastocytosis. Courtesy of Irina Maric (NIH/Clinical Center, Department of Laboratory Medicine). Images were obtained via digital microscopy using an Olympus BH-2 microscope (Olympus, Melville, NY) equipped with a DPlan 10×/0.65 NA objective. Images were captured using an Olympus DP12 digital camera system and recorded on a 3.3V SmartMedia SSFDC card. Imaging software was Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA).
MCL is the leukemic variant of SM, defined by 20% or more atypical mast cells in bone marrow smears. In typical cases, mast cells in the peripheral blood account for more than 10% of leukocytes, but may be lower. Other forms of systemic mastocytosis may progress into a terminal leukemic phase that is indistinguishable from MCL and has the same prognosis.18,19,94
Cytologic abnormalities in hematopoietic cells, as well as mast cells, may be detected in bone marrow aspirates.95 Evidence of dysplastic or neoplastic hematopoietic cells form the basis in most cases for the classification of the associated hematologic disorders. Atypical or poorly differentiated mast cells may be identified in the bone marrow aspirates of patients with MCL and with aggressive forms of mastocytosis.18,19 The cytoplasmic granules of atypical mast cells are often very fine and smaller in number compared with the coarsely granular mast cells from patients with the indolent form of mastocytosis. Nuclear mast cell atypia may take the form of lobated nuclei and have binucleation or multinucleation and mitotic figures. Mitoses are rarely seen in mast cells, even in aggressive forms of mastocytosis, except in patients with MCL.
The relative number of mast cells in the bone marrow aspirate in contrast to the number in the bone marrow biopsy is not always a useful measure of pathologic mastocytosis infiltrates. In some cases, few mast cells are found in the bone marrow aspirate despite evidence of mastocytosis in trephine core biopsy sections because of the increased reticulin associated with the infiltrates in the marrow itself. Mast cell hyperplasia in bone marrow can also be found in conditions other than systemic mast cell disease, including uremia, osteoporosis, and hematologic conditions (lymphomas, preleukemias, and leukemias).
Treatment of mastocytosis
Treatment of mastocytosis is based on amelioration of symptoms with pharmacologic agents that inhibit the actions of mediators identified as mast cell derived, especially histamine and the leukotrienes; treatment of gastric hypersecretion with H2-antihistamines and proton pump inhibitors; treatment of osteoporosis with calcium, vitamin D, and bisphosphonates; treatment of associated inflammation with glucocorticoids; treatment of systemic anaphylaxis-like reactions with epinephrine; and with strategies to treat an associated hematologic disorder or decrease the mast cell compartment.96,97 So far, no standard therapy for patients with aggressive forms of disease has been defined.
IFN-α shows variable efficacy in patients with aggressive mastocytosis,98 and is often combined with glucocorticoids.99 The current recommendation is to restrict use of IFN-α to patients with slowly progressing ASM or smoldering SM with a high mast cell burden or with severe side effects, such as collapse of vertebral bodies associated with severe osteoporosis. The formidable chronic side effects including fever, myalgias, bone pain, anorexia, depression, and fatigue can be an important limitation to the use of this agent.
Case reports have documented that cladribine or 2-CDA may induce clinical remissions in patients with more aggressive forms of mastocytosis.100 In a case series of 9 patients who received 6 courses of therapy, all 9 showed responses concerning symptoms and decreases in serum tryptase but none achieved a complete response.101 Side effects were mostly related to bone marrow suppression. Cladribine may thus offer a reasonable therapeutic approach in treating those patients with aggressive forms of mastocytosis who have IFN-α–resistant advanced disease. Nevertheless, cladribine may induce pancytopenia and immunosuppression, and its potential oncogenicity remains largely unknown. Thus its use in systemic mastocytosis should be approached with caution.
A variety of chemotherapeutic regimens have been used in attempts to achieve control of symptoms and to control disease progression in ASM and SM-AHNMD and MCL as reviewed elsewhere.96,97 Key to the treatment of SM-AHNMD is selection of treatment according to the detection of additional specific targets and recognition of the status and grade of the SM component. In general, patient responses depend on the prognosis of the AHNMD and most remissions are only partial and short-lived. Cytoreductive drugs that have been used include cytosine arabinoside (ARA-C), doxorubicin, daunorubicin, and vincristine, alone or in combination.
Imatinib is a tyrosine kinase inhibitor that inhibits the kinase activity of c-abl, bcr-abl, and platelet-derived growth factor receptor tyrosine kinases as well as KIT. Imatinib has proved effective for treatment of chronic phase chronic myelogenous leukemia, myeloproliferative HES, and most GIST patients who harbor c-KIT mutations. The sensitivity of KIT mutants to imatinib depends on the mutation. Imatinib has been shown to suppress proliferation of an HMC-1 human mast cell line carrying the wild-type codon at 816 but not the mutated 816 codon.102 Growth of COS cells expressing wild-type KIT was inhibited by imatinib at 0.1 μM, but doses of imatinib as high as 10 μM did not suppress growth of COS cells expressing D816V mutated KIT.103 Imatinib was reported to result in histologic or clinical responses in 5 of 12 patients with systemic mastocytosis.104 Although these patients were reported to lack a mutation in c-KIT at codon 816 or a juxtamembrane mutation, it is not clear whether they had any distinguishing histopathologic changes in their bone marrow mast cells or carried other c-KIT mutations. Three of these patients showed dramatic and complete responses and had peripheral blood eosinophilia and other characteristics of patients with MP-HES, but the FIP1L1/PDGFRα fusion gene associated with imatinib-responsive MP-HES had not been described at the time of treatment of this case series. Additional limited clinical data corroborate in vitro studies suggesting that imatinib will prove to be ineffective in the majority of patients with SM associated with c-KIT D816V mutations.105 Imatinib, however, has been shown to dramatically decrease the bone marrow mast cell burden, serum tryptase level, and clinical symptoms in a patient with the (F522C) novel mutation in the transmembrane region of KIT106 and in a patient with systemic mastocytosis with chronic basophilic leukemia and a PRKG2-PDGFRβ fusion.89
Splenectomy has been used in the management of patients with ASM or SM-AHNMD.107 Massive splenomegaly, especially if associated with hypersplenism or portal hypertension, may be an indication for splenectomy. In these patients, splenectomy decreases the mast cell burden and often improves cytopenias, facilitating the subsequent use of myelosuppressive agents such as interferon-alpha (IFN-α) or chemotherapy, if necessary. Nevertheless, it should be considered a high-risk surgical procedure.
Bone marrow transplantation is reserved for those patients with advanced and potentially fatal forms of SM-AHNMD, MCL, and ASM. There is limited experience with this procedure in this patient population and the response has ranged from favorable with engraftment and prolongation of life, to poor in patients with advance disease, with secondary complications such as graft-versus-host disease and death from progressive disease or infection.108 Nonmyeloablative bone marrow transplantation has been performed in select patients with no apparent long-term success to date.109
Reports of resistance of kinase domain codon D816V mutations to imatinib have prompted numerous in vitro studies designed to assay the efficacy of novel tyrosine kinase inhibitors. PKC412 is a novel staurosporine-derived tyrosine kinase inhibitor that inhibits autophosphorylation of KIT. The clinical efficacy of PKC412 was assessed in a patient with MCL with an associated myelodysplastic/myeloproliferative disorder and who was c-KIT D816V positive.110 The patient exhibited a partial response, but died after 3 months of therapy following development of a c-KIT D816V-negative AML, suggesting that clinical efficacy of PKC412 may be limited by clonal evolution in the advanced leukemic phase of this disease. Further studies aimed at assessing the clinical efficacy of this agent in aggressive systemic mastocytosis are in progress. Similar clinical studies are in progress or in the planning stage with BMS-354825 (dasatinib), AMN107, and other agents including monoclonal antibodies and protein trafficking and protein function regulators (such as 17-AAG). Given the experience with PKC-412 and with marrow transplantation, most in the field believe the treatment of aggressive mastocytosis will move to a multiple drug approach using combination therapy with targeted agents that have different mechanisms of action.
Conclusions
The first histologic description of mast cells in the late 19th century was followed in the first half of the 20th century by a gradual recognition that mast cells were a source of histamine and heparin; were involved in allergic responses; and that their pathologic proliferation caused a systemic disease, that of mastocytosis. However, it was not until the late 20th century and the identification of mast cell growth factors that mast cell research truly accelerated. Using cell lines, primary mast cell cultures, and animal models of allergic inflammation and infectious diseases, mast cells were then clearly implicated in both acquired and innate immunity. The ability to culture human mast cells allowed the study of the relationship of human mast cells to other lineages, and elucidation of specific growth factors and cytokines that regulate their responsiveness, and the specific contributions of mast cells to a variety of infections and autoimmune conditions. Much, however, remains to be explored. Challenges such as how to regulate mast cell responsiveness or how to selectively ablate the mast cell compartment remain. Given the techniques now available to gain insight into mast cell function, these are areas that should yield to further study and then for the first time truly allow the development and application of approaches to modifying mast cell function in both health and disease.
Acknowledgment
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: D.D.M. wrote the paper.
Conflict-of-interest disclosure: D.D.M. is the Chief of the Laboratory of Allergic Diseases, NIAID. The author declares no competing financial interests.
Correspondence: Dean D. Metcalfe, Laboratory of Allergic Diseases, NIAID, NIH, Bldg 10/11C207, 10 Center Dr, MSC1881, Bethesda, MD 20892-1881; e-mail: dmetcalfe@niaid.nih.gov.