Abstract
Follicular lymphoma (FL) is a B-cell malignancy characterized by the t(14;18) translocation. Although sensitive to treatment, the disease remains incurable and the reason why tumor cells invariably evade treatment, leading to clinical relapse, is still unknown. Here, we tracked the clonal history of tumor cells by studying mutations introduced by activation-induced cytidine deaminase on the switch μ region of the der(14)t(14;18) during the early phase of the class-switch recombination (CSR) process. We observed frequent intraclonal variations, suggesting that CSR often remains active after the acquisition of the fully transformed phenotype. However, mutations only rarely accumulated over time, but instead showed complex evolutionary scenarios and 2 different progression pathways. The first pathway was a direct and rapid evolution from the dominant clone. The second was indirect, arising from earlier subclones usually after years of remission. A better understanding of these mechanisms might influence the future choice of treatment strategies.
Introduction
Follicular lymphoma (FL) is the most frequent indolent non-Hodgkin B-cell lymphoma. This disease is usually sensitive to therapeutic agents but, paradoxically, most often remains incurable. Its clinical course is marked by recurrent relapses, suggesting that a few FL cells invariably escape treatment. If clinical remission can often be reached, t(14;18)-positive cells persist in the peripheral blood (PB) and bone marrow (BM) during remissions, accounting for the so-called residual disease.1 A better understanding of the clonal history of these cells may help us to understand how they survive therapies.
Clonal evolution in FL was addressed mainly through analysis of the mutations that accumulate in the variable regions of the immunoglobulin heavy chain genes (IgV) in germinal centers (GCs).2-10 Frequent intraclonal variations (ICVs) were initially reported in lymph node (LN) biopsies, suggesting that an antigen-driven selection process may remain active in transformed cells and participate in diversification and progression.2-4 However, this hypothesis was challenged when IgV mutations were compared between diagnosis and relapse. These studies showed that the mutation pattern was not always compatible with linear evolution, and that relapse could arise through the evolution of pre-existing minor subclones.10-12 Unfortunately, as the IgV regions of the rare t(14;18) cells that circulate in PB and BM are difficult to characterize, only LN and a few BM samples with massive involvement were analyzed, giving a limited view of the clonal history of the tumor.
The class-switch recombination process (CSR) is the second physiologic mechanism that somatically modifies the IGH loci in GCs. By reorganizing the constant genes (Cg's), CSR enables B cells to express a different isotype. In FL, the Cg's often show extensive and aberrant rearrangements, suggesting major episodes of CSR activity during lymphomagenesis.13,14 As for somatic hypermutation (SHM), the initial analysis of relatively few tumors suggested that CSR may remain ongoing in fully transformed cells and participate in progression.5,14 However, this hypothesis was challenged when only one case showing convincing evidence for secondary Cg reorganization was identified in a large series of patients.11 Furthermore, even if, in this case, CSR had remained active after the acquisition of the fully transformed phenotype, several features suggested that it was no longer ongoing in the studied sample.15 Due to this stability, CSR does not appear to make a suitable marker for following clonal evolution in this pathology. However, reorganization of the Cg is only the final step in a complex cascade of molecular events. As in SHM, an early step of CSR is controlled by activation-induced cytidine deaminase (AID).16 AID introduces mutations at the 5′ end of the switch μ region (Sμ) that precede but do not necessarily lead to a reorganization of the Cg. Although mutations in the Sμ share several features with SHM mutations, such as a targeting bias to G/C bases and to RGYW motifs, they accumulate independently and are most probably not part of the same process. Each mechanism would require the recruitment of specific AID cofactors that would target mutations specifically to one or the other region.17
In a recent report, we showed that, in agreement with their GC origin, most FL cells are mutated on the Sμ on both alleles.18 In the present study, we used these mutations to follow clonal evolution during progression. Using a polymerase chain reaction (PCR) strategy that specifically targets the der(14)t(14;18), we were able to analyze biologic samples in which only a few lymphoma cells were present. Applied to a large series of patients, this approach allowed us to compare samples obtained both from different tumoral compartments and from different time points during the course of the disease. Our data further demonstrate that FL is a dynamic oligoclonal disease and that tumor progression is often complex and far from linear.
Methods
Patient material
The t(14;18)+ tumors were selected from the files of the Department of Hematology, Center Henri Becquerel (Rouen, France). This study was approved by the institutional review board of our institution, and all biologic material was obtained after patient's consent. Informed consent was obtained in accordance with the Declaration of Helsinki. Patients were selected based on the availability of genomic DNA obtained from any biologic sample at any time point during lymphoma history. All samples had previously tested positive for the amplification of a BCL2-IGH junction using standard methods.19 The 5′Sμ region of the translocated der(14)t(14;18) was specifically amplified as described in Figure 1. To allow for comparisons, at least 2 independent positive samples were necessary for the inclusion of a patient. Fifty cases were included, with a total of 172 biologic samples. Forty-eight patients suffered from FL at diagnosis and 2 (nos. 44 and 153) from diffuse large B-cell lymphoma.
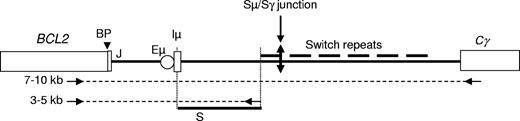
Schematic representation of the der(14)t(14;18). represents the amplified regions and arrows represent the oligonucleotides used for PCR amplifications and sequence reactions. PCR product lengths varied from 3 to 10 kb, depending on the location of the breakpoint on both the BCL2 and IGH loci and on the downstream oligonucleotide. The studied region is shown as a bold line (S). Iμ indicates Iμ exon; Eμ, IGH intronic enhancer; Cγ, constant γ gene; BP, t(14;18) breakpoint; and J, joining element.
represents the amplified regions and arrows represent the oligonucleotides used for PCR amplifications and sequence reactions. PCR product lengths varied from 3 to 10 kb, depending on the location of the breakpoint on both the BCL2 and IGH loci and on the downstream oligonucleotide. The studied region is shown as a bold line (S). Iμ indicates Iμ exon; Eμ, IGH intronic enhancer; Cγ, constant γ gene; BP, t(14;18) breakpoint; and J, joining element.
Schematic representation of the der(14)t(14;18). represents the amplified regions and arrows represent the oligonucleotides used for PCR amplifications and sequence reactions. PCR product lengths varied from 3 to 10 kb, depending on the location of the breakpoint on both the BCL2 and IGH loci and on the downstream oligonucleotide. The studied region is shown as a bold line (S). Iμ indicates Iμ exon; Eμ, IGH intronic enhancer; Cγ, constant γ gene; BP, t(14;18) breakpoint; and J, joining element.
represents the amplified regions and arrows represent the oligonucleotides used for PCR amplifications and sequence reactions. PCR product lengths varied from 3 to 10 kb, depending on the location of the breakpoint on both the BCL2 and IGH loci and on the downstream oligonucleotide. The studied region is shown as a bold line (S). Iμ indicates Iμ exon; Eμ, IGH intronic enhancer; Cγ, constant γ gene; BP, t(14;18) breakpoint; and J, joining element.
Genomic DNA isolations
Genomic DNA was obtained either from fresh biologic samples or from frozen biopsy sections, and extracted with a standard method using proteinase K followed by a salting-out procedure and ethanol precipitation.
Genomic PCR and sequence reactions
Polymerase chain reactions (PCRs) were performed using the 2x Extensor Hi-Fidelity PCR master Mix, Reddy Mix version (ABgene, Epsom, United Kingdom), in a volume of 50 μL with 10 pmol of each primer and 100 ng genomic DNA. The PCR protocol was 94°C for 2 minutes, 35 cycles of amplification (94°C for 10 seconds, 60°C for 45 seconds, and 68°C for 4 minutes), and 68°C for 4 minutes. Products were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA) and were directly sequenced using an ABI PRISM 3130 genetic analyzer (PE Applied Biosystems, Foster City, CA). For each patient, PCR amplification was performed with a couple of primers that surround the BCL2-IGH junction. As the location of the breakpoints on the BCL2 gene varies among cases, 6 forward BCL2 primers were used (BCL2.1: GACCAGCAGATTCAAATCTATGG; BCL2.2: CCTTCTGAAAGAAACGAAAGCA; BCL2.3: GACTCCCTTACGTGCTGGTACC; BCL2.4: GCACCTGCTGGATACAACACTG; BCL2.5: GTAATGACTGGGGAGCAAATCTT; BCL2.6: ACTGGTTGGCGTGGTTTAGAG) in combination with a reverse primer on the Sμ region (SμR: ACTCAGATGGGCAAAACTGACCTAA) or on the Cγ genes (CγR: CTGAGTTCCACGACACCGTCA). Two primers were used for sequence reactions: SeqSμ1: TGCTGAAGACAGGACTGTGG; SeqSμ2: CCCATGCCTTCCAAAGCGATT.
Cloning of PCR products
PCR products were subcloned into pGEM-T EASy vector (Promega, Madison, WI). Recombinant plasmids were purified using the Qiaprep Spin Miniprep Kit (Qiagen).
Sequence analysis
Sequences were analyzed using the UCSC Blat alignment tool.20,21 The sequence origin (+1) was arbitrarily chosen at the 5′ end of the SeqIμ1 primer. This nucleotide is at position 87 328 844 in contig NT_026437.11 (Homo sapiens, Build 36.2). A high-fidelity enzyme was used to avoid PCR artifacts and to be confident in the identity of mutational changes. To remove the possibility that some may be due to PCR errors, the same experiment (PCR amplification and sequencing reaction) was repeated 5 times (patient no. 1129; LN; at diagnosis). No significant difference was noted (data not shown). One recurrent variation, 1067A>G, which corresponds to a known single nucleotide polymorphism (rs980847), was excluded from the analysis.
Cytotogenetic analysis
Statistical analysis
Statistical significance was assessed using the Mann-Whitney U test and the StatView v4.5 software package (Abacus Concepts, Piscataway, NJ).
Results
5′Sμ mutations in serial LNs
Due to the proximity to the BCL2-IGH breakpoint, the Sμ region on the translocated allele can be specifically amplified from t(14;18)-positive cells with a PCR anchored on either side of the hybrid locus (Figure 1). We first took advantage of this specificity to evaluate possible ongoing CSR activity during FL progression. If CSR participates significantly in the evolution of the tumoral population, the total number of mutations at the 5′ end of the Sμ should progressively increase over the course of the disease.10 To test this hypothesis, we first analyzed serial LN biopsies. A total of 67 LNs were analyzed, allowing us to compare at least 2 and up to 4 sequential biopsies for 30 patients (Figure 2). Intervals between the biopsies varied from 1 month to more than 21 years (median interval: 32 months). Consistent with our previous observations, mutations were found in all samples (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This result is in agreement with the GC origin of FL and confirms that, in most if not all cases, CSR is most probably activated either in pre-FL or in fully transformed cells.
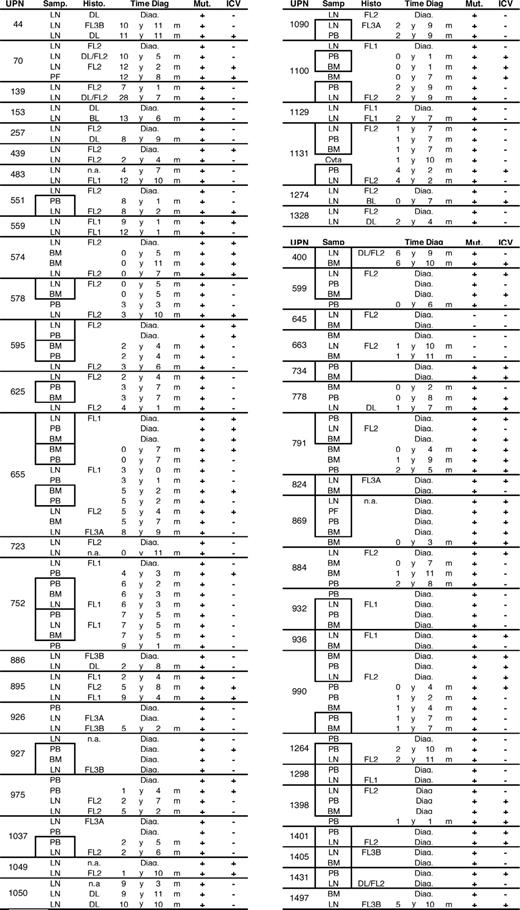
Biologic samples. Patients are identified by their unique patient number (UPN). For each sample, the type (LN indicates lymph node; PB, peripheral blood; BM, bone marrow; and PF, peritoneal fluid), the time from diagnosis (Time diag), and the presence (+) or absence (−) of somatic mutations (Mut) and intraclonal variations (ICVs) are given. Histo indicates LN histology. Paired samples obtained at the same time point (at time intervals shorter than 1 month) are boxed together. The table is divided into 2 groups, the first (patient nos. 44 to 1328) including the cases for which at least 2 LN biopsies were compared.
Biologic samples. Patients are identified by their unique patient number (UPN). For each sample, the type (LN indicates lymph node; PB, peripheral blood; BM, bone marrow; and PF, peritoneal fluid), the time from diagnosis (Time diag), and the presence (+) or absence (−) of somatic mutations (Mut) and intraclonal variations (ICVs) are given. Histo indicates LN histology. Paired samples obtained at the same time point (at time intervals shorter than 1 month) are boxed together. The table is divided into 2 groups, the first (patient nos. 44 to 1328) including the cases for which at least 2 LN biopsies were compared.
For all but 2 patients (nos. 559 and 895), mutations shared by all samples confirmed the clonal origin of the tumors. For the 2 exceptions, we sequenced the BCL2-IGH junctions. In both cases, the serial biopsies showed the same chromosomal breakpoint, confirming that the tumoral populations of the 2 LNs had the same clonal origin (data not shown). In conclusion, we found no evidence for the coexistence or succession of 2 clonally unrelated lymphomas in this series, even for patients who suffered very late relapses.
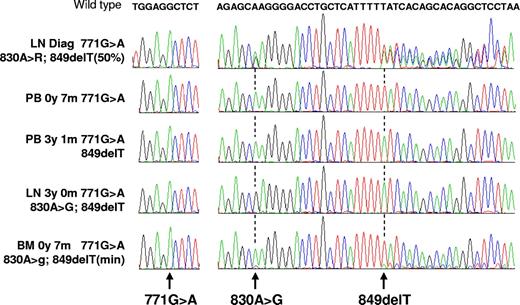
We observed frequent double peaks in the electropherogram sequence traces (from 16/67 LNs; 23.9%; Figure 3). To exclude possible PCR artifacts, we repeated all 16 amplifications and sequencing reactions. Similar patterns were obtained in all cases (data not shown). For 3 patients, identical double peaks were observed with different biopsies (nos. 574; 655; and 895). In addition, as illustrated in Figure 3 with patient no. 655, double peaks sometimes appeared as mutations when we analyzed other biologic samples obtained from different tumoral compartments (as detailed in “Comparison of the 5′Sμ mutations in different tumoral compartments”) or at different time points during progression. Together, these data suggest that double peaks could reflect the existence of clonally related subpopulations with different mutation patterns. To confirm this finding, PCR products from 2 LNs (patient no. 655 [pair no. 1] and patient no. 990) were subcloned and sequenced. In both cases, we were able to isolate subclones that differed by the mutations that corresponded to the double peaks (Figures S1,S2). Furthermore, additional mutations were identified in individual subclones, suggesting a high degree of ICV in the tumoral population and thus a significant ongoing accumulation of mutation in the Sμ. We therefore conclude that clonally related FL cells with different mutation patterns often coproliferate in LNs, suggesting that, as for SHM, an ongoing CSR activity significantly participates in FL cell diversification after the acquisition of the fully transformed phenotype.
Sequencing experiments. Each peak on electropherograms represents one base (green indicates A; red, T; blue, C; and black, G). The wild-type sequence is indicated at the top. All experiments were performed from biologic samples obtained from the same patient (no. 655). For each, the nucleotides around position 771 and the 825 to 869 segment are shown. Each experiment is identified by the nature of the biologic sample (LN indicates lymph node; PB, peripheral blood; and BM, bone marrow) and by the time from diagnosis at which it was obtained (diag). The 771G>A mutation, shared by all samples, shows the clonality of the disease. From the 3 later samples, where different subclones dominate, it can be deduced that the 771G>A mutation was acquired first, followed by the 849del and by the 830A>G. The last example shows that the sensitivity of the technique allows for the detection of minor subclones.
Sequencing experiments. Each peak on electropherograms represents one base (green indicates A; red, T; blue, C; and black, G). The wild-type sequence is indicated at the top. All experiments were performed from biologic samples obtained from the same patient (no. 655). For each, the nucleotides around position 771 and the 825 to 869 segment are shown. Each experiment is identified by the nature of the biologic sample (LN indicates lymph node; PB, peripheral blood; and BM, bone marrow) and by the time from diagnosis at which it was obtained (diag). The 771G>A mutation, shared by all samples, shows the clonality of the disease. From the 3 later samples, where different subclones dominate, it can be deduced that the 771G>A mutation was acquired first, followed by the 849del and by the 830A>G. The last example shows that the sensitivity of the technique allows for the detection of minor subclones.
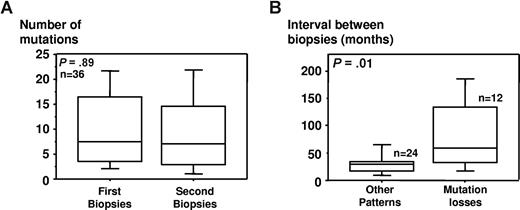
Despite this apparent ongoing CSR activity, our data did not show any significant increase of the total number of mutations during progression when we considered the whole population (370 vs 363 mutations in 36 paired biopsies; median values: 7.5 vs 7; P = .89; Figure 4A). Nevertheless, the analysis of serial samples in individual patients showed frequent variations over time, suggesting various evolutionary schemes. Interestingly, the simple appearance of new mutations was observed only once (no. 926). In this case, 6 and 8 mutations were seen in the first and second biopsies, respectively. For all other pairs, either the mutations had remained unchanged (15 cases: nos. 483; 574; 625; 723; 752; 886; 927; 975; 1037; 1050 [pair nos. 1 and 2]; 1090; 1100; 1129; and 1328), or they suggested more complex evolutionary scenarios.
Evolution of Sμ mutations over time in serial lymph node biopsies. Thirty-six pairs of serial biopsies were studied. One pair was excluded from the analysis as the second biopsy, obtained only 1 month after diagnosis, had not been motivated by a suspicion of transformation but rather to confirm histology (no. 927). (A) Comparison of the median number of mutations in serial lymph nodes (36 pairs). (B) Correlation between the evolution of the mutations and the interval between relapses. Pairs that did (12 pairs) or did not (24 pairs) show mutations losses are compared. Boxes show the range between the 25th and 75th percentiles, with a horizontal line at the median value. Whiskers extend to the 10th and 90th percentile of values.
Evolution of Sμ mutations over time in serial lymph node biopsies. Thirty-six pairs of serial biopsies were studied. One pair was excluded from the analysis as the second biopsy, obtained only 1 month after diagnosis, had not been motivated by a suspicion of transformation but rather to confirm histology (no. 927). (A) Comparison of the median number of mutations in serial lymph nodes (36 pairs). (B) Correlation between the evolution of the mutations and the interval between relapses. Pairs that did (12 pairs) or did not (24 pairs) show mutations losses are compared. Boxes show the range between the 25th and 75th percentiles, with a horizontal line at the median value. Whiskers extend to the 10th and 90th percentile of values.
In 2 cases, mutations seen in the first biopsy were lost in the second (nos. 257 and 752, pair no. 1), whereas in 10 cases we observed both losses and gains (nos. 44 [pair no. 1]; 70 [pair nos. 1 and 2]; 139; 153; 559; 895 [pair nos. 1 and 2]; 1131; and 1274). These losses indicate that the subclones that dominated the relapses had not evolved from those that dominated at the earlier time points, but instead from clonally related but less mutated subclones. Finally, for the last 9 pairs, the mutation patterns suggested complex selection processes. In 3 cases, ICV was seen in the first biopsy but not at relapse, suggesting a subclone selection process (nos. 655 [pair no. 1]; 439; and 595). In 3 cases, ICV was found in the second but not in the first biopsy, suggesting an ongoing diversification process (nos. 551; 44; and 578). Finally, the last 3 pairs showed complex evolutionary schemes with both selections and diversifications (nos. 1049; 655 [pair nos. 1 and 3]). Together, these data indicate that clonal evolution in FL is often not linear and that relapses from minor and less mutated subclones are frequent.
Correlation between the interval between biopsies and the evolution of mutations
For all 4 patients who presented with very late relapse, more than 10 years after diagnosis (nos. 44; 70; 139; and 153), the evolution of the mutations suggested a progression from a less mutated precursor. This observation led us to test a possible correlation between the evolution of the mutations and the interval between biopsies. This analysis revealed that the median interval was significantly longer in serial samples showing differences in mutation patterns (40.5 vs 29 months; P = .01), and confirmed that the time to progression was significantly higher for the pairs that showed mutation losses than for all other pairs (59.5 vs 30 months; P = .009; Figure 4B). In the same way, when mutations remained identical, the second biopsy had almost always been performed in less than 3 years (13/14 pairs, 92.9%). This data strikingly con-trast with serial biopsies obtained at intervals of 3 years or more in which significant differences were almost always seen (14/15 pairs, 93.3%).
Comparison of the 5′Sμ mutations in different tumoral compartments
The concept of relapse from an earlier precursor is reminiscent of the cancer stem cell theory,24 except that, in FL, the Sμ mutations shared by all subclones suggest that the progenitors would not be stem cells but rather mature t(14;18)-positive GC or post-GC B cells. It was recently shown that t(14;18)-positive cells in healthy individuals make an atypical population of post-GC FL-like cells.25 During the early phases of lymphomagenesis, before the acquisition of the fully transformed phenotype, these cells, like memory B cells, may be prone to intense trafficking and take advantage of niches where support for maintenance and proliferation may be provided by their microenvironment. Based on IgV analysis, other authors also showed that, in established FL, different subclones expanded in LN and BM.9,15,26 These observations raise the possibility that the tumoral cells that dominate at relapse may evolve from other tumoral compartments.
To test this hypothesis, we extended our approach to different disease sites. We first addressed whether Sμ mutations may distinguish different cell populations in LN, PB, and BM. For the 30 patients in the first series, we analyzed 24 PB, 15 BM, and 1 peritoneal fluid (PF). In addition, 20 additional patients, for whom no serial biopsies were available, were included. For these, 19 LN, 21 PB, 23 BM, and 1 PF were analyzed.
In the whole series, we found mutations in all compartments (84/86 LN; 45/45 PB; 35/38 BM; and 2/2 PF). Only 2 patients had no mutations in any biologic sample. Notably, significant ICVs were observed in all compartments (25/86 LN; 19/45 PB; 17/38 BM; 2/2 PF) and in at least one sample in a majority of cases (31/50; 62%). This result again reinforces the hypothesis that an ongoing CSR process often participates in the diversification of FL cells.
To test whether Sμ mutations vary significantly between tumoral compartments, we compared biologic samples obtained at the same time point (at time intervals shorter than 1 month; Figure 2 boxed samples). Thirty-four paired samples from 27 patients were compared (10 LN/PB, 6 LN/BM, 10 LN/BM/PB, 7 BM/PB, and 1 LN/BM/PB/PF). We observed that the mutations often differed between LN and PB (9/21 cases), LN and BM (9/17 cases), and, more unexpectedly, between BM and PB (9/18 cases). These results indicate that FL is often an oligoclonal disease where different subclones spread or proliferate asymmetrically between the tumoral compartments.
Genealogic trees of FL evolution
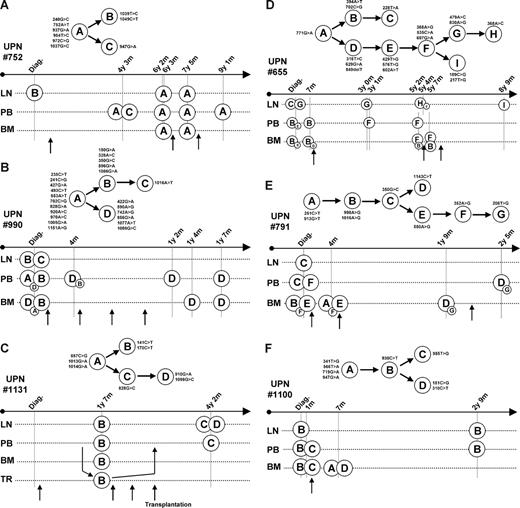
To gain more insight into the clonal history of FL, we next focused on the 6 patients for whom we had analyzed more than 5 samples (nos. 655; 752; 791; 990; 1100; and 1131). For these cases, the segregation of the mutations allowed us to construct genealogic trees and to identify the dominant subclones in each biologic sample (Figure 5). For 2 patients (nos. 752 and 990), we could also compare these trees with cytogenetic data (Table 1).
Genealogic trees. Subclones were identified by sequence alignments and by comparisons between biologic samples. Genealogic trees were then constructed (top panels, where nucleotide changes are given for each clone). Dominant subclones were identified in all samples (bottom panels). For each sample, the biologic type (LN indicates lymph node; PB, peripheral blood; BM, bone marrow; PF, peritoneal fluid; and TR, autologous transplant) and time from diagnosis are indicated. For all patients, the first vertical bar corresponds to diagnosis. When different subclones coexist, minor subclones are presented as small circles. Treatments are indicated by arrows. For patient no. 1131, transplanted cells (TR) were obtained before the first relapse, as indicated by arrows.
Genealogic trees. Subclones were identified by sequence alignments and by comparisons between biologic samples. Genealogic trees were then constructed (top panels, where nucleotide changes are given for each clone). Dominant subclones were identified in all samples (bottom panels). For each sample, the biologic type (LN indicates lymph node; PB, peripheral blood; BM, bone marrow; PF, peritoneal fluid; and TR, autologous transplant) and time from diagnosis are indicated. For all patients, the first vertical bar corresponds to diagnosis. When different subclones coexist, minor subclones are presented as small circles. Treatments are indicated by arrows. For patient no. 1131, transplanted cells (TR) were obtained before the first relapse, as indicated by arrows.
Karyotypes of patient nos. 752 and 990
| Sample . | Time from diagnosis . | Cytogenetics . |
|---|---|---|
| UPN 752 | ||
| LN | At diagnosis | 46,XX,del(6)(q13q16),add(7)(p21),t(14;18)(q32;q21)[18] |
| LN | 6 y 3 m | 46,XX,del(3)(q26q27),add(7)(p21),t(14;18)(q32;q21)[13], 46,XX,del(3)(q26q27),add(7)(p21),add(14)(p12),t(14;18)(q32;q21)[3] |
| LN | 7 y 5 m | 46,XX,add(7)(p21),t(14;18)(q32;q21)[12], 46,XX,add(7)(p21),der(13)t(1;13)(q11;q32),t(14;18)(q32;q21)[2] |
| UPN 990 | ||
| LN | At diagnosis | 46,X,der(X)t(1;X)(p21;q21),del(6)(q12q21),t(14;18)(q32;q21)[4], 45,X,der(X)t(1;X)(p21;q21),−4,del(6)(q12q21),add(9)(p13),add(9)(q34),add(13)(q33), t(14;18)(q32;q21),add(17)(q11)[9] |
| BM | At diagnosis | 46,X,der(X)t(1;X)(p21;q21),del(6)(q12q21),t(14;18)(q32;q21)[4], 46,X,der(X)t(1;X)(p21;q21),t(3;6)(p21;p22),del(6)(q12q21),t(14;18)(q32;q21)[1] |
| PB | 0 y 4 m | 46,X,der(X)t(1;X)(p21;q21),t(3;6)(p21;p22),del(6)(q12q21),t(14;18)(q32;q21)[1] |
| Sample . | Time from diagnosis . | Cytogenetics . |
|---|---|---|
| UPN 752 | ||
| LN | At diagnosis | 46,XX,del(6)(q13q16),add(7)(p21),t(14;18)(q32;q21)[18] |
| LN | 6 y 3 m | 46,XX,del(3)(q26q27),add(7)(p21),t(14;18)(q32;q21)[13], 46,XX,del(3)(q26q27),add(7)(p21),add(14)(p12),t(14;18)(q32;q21)[3] |
| LN | 7 y 5 m | 46,XX,add(7)(p21),t(14;18)(q32;q21)[12], 46,XX,add(7)(p21),der(13)t(1;13)(q11;q32),t(14;18)(q32;q21)[2] |
| UPN 990 | ||
| LN | At diagnosis | 46,X,der(X)t(1;X)(p21;q21),del(6)(q12q21),t(14;18)(q32;q21)[4], 45,X,der(X)t(1;X)(p21;q21),−4,del(6)(q12q21),add(9)(p13),add(9)(q34),add(13)(q33), t(14;18)(q32;q21),add(17)(q11)[9] |
| BM | At diagnosis | 46,X,der(X)t(1;X)(p21;q21),del(6)(q12q21),t(14;18)(q32;q21)[4], 46,X,der(X)t(1;X)(p21;q21),t(3;6)(p21;p22),del(6)(q12q21),t(14;18)(q32;q21)[1] |
| PB | 0 y 4 m | 46,X,der(X)t(1;X)(p21;q21),t(3;6)(p21;p22),del(6)(q12q21),t(14;18)(q32;q21)[1] |
For patient no. 752 (Figure 5A), we analyzed 3 independent LNs. We did not observe any evidence for the coexistence of different subpopulations in these biopsies. Interestingly, only 6 of the 8 mutations identified at diagnosis were found in the 2 relapses, suggesting that both had developed from less mutated precursors. To further investigate this possibility, we compared these results with cytogenetic data. At diagnosis, all cells shared the same chromosomal rearrangements, with the t(14;18), a 6q deletion, and a 7p abnormality. At relapse, 2 different subpopulations were identified in each LN. This heterogeneity, which we had not detected previously, indicates that sole analysis of the Sμ can underestimate the clonal complexity of the disease. At relapse, all cells shared the t(14;18) and the 7p abnormality, some showed 3q, 14p, and 13q abnormalities, but none had the 6q deletion. This loss, in agreement with our previous data, supports the hypothesis that none of the relapses had evolved from the cells that dominated in the LN at diagnosis. For this patient, we also analyzed 4 PB and 2 BM obtained at different time points during follow-up. None of these samples showed the 8 mutations seen at diagnosis, further indicating that the population that dominated in the LN at diagnosis had not been responsible for progression.
