ADAMTS13, the metalloprotease that regulates the size of von Willebrand factor (VWF),1 is critical in preventing microvascular platelet clumping, such as occurs in thrombotic thrombocytopenic purpura. The article in this issue of Blood by Gao et al addresses how these two proteins use multiple binding sites to facilitate proteolytic regulation of VWF.
ADAMTS13 cleaves only a single bond, Tyr1605-Met1606, buried within the VWF A2 domain. Shear forces unravel the normally tightly packed globular VWF, exposing this cleavage site. How does ADAMTS13 position itself on this flexible and dynamic multimeric glycoprotein, in such a way that it remains in place throughout the shear-induced folding and unfolding that occurs during transit through microcirculatory beds?
Binding is known to take place directly on the A2 domain of VWF spatially adjacent to the cleavage site,2 but also on a more remote site of the A2 domain.3,4 There might be additional domain interaction sites on VWF. Complementary binding sites have been identified on ADAMTS13, certainly within its Spacer5 and Cys-rich regions,6 but also within the TSR/CUB domains,7 depending on shear conditions. Binding between each distinct remote site (exosite) ramps up the specificity constant of cleavage. According to Gao et al, collective exosite-driven proteolysis of the Tyr1605-Met1606 bond accounts for approximately 2 orders of magnitude increase in cleavage of a small VWF substrate.
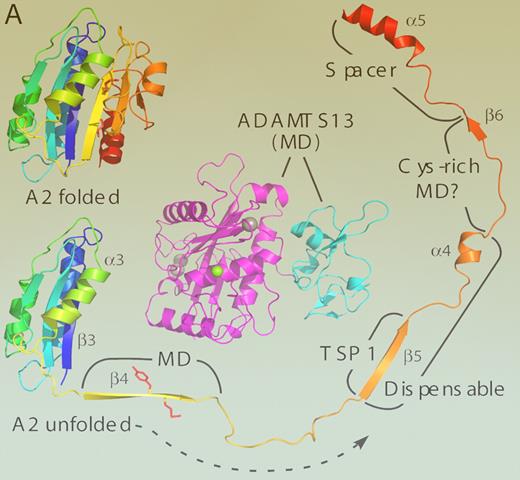
Remote exosite interactions are common in the hemostatic process and are exemplified by thrombin,8 which uses exosites to direct and enhance cleavage of multiple protein and cellular substrates. In order for ADAMTS13 to utilize a similar mechanism of exosite recognition to partner its conformationally-responsive substrate during its conformational dance to shear, this enhanced proteolysis also requires that the protease can retain its binding under changing shear conditions during its transit through vessels of different sizes. Gao et al introduce an important concept of portability of exosites. This can be seen in the figure. The A2 domain model is shown folded as a compact globular molecule with the cleavage site hidden within the structure. When unfolded, it presents several distinct interaction sites near the cleavage site and extending to its C-terminus. Flexibility in spatial relationships (portability) between interacting sites is provided and/or enhanced by a redundant (dispensable) sequence. If there is no fixed distance between exosite and protease cleavage site, then the 2 partner macromolecules can be tightly bound but move together, elongating when shear stress is high in the microcirculation, and contracting in length to globular form in larger vessels when shear stress is low. Such a rhythmic dance depends on mutual and multiple exosite engagements on dynamical structures that nevertheless precisely position the active site within the protease domain. This makes it poised to strike when the cleavage site is revealed by the shear-induced unfolding of the A2 domain.
A homology model of the ADAMTS13 metalloprotease (magenta) and disintegrin-like domains (cyan) is shown (MD) to provide a sense of scale, with active site Zn2+ ion (green) and 3 structural Ca2+ ions (gray) as spheres. The VWF A2 domain is predicted to consist of a 6-stranded β-sheet surrounded by 5 α-helices. Residues Tyr1605-Met1606 (side chains in red) are buried in strand β4. Exposure of this bond to ADAMTS13 requires substantial unfolding of domain A2; more distal segments that interact with specific domains of ADAMTS13 are labeled. The locations of these ADAMTS13 domains relative to the MD moiety are not known. Deletion of strand β5 through helix α4 (dispensable) has a minimal effect on the rate of substrate cleavage. Molecular graphics prepared with PyMOL (DeLano Scientific, Palo Alto, CA). See the complete figure in the article beginning on page 1713.
A homology model of the ADAMTS13 metalloprotease (magenta) and disintegrin-like domains (cyan) is shown (MD) to provide a sense of scale, with active site Zn2+ ion (green) and 3 structural Ca2+ ions (gray) as spheres. The VWF A2 domain is predicted to consist of a 6-stranded β-sheet surrounded by 5 α-helices. Residues Tyr1605-Met1606 (side chains in red) are buried in strand β4. Exposure of this bond to ADAMTS13 requires substantial unfolding of domain A2; more distal segments that interact with specific domains of ADAMTS13 are labeled. The locations of these ADAMTS13 domains relative to the MD moiety are not known. Deletion of strand β5 through helix α4 (dispensable) has a minimal effect on the rate of substrate cleavage. Molecular graphics prepared with PyMOL (DeLano Scientific, Palo Alto, CA). See the complete figure in the article beginning on page 1713.
Ultimately, then, such flexible proximity enables the catalytic center of ADAMTS13 to proteolyse the Tyr1605-Met1606 bond as it becomes exposed and thereby reduces the size and reactivity of the VWF polymer. The unique preference of ADAMTS13 for a single bond contrasts with that of thrombin, the latter being an example of exosite-enhanced (almost) promiscuous proteolysis. Numerous crystal structures have given insight into the mechanisms of functional specificity of thrombin. What precisely determines the unique cleavage specificity of ADAMTS13 remains to be determined.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal