Ramping up and maintaining a high rate of iron delivery to support heme biosynthesis is both unique and fundamental to erythroid maturation. In this issue, Zhu and colleagues reveal a previously unappreciated role of the cytokine-activated transcription factor STAT5A/B as a regulator of iron delivery through transcriptional activation of the Tfr1 gene.
The transferrin receptor gene (Tfr1) has previously been shown to be essential for iron delivery during erythroid development, with knockout mice dying at midgestation with severe anemia, and haplo-insufficient mice displaying a compensated microcytosis secondary to iron-deficient erythropoiesis.1 Similarly, the importance of STAT5A/B in erythropoiesis was demonstrated by whole animal knockout studies that generated severely anemic animals with perinatal lethality.2 However, revealing a direct role for STAT5A/B in iron metabolism through regulation of Tfr1 required a refinement of technique.
Zhu et al employed a conditional knockout strategy using a Tie2-Cre transgene (known to be active in hematopoietic stem cells) to produce a hematopoietic cell-specific knockout of both Stat5 isoforms. The resultant animals (designated Stat5a/bf/f:TC) were born at the expected Mendelian ratio with a hematocrit of approximately 25% compared with 47% in control animals, with the anemia persisting into adulthood. Red-cell morphology in Stat5a/bf/f:TC animals revealed microcytosis and hypochromia. Decreased peripheral red- cell numbers and lack of a compensatory erythrocytosis in marrow was suggestive of iron-deficient erythropoiesis. Serum and tissue iron studies revealed that Stat5a/bf/f:TC mice have elevated transferrin saturation, elevated serum iron, and increased nonheme iron content in the liver. Having ruled out iron deficiency, Zhu et al searched for a mechanism impacting erythroid iron assimilation.
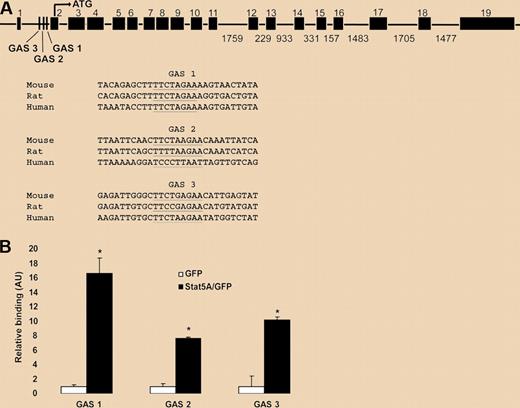
Flow cytometric comparison of Stat5a/bf/f:TC and control reticulocytes revealed a 50% decrease in Tfr1 protein on the surface of mutant cells. Parallel analyses of Tfr1 mRNA expression in fetal liver cells, marrow and spleen using microarray, and qPCR all pointed to reduced transcription in Stat5a/bf/f:TC cells by 30% to 80% when compared with control cells. Zhu et al then tackled the question of whether STAT5 directly regulates transcription of the Tfr1 gene. They first demonstrated that expression of a constitutively active STAT5A construct in the murine erythroleukemia (MEL) cell line resulted in a 2-fold increase in Tfr1 expression. Prior analyses of transcriptional control of Tfr1 had defined a promoter region upstream of the first exon that includes a hypoxia response element and binding sites for AP1, CREB/ATF, and Ets family transcription factors. A search for Stat binding sites–GAS motifs (for interferon Gamma-Activated Sequence)–identified 3 consensus elements within the first intron of Tfr1. Chromatin immunoprecipitation experiments in MEL cells using a constitutively active STAT5A protein demonstrated specific binding to the intronic GAS elements, as displayed in the figure. Zhu et al conclude that Stat5a/b controls erythropoiesis in part through direct regulation of Tfr1 transcription.
STAT5A/B binding sites in a putative Tfr1 enhancer. (A) Putative STAT5A/B binding (GAS) sites within intron 1 of the Tfr1 gene (underlined). Three conserved GAS sites were identified with GAS 1 having the highest interspecies conservation. Sites are shown aligned with sequences from other species (www.ensembl.org/index.html). (B) Chromatin immunoprecipitation analysis of binding of active STAT5A to the putative GAS sites within intron 1 of Tfr1. See the complete figure in the article beginning on page 2071.
STAT5A/B binding sites in a putative Tfr1 enhancer. (A) Putative STAT5A/B binding (GAS) sites within intron 1 of the Tfr1 gene (underlined). Three conserved GAS sites were identified with GAS 1 having the highest interspecies conservation. Sites are shown aligned with sequences from other species (www.ensembl.org/index.html). (B) Chromatin immunoprecipitation analysis of binding of active STAT5A to the putative GAS sites within intron 1 of Tfr1. See the complete figure in the article beginning on page 2071.
The microcytosis observed in Stat5a/bf/f:TC mice is similar to that seen in mice haplo-insufficient for Tfr1: in agreement with the approximately 50% decrease in Tfr1 expression reported by Zhu et al. However, the inability of Stat5a/bf/f:TC hemopoietic cells to mount a hyperplastic erythroid response distinguishes between these lesions and points to additional functions of STAT5A/B in erythroid development. Previous work had demonstrated that erythroid progenitors from a separately derived line of Stat5a/b null mice had impaired survival, in part because of reduced expression of the antiapoptotic protein, Bcl-xL.3 In the current study, there is evidence of increased apoptosis in cells lacking STAT5A/B (but little change in Bcl-xL levels). This suggests that ineffective erythropoiesis and impaired iron delivery are both important in the development of anemia in Stat5a/bf/f:TC mice.
The finding of STAT5 responsive GAS elements in the first intron of Tfr1 has implications beyond erythropoiesis. Iron delivery is critical to maintain active cell division, and Tfr1 expression is increased across a wide range of cancer cells. Previous work had demonstrated that the oncogenic transcription factor c-Myc activates Tfr1 transcription through a conserved c-Myc binding site also located within the first intron of the Tfr-1 gene.4 Having elegantly demonstrated a role for STAT5 in regulation of Tfr1 expression in erythroid cells, Zhu et al leave us to ponder the role of STAT5 in GASing up other cell types that require iron for proliferation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal