While the FIP1L1/PDGFRA fusion gene is mainly known for its association with hypereosinophilia, this abnormal gene was also identified in patients with systemic mastocytosis. In this issue of Blood, Yamada and colleagues' study of an elegant mouse model for FIP1L1/PDGFRA–induced eosinophilia reveals that FIP1L1/PDGFRα synergizes with activated Kit, the receptor for stem cell factor, to promote mast cell development, activation, and survival.
Five years ago, the FIP1L1/PDGFRA fusion gene was identified in patients with hypereosinophilic syndrome (HES). Since then, many other studies have confirmed the presence of the FIP1L1/PDGFRA fusion gene in a significant fraction of hypereosinophilia patients and have shown that these patients have excellent responses to imatinib treatment.1,2 Given the documented clonality of the disease in HES patients, the preferred nomenclature for this disease is FIP1L1/PDGFRA chronic eosinophilic leukemia (CEL). Eosinophilia is also frequently observed in systemic mastocytosis, and up to half of these cases are also positive for the FIP1L1/PDGFRA fusion gene.3 It is currently undecided whether to call this disease systemic mastocytosis with eosinophilia, or rather FIP1L1/PDGFRA–positive CEL,3,4 illustrating the close relationship between both FIP1L1/PDGFRA–positive diseases.
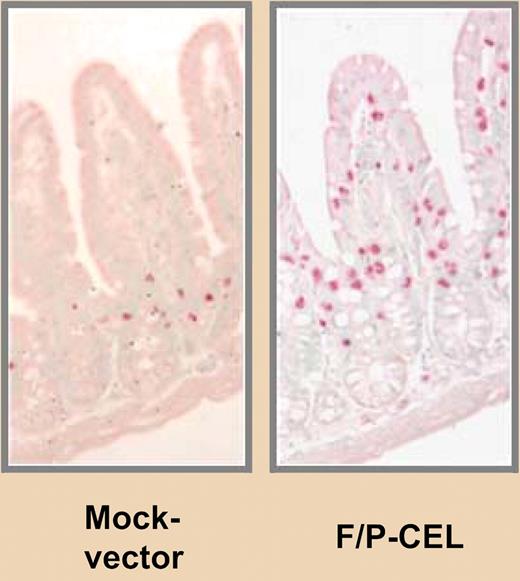
Infiltration of mast cells in the small intestine of FIP1L1/PDGER α expressing mice versus FN “AND” replaced with “vs.” control mice. See the complete figure in the article beginning on page 2500.
Infiltration of mast cells in the small intestine of FIP1L1/PDGER α expressing mice versus FN “AND” replaced with “vs.” control mice. See the complete figure in the article beginning on page 2500.
On the other hand, there is no doubt about the specific association of the FIP1L1/PDGFRA gene with eosinophilia. Attempts to model this in mice have, however, been difficult. Simple expression of FIP1L1/PDGFRA in the bone marrow cells of mice using the classic bone marrow transplantation model results in the generation of a myeloproliferative disease without striking eosinophilia.5 Yamada et al modified this mouse model and showed that FIP1L1/PDGFRA expression, when combined with overexpression of interleukin-5, was able to recapitulate typical features of HES/CEL, including hypereosinophilia and tissue infiltration of eosinophils.6 In addition to this study, recent work from the group of Nicholas C. P. Cross suggests that variations in IL5RA expression are linked to constitutional IL5RA genotype and severity of FIP1L1/PDGFRA–positive CEL.7 Together, these data may suggest that FIP1L1/PDGFRA alone is not sufficient to cause CEL, at least in mice, and that other cooperating factors are required and may be additional targets for therapy.
In this issue of Blood, Yamada et al extend their previous findings on the FIP1L1/PDGFRα/interleukin-5 mouse model and present a detailed analysis of the effect of FIP1L1/PDGFRα on mast cell biology. Their analyses reveal that FIP1L1/PDGFRα synergizes with stem cell factor (SCF) stimulation via Kit to promote mast cell development, activation, and survival. SCF is a key factor for the development of mast cells from bone marrow precursors but normally requires cofactors, such as IL-3 and IL-4. The work by Yamada et al suggests that signaling by the activated tyrosine kinase FIP1L1/PDGFRα may replace the need for these cofactors. They also nicely demonstrate that interference with activation of Kit by administration of an anti-Kit antibody diminished infiltrations of mast cells in the intestine and spleen. Since KIT is potently inhibited by imatinib, the translation of these results toward clinical practice may already have been accomplished, as this kinase inhibitor is the standard treatment for FIP1L1/PDGFRA–positive disease. It remains to be determined whether polymorphisms that influence the expression levels of the KIT or SCF genes may determine phenotypic differences within the group of FIP1L1/PDGFRA–positive diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal