Abstract
Adoptive immunotherapy with T cells expressing a tumor-specific chimeric T-cell receptor is a promising approach to cancer therapy that has not previously been explored for the treatment of lymphoma in human subjects. We report the results of a proof-of-concept clinical trial in which patients with relapsed or refractory indolent B-cell lymphoma or mantle cell lymphoma were treated with autologous T cells genetically modified by electroporation with a vector plasmid encoding a CD20-specific chimeric T-cell receptor and neomycin resistance gene. Transfected cells were immunophenotypically similar to CD8+ effector cells and showed CD20-specific cytotoxicity in vitro. Seven patients received a total of 20 T-cell infusions, with minimal toxicities. Modified T cells persisted in vivo 1 to 3 weeks in the first 3 patients, who received T cells produced by limiting dilution methods, but persisted 5 to 9 weeks in the next 4 patients who received T cells produced in bulk cultures followed by 14 days of low-dose subcutaneous interleukin-2 (IL-2) injections. Of the 7 treated patients, 2 maintained a previous complete response, 1 achieved a partial response, and 4 had stable disease. These results show the safety, feasibility, and potential antitumor activity of adoptive T-cell therapy using this approach. This trial was registered at www.clinicaltrials.gov as #NCT00012207.
Introduction
Several lymphoma subtypes are incurable with standard chemotherapy and radiation, but immune-based therapies have emerged as effective treatment and offer a potential for cure. Monoclonal antibodies (Abs) against the B-cell lymphoma marker CD20 have activity alone,1,2 in combination with chemotherapy,3-5 or conjugated with radiation-emitting nuclides.6-8 Adoptive cellular therapy with nonmyeloablative allogeneic stem cell transplantation (SCT) or donor lymphocyte infusion (DLI) can eradicate tumors, resulting in long-term survival, even in highly chemotherapy-refractory lymphomas.9-11 Both of these immunotherapy approaches have limitations, however, because antibodies fail to cure many types of lymphoma, and SCT and DLI, although potentially curative, cannot be used in many patients because of significant toxicity and transplantation-related mortality
Because the graft-versus-tumor effect of SCT and DLI appears to be mediated by alloreactive donor T lymphocytes,12,13 generating T cells specific for tumor antigens minimally expressed in normal tissues is an attractive strategy for harnessing this antitumor effector activity. One technique involves genetically modifying autologous T cells to express a chimeric T-cell receptor (cTCR) that targets a tumor antigen and induces antigen-specific T-cell activation, proliferation, and killing. Because this antigen-induced activation of the T cell occurs in an MHC-independent fashion, a single vector can be used universally to confer recognition of a selected target antigen. By introducing the cTCR into autologous T cells, the risk of graft-versus-host disease is eliminated. Such genetically modified T cells have been designed to target antigens associated with a variety of tumors, with success in animal models14-16 and some early evidence of clinical efficacy in human subjects.17
Our group has developed a technique to manufacture CD20-specific T cells by transfecting peripheral blood mononuclear cells (PBMCs) with a linearized naked DNA plasmid encoding a cTCR derived from a murine anti–human CD20 Ab.18-20 The cell-surface antigen CD20 is an attractive target for immune-based therapies because it is present in more than 90% of B-cell lymphomas, is expressed at a high copy number, is stable on the cell surface, and does not internalize on binding Abs.21 These modified T cells secrete interleukin-2 (IL-2) in an antigen-dependent manner,19 selectively kill CD20+ target cells in vitro,20 and eradicate human xenograft tumors in mice.22 Application of this approach to the treatment of lymphoma in human subjects has not yet been described. We report here the results of a proof-of-concept clinical trial in which ex vivo–expanded, genetically modified autologous CD20-specific T cells were used as adoptive cellular therapy for patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma (NHL) and mantle cell lymphoma (MCL). We show that these T cells can be reproducibly generated and expanded to therapeutic numbers, exhibit in vitro antitumor cytotoxicity, persist in vivo for up to 9 weeks, and appear to be safe, well tolerated, and potentially capable of mediating in vivo antitumor activity.
Methods
Clinical protocol
This clinical protocol was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, the University of Washington Institutional Biosafety Committee, the US Food and Drug Administration, and the Recombinant DNA Advisory Committee of the National Institutes of Health. Informed consent was obtained in accordance with the Declaration of Helsinki. Patients were eligible if they had a pathologically confirmed diagnosis of CD20+ MCL or indolent B-cell lymphoma, had relapsed or refractory disease after at least one prior chemotherapy, were deemed not to be candidates for (or refused) stem cell transplantation, and had serologic evidence of prior Epstein-Barr virus (EBV) exposure (because the TM-LCL cell line used in T-cell culture is EBV-transformed). Patients were excluded if they received fludarabine or cladribine within 2 years before apheresis (but could receive these drugs as cytoreductive therapy after apheresis), anti-CD20 Ab within 4 months of T-cell infusions, or chemotherapy within 4 weeks of T-cell infusions; had lymph nodes more than 5 cm or more than 5000 circulating lymphoma cells in the peripheral blood at the time of T-cell infusions, a previous allogeneic stem cell transplantation, or human anti–mouse Ab (HAMA) seropositivity; required corticosteroids during the study period; had pulmonary or central nervous system involvement with lymphoma; were HIV-seropositive; or were pregnant.
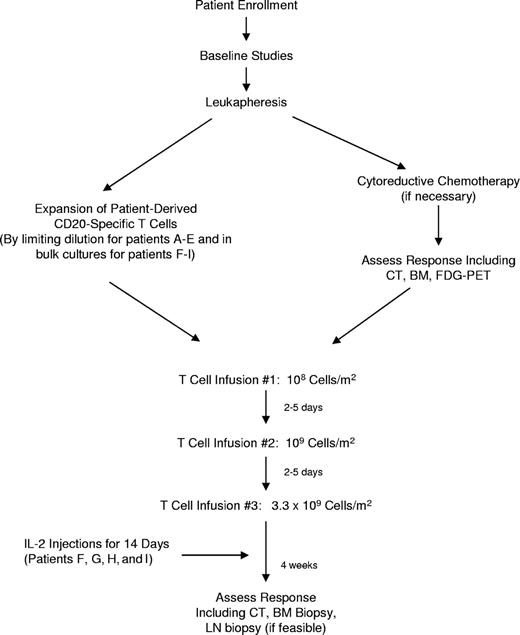
Patients underwent leukapheresis after signing informed consent, and then they were allowed to receive cytoreductive chemotherapy for disease control or debulking during the 2- to 4-month period of T-cell generation, at the discretion of their referring physician. For patients A to E, PBMCs were activated, transfected, and plated at limiting dilution with the intention of isolating and subsequently expanding T-cell clones. This approach proved to be laborious and inefficient, however, and the protocol was modified for patients F to I to allow expansion of modified cells in bulk culture. Patients subsequently received 3 infusions of autologous CD20-specific T cells 2 to 5 days apart in escalating doses (108 cells/m2, 109 cells/m2, and 3.3 × 109 cells/m2) followed by 14 days of subcutaneous low-dose (500 000 IU/m2) interleukin-2 (IL-2) injections twice daily (patients F-I only). Patients then underwent clinical follow-up to evaluate toxicities related to therapy, which were assessed according to National Institutes of Health Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/). A Data and Safety Monitoring Board was assembled that performed reviews of the safety data every 6 months. Clinical responses were assessed according to International Working Group criteria.23
T-cell transfection, selection, and expansion
All cell culture for therapeutic use was performed in the Cell and Gene Therapy Core Laboratory at the University of Washington General Clinical Research Center, under current good manufacturing practice standards. PBMCs collected by apheresis were diluted 1:2 with PBS containing 200 mg/L EDTA, isolated by density gradient centrifugation over Ficoll-Paque (GE Healthcare, Little Chalfont, United Kingdom), washed, and resuspended in RPMI 1640 medium containing 2 mmol of l-glutamine, 25 mmol HEPES, and 10% fetal calf serum. Cells were activated with 30 ng/mL OKT3, and after overnight incubation recombinant human IL-2 was added (50 U/mL).
Electroporation and selection.
On day 4 of culture, cells were harvested and resuspended in chilled hypo-osmolar electroporation buffer (Eppendorf North America, New York, NY) at 20 × 106 cells/mL. Cell suspensions were mixed with linearized plasmids (25 μg/mL) encoding a CD20-specific scFvFc:ζ cTCR,18-20,24 and divided into aliquots into chilled 0.2-cm electroporation cuvettes. Cells were electroporated with an Eppendorf Multiporator at 250 V for 40 microseconds (μsec) as previously described.20 Approximately 3 days after electroporation, G418 was added to flasks (at 0.8 mg/mL). Cells were selected by G418 for 8 days before generating cells by limiting dilution.
Generation and expansion of genetically modified T cells.
Transfected cells from patients A through E were selected in G418, and attempts were made to generate T-cell clones by limiting dilution as previously described.24,25 Although the intention was to isolate clonal populations derived from a single progenitor cell, the plating density required to yield reliable growth of T cells resulted in the presence of 1 to 3 clones per well, as subsequently determined by Vβ TCR spectratyping. For patients F through I, G418-resistant transfected cells were grown in bulk cultures as previously described.25 As cell numbers increased, T cells were transferred to 1-L or 3-L tissue culture bags (Lifecell, Branchburg, NJ). During the expansion, 5 to 8 stimulation cycles were performed. Fresh T cells were infused in patients A and B. For logistic reasons, T cells from patients D, F, G, H, and I were cryopreserved between days 70 and 132 after apheresis in Plasmalyte-A containing 5% HSA and 10% DMSO and thawed 3 to 4 hours before infusion (48 hours before infusion for patient D). Release criteria included detectable cTCR expression by flow cytometry, negative bacterial, fungal, and Mycoplasma cultures, endotoxin level no more than 5 EU/kg per hour, Gram stain–negative on day of infusion, greater than 80% cell viability, TCRα/β+ and CD3+ phenotype by flow cytometry, IL-2 growth dependence, and CD20-specific cytotoxicity.
T-cell clonality assays
T-cell clonality was determined by polymerase chain reaction (PCR) amplification of rearrangements at the T-cell receptor gamma (TCRγ) locus as previously described,26 except that VγI-Jγ1/2, VγII-Jγ1/2, VγI-JγP1/2, and VγII-JγP1/2 rearrangements were amplified in a single multiplex PCR reaction and analyzed by capillary electrophoresis on an Applied Biosystems Model 3130 (Foster City, CA). See Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) for detailed methods.
Vβ spectratyping was also performed by flow cytometry. Cells were labeled with monoclonal antibodies CD8 ECD and IOTest Beta Mark Kit (Beckman Coulter, Fullerton, CA). The expression of each of the 24 T-cell receptor isoforms present in the Beta Mark Kit (approximately 70% coverage of the normal human TCR Vβ repertoire) were determined independently on the CD8+ T-cell populations, and a threshold of 85% positivity for a single isoform or an absence of expression of all 24 isoforms outside the reference range was considered to represent a clonal expansion. Samples showing 2 or more isoforms outside the reference range were considered oligoclonal.
Western blot assay
Whole cell lysates of modified T cells were probed with a mouse anti–human CD3ζ monoclonal Ab (BD PharMingen, San Diego, CA) as previously described.20
Cytotoxicity assays
T-cell cytotoxicity was analyzed 2 to 7 weeks before planned T-cell infusions to permit selection of optimal “clones” of T cells for expansion. CD20-specific cytotoxicity was assessed with the use of standard chromium-release assays with the following target cell lines: EL4-CD20 (a murine T-cell lymphoma line transfected to express the human CD20 molecule), the parental CD20–nontransfected EL4 cell line, or the Daudi Burkitt lymphoma cell line, as previously described.25 Cytotoxicity assays were repeated in some patients just before T-cell infusions and showed levels of cytotoxicity comparable to assays performed 2 to 7 weeks before infusion.
Flow cytometry for immunophenotypic characterization of T cells and lymphocyte subset analysis
Flow cytometry was performed with the use of standard methods. Briefly, cells cryopreserved within 1 day of the first T-cell infusion were thawed, washed, and labeled with the indicated monoclonal Ab for 15 minutes at room temperature in the dark. The samples were then washed once, resuspended in a dilute DNA binding dye (DAPI), incubated for 10 minutes, and approximately 20 000 events acquired on an LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using software developed in our laboratory (WoodList). Positivity for DAPI was used to exclude nonviable cells, and thresholds for positivity were determined with unstained cells and isotype control Ab, as appropriate. Antibodies were used at the manufacturer's recommended concentrations. A complete list of Abs used is included in Document S1. Flow cytometry to detect cTCR expression was performed using a FITC-labeled polyclonal goat anti–mouse IgG Fab-specific Ab (Sigma-Aldrich, St Louis, MO) as previously described.25
Detection of modified T cells in vivo
PBMCs collected serially after T-cell infusions were isolated by Ficoll density-gradient centrifugation, and genomic DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA). The standard consisted of 10-fold serial dilutions of purified scFvFc:ζ plasmid DNA starting at 106 copies/μL, with each sample containing 1 μg of preinfusion PBMC DNA to control for background signal. The negative control was preinfusion PBMC genomic DNA. A 72-bp (base pair) fragment containing portions of the CD3ζ chain and adjacent CD4 transmembrane domain sequences was amplified using forward primer 5′-TCGCCGGCCTCCTGCTTT-3′ and reverse primer 5′-CGTCTGCGCTCCTGCTGA-3′. The probe used was 5′-FAM-TGGGCTAGGCATCTTCTTCAGAGTGAA-TAMRA-3′. Primers that amplify a fragment of the β-actin gene (TaqMan B-actin Detection Reagent Kit; Applied Biosystems) were used as an internal control and for normalization of DNA quantities. Quantitative real-time PCR was performed in triplicate with 1 μg DNA in each reaction, using TaqMan Universal PCR Master Mix in a 7900HT Sequence Detection System (all Applied Biosystems).
Immune response assays
Two assays were performed to test for humoral immune responses to the cTCR. In the first assay, 96-well enzyme-linked immunoabsorbent assay (ELISA) plates were coated with 0.5 μg Leu-16 murine anti–human CD20 Ab (BD Biosciences, San Diego, CA) in pH 9.6 carbonate buffer and blocked with 5% milk before adding samples of goat anti–mouse IgG Fab-specific Ab (standard curve; Jackson ImmunoResearch Laboratories, West Grove, PA), serially diluted 2% BSA/PBS (negative control), baseline patient serum (negative control), HAMA+ patient serum (positive control), or study subject serum from serial postinfusion time points. Biotinylated Leu-16 murine anti–human CD20 Ab (BD Biosciences; 10 μg/mL) was added to each well as the primary Ab, followed by 1:1000 horseradish peroxidase-Avidin D (BD Biosciences). Samples were incubated for 30 minutes at room temperature and washed 3 times with 0.01 M PBS/0.3% Tween between each step. Color reagent (2,2,-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid] diammonium salt; Sigma-Aldrich) at 0.42 mg/mL in citrate buffer (citrate 10.5 mg/mL, pH 4.0) plus hydrogen peroxide (100 μL/12 mL buffer) was added to each well; absorbency was read with a Bio-Tek XS ELISA reader (Bio-Tek Instruments, Winooski, VT). Optical density measurements were converted to concentration values as calculated from the standard curve. In the second assay, flow cytometry was used to assess the presence of anti-cTCR Ab in posttreatment patient serum samples (see Document S1 for detailed methods).
Cellular immune response assays were performed by coincubating patient-derived PBMCs (106 cells/mL) serially collected after T-cell infusions with irradiated anti-CD20 cTCR-expressing T cells (106 cells/mL) from infused batches, at a 2:1 ratio. After 2 rounds of stimulation 1 week apart, the PBMCs were tested in 51Cr release cytotoxicity assays using either autologous T cells transfected with the cTCR-encoding plasmid or nontransfected autologous PBMC as target cells at a 25:1 E/T ratio. In the first 2 patients treated we also assessed the responsiveness of recovered T cells to histocompatibility locus antigen–disparate cells as a positive control.
Results
Study design and patient characteristics
The primary objective of this study was to assess the feasibility, safety, and toxicity of adoptive therapy using patient-derived T cells bearing a CD20-specific cTCR to treat indolent and mantle cell lymphomas. Autologous PBMCs were collected by apheresis, genetically modified, and expanded ex vivo, a process that typically required 2 to 4 months. During this interval patients underwent cytoreductive chemotherapy if necessary for tumor debulking or to maintain disease control. Subjects were then treated with 3 infusions of modified CD20-specific T cells, 2 to 5 days apart, at incremental doses (108 cells/m2, 109 cells/m2, and 3.3 × 109 cells/m2) similar to those used in previous adoptive T-cell therapy trials,27 but with a shorter interval between infusions to limit the potential for development of an immune response against the transfected cells. The last 4 patients received low-dose subcutaneous injections of IL-2 twice daily for 14 days after the final T-cell infusion to enhance in vivo T-cell survival and proliferation. Patients then underwent follow-up for clinical and research end points, and long-term monitoring for adverse events for 2 years. The study design is outlined in Figure 1.
Nine patients with relapsed or refractory indolent B-cell NHL or MCL were enrolled: 8 men and 1 woman between the ages of 43 and 77 years; 8 had relapsed follicular lymphoma, and 1 had relapsed MCL. Patients had been treated with a median of 2 prior therapies (range, 1-7 therapies; Table 1).
Patient characteristics
| Patient . | Age, y . | Sex . | Diagnosis . | Stage . | Prior therapies . | Cytoreductive therapy before T-cell infusions . |
|---|---|---|---|---|---|---|
| A | 44 | F | FL | IV-B | R-CHOP | CVP |
| B | 70 | M | FL | II-A | CHOP, rituximab, 131I-tositumomab | CVP |
| C | 47 | M | FL | IV-B | ProMACE/MOPP, ASCT, fludarabine (10 cycles) | CVP |
| D | 60 | M | FL | IV-A | Rituximab | CVP |
| E | 63 | M | MCL | IV-A | R-HyperCVAD, GCD-R | None |
| F | 46 | M | FL | IV-A | R-CVP | FND |
| G | 43 | M | FL | IV-A | CHOP, IFN, CY + VP16, R-CY, CY + DEX, GCD-R, ASCT | None |
| H | 46 | M | FL | IV-B | R-CHOP, fenretinide | FND |
| I | 77 | M | FL | III-A | R-CVP, R-CHOP, GCD-R | 131I-tositumomab |
| Patient . | Age, y . | Sex . | Diagnosis . | Stage . | Prior therapies . | Cytoreductive therapy before T-cell infusions . |
|---|---|---|---|---|---|---|
| A | 44 | F | FL | IV-B | R-CHOP | CVP |
| B | 70 | M | FL | II-A | CHOP, rituximab, 131I-tositumomab | CVP |
| C | 47 | M | FL | IV-B | ProMACE/MOPP, ASCT, fludarabine (10 cycles) | CVP |
| D | 60 | M | FL | IV-A | Rituximab | CVP |
| E | 63 | M | MCL | IV-A | R-HyperCVAD, GCD-R | None |
| F | 46 | M | FL | IV-A | R-CVP | FND |
| G | 43 | M | FL | IV-A | CHOP, IFN, CY + VP16, R-CY, CY + DEX, GCD-R, ASCT | None |
| H | 46 | M | FL | IV-B | R-CHOP, fenretinide | FND |
| I | 77 | M | FL | III-A | R-CVP, R-CHOP, GCD-R | 131I-tositumomab |
F indicates female; M, male; FL, follicular lymphoma; MCL, mantle cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; R, rituximab; CVP, cyclophosphamide, vincristine, and prednisone; ProMACE/MOPP, procarbazine, methotrexate with leucovorin, doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, and prednisone; ASCT, high-dose therapy followed by autologous stem cell transplantation; HyperCVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with cycles of high-dose cytarabine and methotrexate; GCD, gemcitabine, carboplatin, and dexamethasone; FND, fludarabine, mitoxantrone, and dexamethasone; IFN, interferon-α; CY, cyclophosphamide; VP16, etoposide; and DEX, dexamethasone.
Generation and expansion of autologous CD20-specific T cells
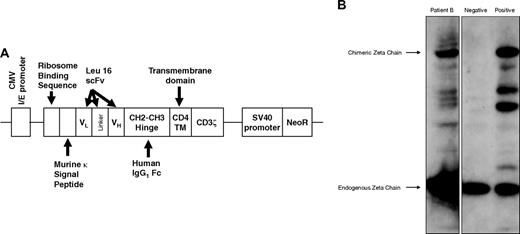
PBMCs collected by apheresis were stimulated with anti-CD3 Ab (OKT3) and IL-2 and transfected by electroporation with a naked DNA plasmid encoding a cTCR consisting of a murine kappa leader sequence, CD20-specific scFv derived from the Leu16 murine Ab, human IgG1 CH2CH3 hinge, human CD4 transmembrane, and human CD3ζ intracellular signaling domain, as well as a neomycin resistance gene (neoR) under a separate promoter (Figure 2A).20,25 Anti-CD20 cTCR surface expression was confirmed by Western blot (Figure 2B) and flow cytometry (Figure S3).
Expression of the CD20-specific cTCR. (A) Schematic diagram of the CD20-specific scFvFc:ζ chimeric T-cell receptor cDNA plasmid. (B) A representative Western blot analysis of cTCR expression performed using whole-cell lysates of preinfusion T cells from patient B, probed with mouse anti–human CD3ζ monoclonal Ab. Negative control was parental PBMCs, and positive control was transfected Jurkat cell line. A 21-kDa band corresponding to the endogenous CD3ζ chain and a 66-kDa band representing the expected cTCR protein were detected. The intermediate bands indicate degradation products or truncated forms of the cTCR.
Expression of the CD20-specific cTCR. (A) Schematic diagram of the CD20-specific scFvFc:ζ chimeric T-cell receptor cDNA plasmid. (B) A representative Western blot analysis of cTCR expression performed using whole-cell lysates of preinfusion T cells from patient B, probed with mouse anti–human CD3ζ monoclonal Ab. Negative control was parental PBMCs, and positive control was transfected Jurkat cell line. A 21-kDa band corresponding to the endogenous CD3ζ chain and a 66-kDa band representing the expected cTCR protein were detected. The intermediate bands indicate degradation products or truncated forms of the cTCR.
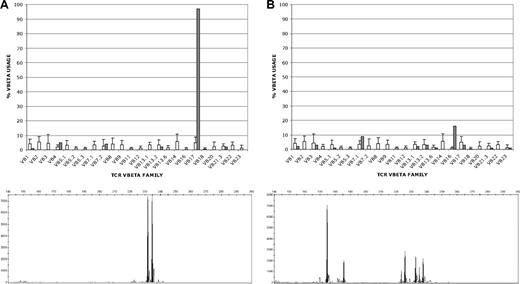
Modified T cells were generated for the first 5 patients by limiting dilution and selected for CD20 cytotoxicity by chromium release assay and cTCR expression by flow cytometry. At the plating density required to reproducibly generate modified T cells, the resulting T-cell populations consisted of cells derived from 1 to 3 clones of T cells as assessed by Vβ TCR spectratyping and TCRγ clonality testing by PCR (Figure 3A; Table S1). This expansion and selection process proved to be laborious and inefficient, requiring approximately 4 months to achieve the target cell dose. Moreover, T cells generated by limiting dilution could not be expanded adequately for infusions in 2 of these initial 5 patients, and in 2 of the other 3 patients the target cell doses could not be reached (Table 2).
Clonality of T cells produced by limiting dilution and in bulk culture. T-cell clonality was determined by flow cytometric T-cell receptor (TCR) Vβ spectratyping (top) and by PCR amplification of clonal V-J rearrangements at the TCRγ locus (bottom). Representative results for T cells produced by limiting dilution (A) and in bulk culture (B) are shown. (A) T cells produced by limiting dilution (patient B), showing clonal expression of Vβ17 in 98% of CD8+ T cells by Vβ spectratyping (top; ■) and showing 2 predominant TCRγ rearrangements (bottom). Because each T-cell clone can rearrange one or both of its TCRγ alleles, the 2 PCR products could represent either 1 T-cell clone with biallelic TCRγ rearrangements or 2 singly rearranged clones, although the single predominant Vβ17 clone identified by spectratyping would favor a single doubly rearranged clone. (B) T cells produced in bulk culture (patient G) showing oligoclonal Vβ expression in CD8+ T cells (16% Vβ16; 9% Vβ7.1; 3% each Vβ3, Vβ13.2 and Vβ17; 2% each Vβ1 and Vβ13.1; and 1% each Vβ5.1, Vβ13.6, Vβ21.3, and Vβ23) and 7 distinct TCRγ rearrangements by PCR (bottom; ■) that could correspond to between 4 and 7 different T-cell clones, depending on the number of singly and doubly rearranged clones (see Table S1). The □ in both top panels represent the average expression levels for each Vβ chain in normal polyclonal T-cell populations.
Clonality of T cells produced by limiting dilution and in bulk culture. T-cell clonality was determined by flow cytometric T-cell receptor (TCR) Vβ spectratyping (top) and by PCR amplification of clonal V-J rearrangements at the TCRγ locus (bottom). Representative results for T cells produced by limiting dilution (A) and in bulk culture (B) are shown. (A) T cells produced by limiting dilution (patient B), showing clonal expression of Vβ17 in 98% of CD8+ T cells by Vβ spectratyping (top; ■) and showing 2 predominant TCRγ rearrangements (bottom). Because each T-cell clone can rearrange one or both of its TCRγ alleles, the 2 PCR products could represent either 1 T-cell clone with biallelic TCRγ rearrangements or 2 singly rearranged clones, although the single predominant Vβ17 clone identified by spectratyping would favor a single doubly rearranged clone. (B) T cells produced in bulk culture (patient G) showing oligoclonal Vβ expression in CD8+ T cells (16% Vβ16; 9% Vβ7.1; 3% each Vβ3, Vβ13.2 and Vβ17; 2% each Vβ1 and Vβ13.1; and 1% each Vβ5.1, Vβ13.6, Vβ21.3, and Vβ23) and 7 distinct TCRγ rearrangements by PCR (bottom; ■) that could correspond to between 4 and 7 different T-cell clones, depending on the number of singly and doubly rearranged clones (see Table S1). The □ in both top panels represent the average expression levels for each Vβ chain in normal polyclonal T-cell populations.
T-cell infusions
| Patient . | Infusion 1, cells/m2* . | Infusion 2, cells/m2† . | Infusion 3, cells/m2‡ . | Fresh versus thawed cells . | Time from apheresis to target cell number, d . | No. of stimulation cycles§ . |
|---|---|---|---|---|---|---|
| A | 108 | 109 | 3.3 × 109 | Fresh | 130 | 7 |
| B | 108 | 109 | 2 × 109 | Fresh | 129+ | 7 |
| C | Expansion failed | 5 | ||||
| D | 108 | 4 × 108 | Thawed | 159+ | 7 | |
| E | Expansion failed | 5 | ||||
| F | 108 | 109 | 3.3 × 109 | Thawed | 96 | 6 |
| G | 108 | 109 | 3.3 × 109 | Thawed | 90 | 5 |
| H | 108 | 109 | 3.3 × 109 | Thawed | 81 | 5 |
| I | 108 | 109 | 2 × 109‖ | Thawed | 104 | 8 |
| Patient . | Infusion 1, cells/m2* . | Infusion 2, cells/m2† . | Infusion 3, cells/m2‡ . | Fresh versus thawed cells . | Time from apheresis to target cell number, d . | No. of stimulation cycles§ . |
|---|---|---|---|---|---|---|
| A | 108 | 109 | 3.3 × 109 | Fresh | 130 | 7 |
| B | 108 | 109 | 2 × 109 | Fresh | 129+ | 7 |
| C | Expansion failed | 5 | ||||
| D | 108 | 4 × 108 | Thawed | 159+ | 7 | |
| E | Expansion failed | 5 | ||||
| F | 108 | 109 | 3.3 × 109 | Thawed | 96 | 6 |
| G | 108 | 109 | 3.3 × 109 | Thawed | 90 | 5 |
| H | 108 | 109 | 3.3 × 109 | Thawed | 81 | 5 |
| I | 108 | 109 | 2 × 109‖ | Thawed | 104 | 8 |
For patients A through E, T cells were selected and expanded by limiting dilution. For patients F through I, T cells were expanded in bulk culture. Patients C and E did not receive T-cell infusions. Patients B and D received infusions but did not reach the target cell dose.
Target dose was 108 cells/m2.
Target dose was 109 cells/m2.
Target dose was 3.3 × 109 cells/m2.
Defined as the number of times cells were stimulated with OKT3 in the presence of irradiated feeder PBMCs and LCL (repeated every 14 days, first cycle at day 14).
The target number of cells was generated for this patient, but nearly half of the last cell dose was lost during a quality control assay before infusion.
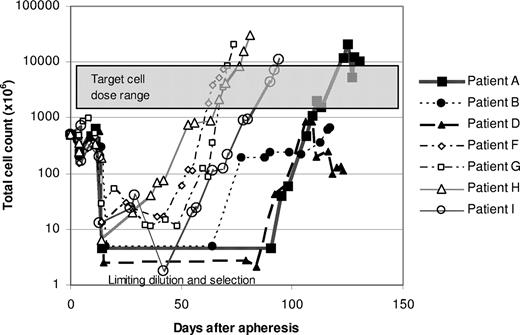
We subsequently elected to modify the protocol to include expansion of T-cell transfectants in bulk culture to circumvent the difficulties of expanding T cells after limiting dilution. Successful expansion of modified T cells was achieved for the subsequent 4 patients using this approach, and the time required to reach the target cell dose was reduced by approximately 50% (Figure 4). Vβ TCR spectratyping and TCRγ clonality testing by PCR showed more heterogeneous T-cell populations in these bulk cultures, although several of the cultures contained prominent T-cell clones (Figure 3B; Table S1). Three of these 4 patients received all planned doses of T cells. The target cell number was reached for the fourth patient as well, but the third infusion consisted of only 2 × 109 cells/m2 because of a loss of cells during a quality control assay.
Growth curves of genetically modified T cells. Patient PBMCs were transfected with the scFvFc:ζ plasmid by electroporation after stimulation with OKT3. For patients A, B, and D, populations of G418-resistant T cells were generated by limiting dilution, and T-cell cultures exhibiting the most favorable cytotoxicity and growth profiles were selected for expansion to therapeutic numbers. For patients F through I, G418-resistant cells were grown as bulk cultures.
Growth curves of genetically modified T cells. Patient PBMCs were transfected with the scFvFc:ζ plasmid by electroporation after stimulation with OKT3. For patients A, B, and D, populations of G418-resistant T cells were generated by limiting dilution, and T-cell cultures exhibiting the most favorable cytotoxicity and growth profiles were selected for expansion to therapeutic numbers. For patients F through I, G418-resistant cells were grown as bulk cultures.
Immunophenotype of modified T cells
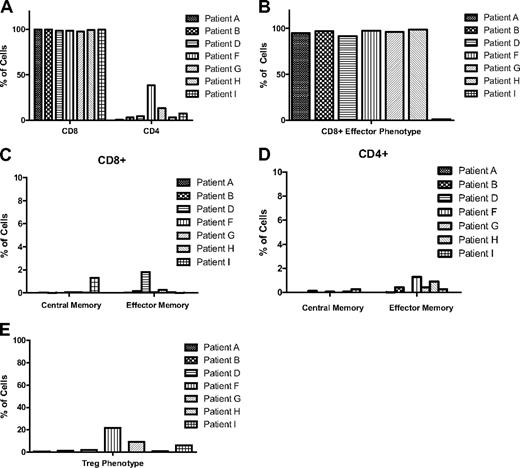
The phenotype of ex vivo–expanded cTCR-bearing T cells has not been well described. We analyzed the immunophenotype of the infused T cells using multicolor flow cytometry and found it to be similar to that of activated effector T cells,28,29 expressing CD3, CD8, and CD45RO and lacking CD62L, CCR7, and CD127 (Figure 5). As expected, patients treated with CD8+ T cells derived by limiting dilution received negligible numbers of CD4-expressing cells (0.67%-4.5%), whereas patients receiving infusions of T cells grown in bulk culture received 3.4% to 38.6% CD4+ cells. Infused T cells also expressed the activation marker CD95, but relatively few cells (1.3%-6.2%) expressed CD134 (OX40; Table 3). We found negligible numbers of cells expressing central memory (CD62L+/CCR7+/CD45RA−/CD127+) and effector memory (CD62L−/CCR7−/CD45RA−/CD127+) phenotypes.T cells resulting from bulk culture and from limiting dilution were phenotypically similar, although the former generally contained higher proportions of CD4+ cells (3.4%-38.6% compared with 0.67%-4.5%) and cells with a regulatory T (Treg)–like phenotype (CD4+/CD25+/FoxP3+) (0.54%-1.9% compared with 0.61%-21.6%). Treg functionality studies were not performed, however. All T cells exhibited low expression of costimulatory markers CD28 (0.92%-5.4%) and CD137 (0.47%-4.4%). High proportions of cells from all patients expressed adhesion molecules such as CD11a (98.7%-100%), CD44 (99.8%-100%), and CD49d (85.8%-99.6%).
Immunophenotypes of infused T cells. The phenotypes shown were determined using multicolor flow cytometry and are expressed in terms of percentage of the population of infused cells. (A) CD8+ versus CD4+ cells, (B) cells with a CD8+ effector T-cell phenotype (CD8+/CD62L−/CCR7−/CD45RA−/CD127−), (C) cells with CD8+ central memory (CD62L+/CCR7+/CD45RA−/CD127+) versus effector memory (CD62L−/CCR7−/CD45RA−/CD127+) T-cell phenotypes, (D) cells with CD4+ central memory versus effector memory T-cell phenotypes, and (E) cells with a regulatory T-cell (Treg) phenotype (CD4+/CD25+/FoxP3+) are shown.
Immunophenotypes of infused T cells. The phenotypes shown were determined using multicolor flow cytometry and are expressed in terms of percentage of the population of infused cells. (A) CD8+ versus CD4+ cells, (B) cells with a CD8+ effector T-cell phenotype (CD8+/CD62L−/CCR7−/CD45RA−/CD127−), (C) cells with CD8+ central memory (CD62L+/CCR7+/CD45RA−/CD127+) versus effector memory (CD62L−/CCR7−/CD45RA−/CD127+) T-cell phenotypes, (D) cells with CD4+ central memory versus effector memory T-cell phenotypes, and (E) cells with a regulatory T-cell (Treg) phenotype (CD4+/CD25+/FoxP3+) are shown.
Flow cytometric immunophenotypes of infused T cells
| Marker . | Patient A . | Patient B . | Patient D . | Patient F . | Patient G . | Patient H . | Patient I . |
|---|---|---|---|---|---|---|---|
| CD2 | 100 | 100 | 99 | 100 | 99.9 | 100 | 99.8 |
| CD3 | 99 | 99.8 | 99 | 99.9 | 99.9 | 99.9 | 99.8 |
| CD4 | 0.67 | 3.2 | 4.5 | 38.6 | 13.6 | 3.4 | 7.8 |
| CD5 | 5.4 | 25.3 | 93 | 85.8 | 97.3 | 93.8 | 92.3 |
| CD8 | 99.8 | 99.7 | 98.5 | 98.5 | 97.7 | 99.4 | 99.6 |
| CD11a | 100 | 100 | 98.7 | 99.9 | 99.8 | 99.9 | 99.8 |
| CD25 | 2.4 | 45.9 | 22.1 | 23.2 | 52.5 | 52.7 | 16.6 |
| CD28 | 0.92 | 3.2 | 4.4 | 2.6 | 5.4 | 1.9 | 2.7 |
| CD44 | 99.8 | 100 | 99.5 | 99.9 | 100 | 99.9 | 99.9 |
| CD45RA | 61.5 | 14.1 | 2.3 | 3.1 | 9.5 | 2.2 | 23.0 |
| CD45RO | 96.8 | 99.3 | 99.1 | 99.4 | 99.4 | 99.2 | 99.8 |