For patient no. 1131 (Figure 5C), only samples from the first and second relapses were available. At the first relapse, an identical pattern of 5 mutations was seen in LN, PB, and BM (141C>T; 170C>T; 857C>G; 1013G>A; and 1014G>A). At the second relapse, 3 of these mutations were seen in LN and PB (857C>G; 1013G>A; and 1014G>A) but 2 were absent (141C>T and 170C>T). This result indicates that, as seen with patient no. 752, this relapse had not developed from the cells that dominated in the first LN. For this patient, we did not detect less mutated progenitors either in PB or in BM at the first time point, suggesting that these cells were probably located in another compartment or were masked by the dominant tumoral population. Interestingly, this patient was treated by autologous stem cell transplantation with a transplant in which t(14;18)-positive cells had been detected by conventional PCR analysis. Surprisingly, our data indicate that the dominant t(14;18)-positive cells in the transplant showed the pattern of 5 mutations, excluding the possibility that they may have been responsible for progression.
Patient no. 990 (Figure 4B) presented a clinically aggressive FL with a leukemic phase. At diagnosis, Sμ mutations were compared among the LN, PB, and BM. All electropherogram traces showed complex patterns with double peaks, suggesting the coexistence of different subpopulations. The disease was refractory to all treatments, and leukemic cells remained detectable in PB and BM throughout the course of the disease. Five mutations seen in LN at diagnosis (150G>A; 328A>C; 350G>C; 596G>A; and 1086G>A) were absent in all samples obtained after treatments. This result suggests that progression had not developed from those cells that dominated in the biopsy. At progression, all biologic samples instead showed an identical pattern of 16 mutations. At diagnosis, 10 of these mutations were found in all compartments, but 6 were detected only in the BM (422G>A; 590A>G; 742A>G; 856G>A; 1077A>T; and 1086G>C). This observation suggests that the lymphoma cells that escaped treatments may have emerged from this compartment. To verify this hypothesis, PCR products from LN, BM, and PB at diagnosis were subcloned and sequenced. Again, cells with the 6 mutations seen at progression were found only in the BM (Figure S1). We next compared these results with cytogenetic data obtained from LN and BM at diagnosis and from the first PB sample obtained during follow-up. In these samples, all tumoral cells shared the t(14;18), a 6q deletion, and an Xq abnormality. Consistent with our previous results, 2 subpopulations were identified in LN and BM at diagnosis. One population, which showed only the t(14;18), the del(6q), and the Xq abnormality, was present in both compartments. One population, which showed 9q, 9p, 13q, and 17q abnormalities, was seen only in the LN. Interestingly, we observed a second subpopulation in BM that showed a t(3;6) translocation, which was also identified in PB after the first line of treatment. This result again reinforces the hypothesis that progression had emerged from the BM but not from LN.
Finally, the most complete view of the clonal evolution of a FL was obtained from patient no. 655 (Figure 5D; Figure S2). In this case, 12 independent biologic samples were obtained over a period of nearly 9 years, covering diagnosis and 3 relapses. For this patient, the clonal evolution was addressed by the analysis of sequences of whole PCR products and of plasmid subclones obtained from LN, BM, and PB at diagnosis. At diagnosis, the LN contained 2 dominant subpopulations, each containing unrelated mutations. Different populations were identified in both PB and BM, with one (subclone B) dominating all others. After a 7-month period of observation, few changes were noted in PB and BM, where subclone B remained dominant. When the first relapse occurred, 3 years after the initial remission, subclones from only one branch were identified in LN and PB (subclones F and G). Interestingly, the cells that dominated in PB showed fewer mutations than in the LN. At the second relapse, a more advanced subclone (H) emerged in LN, whereas PB apparently remained unchanged. At this time, cells resembling those that dominated in PB and BM at diagnosis (subclone B) were detected in 2 BM samples. These cells descended from a different progenitor than the ones that proliferated in the LN (subclones F and H), indicating that different arms of the disease can coexist for years, even after multiple lines of treatment. Finally, at the third relapse, a new subclone (I) was identified in LN, which had emerged from less mutated progenitors, but not from those cells that dominated in the previous LN samples.
Discussion
FL development probably initiates in the BM during VDJ recombination by the acquisition of a t(14;18) translocation. The presence of circulating t(14;18)-positive cells or FL-like cells in the blood of healthy individuals indicates that this translocation is not sufficient for complete transformation.27 As many patients have widespread disease at diagnosis, a long preclinical period may allow these cells to gain secondary genomic abnormalities and the capacity to invade new compartments. Next, when the fully transformed phenotype is gained, they may retain this capacity, localize to different biologic niches, and contribute to subsequent relapses. Besides this widespread distribution, an additional layer of complexity comes from the ICV shown by the tumoral population. This ICV was initially reported in LN, but little is known about the other tumoral compartments. Interestingly, a recent report based on VDJ mutation analysis has suggested that BM involvements may not always result from the migration of cells from LN, but that the 2 compartments may evolve independently.9 Unfortunately, due to the difficulty in characterizing the IgV regions of the rare t(14;18)-positive cells in PB and BM, this approach allows the study of only a few samples with significant involvements. To circumvent this limitation, we adopted an alternate strategy and analyzed the mutations that accumulate on the Sμ region on the der(14)t(14;18) allele during the early steps of CSR.
It is likely that IgV mutations directly participate in the selection of FL cells by modulating BCR signaling.2-4,10 In contrast, Sμ mutations, on the translocated allele, probably do not interfere with this process. Once their frequency is high enough to allow discrimination of different subpopulations, Sμ mutations can serve as independent markers of clonal evolution. In our series, the frequency of Sμ mutations was 0.8%, roughly 10-fold lower than for IgV regions.11 In many cases, this lower frequency probably led us to underestimate the clonal diversity. As indicated by the analysis of individual subclones, sequencing whole PCR products does not reveal the true ICV but allows the identification of only the mutations of the major tumoral subpopulations. However, it still allowed us to obtain a clonal signature from the rare t(14;18) cells that populate PB and the BM, including those cells that can often be detected during clinical remission. By showing that different subpopulations often coexist in these different compartments at a given time point, our data further confirm that FL is a complex oligoclonal disease. Obtaining the clonal signature of t(14;18)-positive cells both at different time points and from different compartments also gave us new insights into clonal evolution during progression.
If the presence of clonal Sμ mutations in all biologic samples from individual patients confirmed that FL evolution probably initiates from unique t(14;18)-positive GC cells,25 our data also unambiguously confirm that the linear view of progression is too restrictive. We instead observed that the clonal history often follows complex Darwinian schemes, where different clonally related subpopulations proliferate and spread asymmetrically among different tumoral compartments.28 In showing that less mutated and apparently earlier subpopulations often exist in these compartments both at diagnosis and during remission, our data challenge the current view of residual disease in FL. Our results suggest that spreading of the tumoral cells may often be different than usually thought, originating from precursor cells that circulate in the PB or BM that could gain the capacity to proliferate in secondary lymphoid organs.
For 4 of 6 patients for whom we could reconstitute a detailed history (nos. 1131; 752; 655; and 990), major subclones in at least one LN appeared as dead ends on the evolution trees. This observation suggests that these subclones, which may have had some proliferative advantage, may have been eliminated by treatment. However, their eradication was not sufficient to cure the disease, which reemerged from an earlier subpopulation containing fewer mutations. In line with this observation, for each of these 4 patients, we were able to identify less mutated subclones in at least one other biologic compartment over the course of the disease. Together, our data thus strongly support the hypothesis raised after the analysis of the mutations on the IgV regions, that some relapse could arise through the evolution of preexisting minor subclones.10-12 This would explain why we and others have observed that chromosomal abnormalities present at diagnosis can be lost at relapse.29,30 This hypothesis would also explain why, despite an apparent ongoing CSR activity in fully transformed cells suggested by the frequent ICV, the total number of mutations rarely increases over time.
As FL progression most likely depends on alterations in non-Ig genes, the identification of subpopulations that differ in mutations of the Sμ does not necessarily imply that precursors with fewer mutations are less transformed. However, the clinical behavior of the tumors provides several lines of evidence to support this hypothesis. In this study, we observed a significant correlation between the interval between biopsies and the evolution of the mutation patterns, which suggests that the time to progression is related to different evolutionary pathways. In one group, the stability of the mutations, together with the shorter interval to progression, suggests that therapy sometimes fails to eradicate the fully transformed population, which then rapidly reexpands. In the second group, the differences between the mutation patterns evoke an ongoing evolutionary process where “new” tumoral populations would emerge from precursors. Because the time to progression is significantly longer in this group, it is tempting to speculate that at least one additional genetic event was necessary to reach a fully transformed phenotype and to develop a relapse.
Despite the relatively large number of cases we studied, we could not find any reliable indicator of the evolution of mutations or of histologic transformation. It should, however, be noted that, because we studied archival materials, we got access to only a limited number of samples. We therefore often obtained a very incomplete view of the clonal history of the tumors. Another limitation to our study comes from the period of recruitment, which covers more than 20 years. Patients thus received heterogeneous treatment regimens, which most probably exerted different selective pressures on the tumoral populations, thus preventing direct comparisons or any reliable definition of clinical subgroups. Additional investigations on homogeneous cohorts would be necessary to detect correlations that might exist between clonal evolution and clinical progression.
In conclusion, our data suggest that many relapses in FL arise from a pool of pretumoral cells, less clonally evolved than the ones that proliferate in LN at diagnosis. It was recently proposed that the atypical post-GC mature t(14;18)-positive cells (named FL-like cells) that circulate in the blood of healthy individuals could constitute a such pool.25 However, as the incidence of this translocation in the whole population is far higher than the lifetime risk of developing a lymphoma,31 the probability of these cells evolving into a neoplasm appears extremely low. In contrast, our data suggest that a population of more advanced FL “precursor cells” with a high probability of reaching a fully transformed phenotype may almost invariably escape treatment and persist in the PB or BM during remissions. Control over this population of cells may thus be a necessary step toward a cure for FL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ligue contre le Cancer, Paris, France (comité de Seine Maritime) and the Institut National du Cancer (INCa; grant no. PL070-2005, Prof T. Fest, Rennes, France).
Authorship
Contribution: P.R. designed research, performed experiments, analyzed the data, and wrote the paper; F.J. provided clinical samples, analyzed the data, and contributed to writing the paper; J.-M.P. reviewed histologies; F.P. performed experiments and sequence analysis; N.C. and G.B. provided clinical samples and contributed to data analysis; S.T. and V.R. performed experiments and participated in patient selection; H.T. supervised the study and contributed to writing the paper; and C.B. designed research, analyzed the data, and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Ruminy, Inserm U918, Centre Henri Becquerel, Rue d'Amiens, 76000 Rouen, France; e-mail: phirum@rouen.fnclcc.fr.