| CD49d | 85.8 | 98.7 | 91.2 | 95.2 | 99.6 | 98.1 | 97.3 |
| CD56 | 99.9 | 98.4 | 98.7 | 95.7 | 91.7 | 95.8 | 99.3 |
| CD62L | 1.3 | 6.8 | 4.6 | 2.1 | 6.2 | 2.7 | 2.5 |
| CD94 | 98.8 | 67.3 | 38.7 | 32.8 | 7.1 | 17.6 | 33.6 |
| CD95 | 99.4 | 99.5 | 99.8 | 98.1 | 98.4 | 99.6 | 98.6 |
| CD134 | 1.3 | 2.9 | 6.2 | 4.1 | 5.3 | 3.2 | 4.5 |
| CD137 (4-1BB) | 0.47 | 1.9 | 4.4 | 1.2 | 3.1 | 0.69 | 3.7 |
| CD154 (CD40L) | 12.5 | 3.3 | 3.2 | 4.4 | 2.6 | 0.64 | 24.4 |
| CD314 (NKG2D) | 99.5 | 97.7 | 92.6 | 93.7 | 99.2 | 95.3 | 97.4 |
| CCR5 (CD195) | 58.7 | 43.1 | 58.1 | 27.9 | 85.9 | 6.0 | 96.8 |
| CCR6 | 5.5 | 31.1 | 10.8 | 3.4 | 10.7 | 12.3 | 9.9 |
| CCR7 | 0.21 | 2.3 | 4.2 | 2.1 | 4.6 | 0.75 | 5.4 |
| CXCR3 | 97 | 89.9 | 91.9 | 95.2 | 97.5 | 93.1 | 98.1 |
| CXCR4 | 6.5 | 9.6 | 6.1 | 3.9 | 21.1 | 9.7 | 2.6 |
| CXCR5 | 1.1 | 3.1 | 3.8 | 2.7 | 6.1 | 2.0 | 3.5 |
| TCR α/β | 92.8 | 99.2 | 98.7 | 99 | 96 | 99.7 | 99.7 |
| Marker . | Patient A . | Patient B . | Patient D . | Patient F . | Patient G . | Patient H . | Patient I . |
|---|---|---|---|---|---|---|---|
| CD2 | 100 | 100 | 99 | 100 | 99.9 | 100 | 99.8 |
| CD3 | 99 | 99.8 | 99 | 99.9 | 99.9 | 99.9 | 99.8 |
| CD4 | 0.67 | 3.2 | 4.5 | 38.6 | 13.6 | 3.4 | 7.8 |
| CD5 | 5.4 | 25.3 | 93 | 85.8 | 97.3 | 93.8 | 92.3 |
| CD8 | 99.8 | 99.7 | 98.5 | 98.5 | 97.7 | 99.4 | 99.6 |
| CD11a | 100 | 100 | 98.7 | 99.9 | 99.8 | 99.9 | 99.8 |
| CD25 | 2.4 | 45.9 | 22.1 | 23.2 | 52.5 | 52.7 | 16.6 |
| CD28 | 0.92 | 3.2 | 4.4 | 2.6 | 5.4 | 1.9 | 2.7 |
| CD44 | 99.8 | 100 | 99.5 | 99.9 | 100 | 99.9 | 99.9 |
| CD45RA | 61.5 | 14.1 | 2.3 | 3.1 | 9.5 | 2.2 | 23.0 |
| CD45RO | 96.8 | 99.3 | 99.1 | 99.4 | 99.4 | 99.2 | 99.8 |
| CD49d | 85.8 | 98.7 | 91.2 | 95.2 | 99.6 | 98.1 | 97.3 |
| CD56 | 99.9 | 98.4 | 98.7 | 95.7 | 91.7 | 95.8 | 99.3 |
| CD62L | 1.3 | 6.8 | 4.6 | 2.1 | 6.2 | 2.7 | 2.5 |
| CD94 | 98.8 | 67.3 | 38.7 | 32.8 | 7.1 | 17.6 | 33.6 |
| CD95 | 99.4 | 99.5 | 99.8 | 98.1 | 98.4 | 99.6 | 98.6 |
| CD134 | 1.3 | 2.9 | 6.2 | 4.1 | 5.3 | 3.2 | 4.5 |
| CD137 (4-1BB) | 0.47 | 1.9 | 4.4 | 1.2 | 3.1 | 0.69 | 3.7 |
| CD154 (CD40L) | 12.5 | 3.3 | 3.2 | 4.4 | 2.6 | 0.64 | 24.4 |
| CD314 (NKG2D) | 99.5 | 97.7 | 92.6 | 93.7 | 99.2 | 95.3 | 97.4 |
| CCR5 (CD195) | 58.7 | 43.1 | 58.1 | 27.9 | 85.9 | 6.0 | 96.8 |
| CCR6 | 5.5 | 31.1 | 10.8 | 3.4 | 10.7 | 12.3 | 9.9 |
| CCR7 | 0.21 | 2.3 | 4.2 | 2.1 | 4.6 | 0.75 | 5.4 |
| CXCR3 | 97 | 89.9 | 91.9 | 95.2 | 97.5 | 93.1 | 98.1 |
| CXCR4 | 6.5 | 9.6 | 6.1 | 3.9 | 21.1 | 9.7 | 2.6 |
| CXCR5 | 1.1 | 3.1 | 3.8 | 2.7 | 6.1 | 2.0 | 3.5 |
| TCR α/β | 92.8 | 99.2 | 98.7 | 99 | 96 | 99.7 | 99.7 |
Values represent percentages of total cell population.
Cytotoxicity of modified T cells in vitro
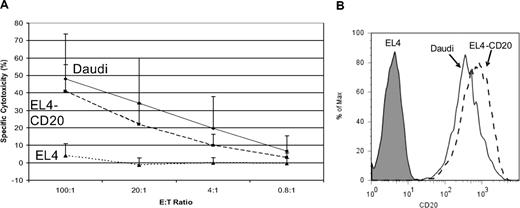
Our group showed in preclinical studies that T-cell clones bearing CD20-specific cTCRs exhibit antigen-specific cytotoxicity.20 We assessed the cytotoxicity of T cells used in this trial by coincubation with 51Cr-labeled CD20+ target cells (Daudi lymphoma cells and EL4 mouse lymphoma cells transfected to express human CD20), and the expanded T cells used for all 7 patients killed CD20+ lymphoma cells in an antigen-specific manner (Figure 6).
Cytotoxicity of modified T cells. Standard chromium release assays were performed using preinfusion-modified T cells at 8 to 12 days after restimulation, using the following MHC-mismatched target cells: EL4-CD20, a murine T-cell lymphoma line transfected to express the human CD20 molecule (----), the parental EL4 nontransfected CD20− line (…), and the Daudi CD20+ Burkitt lymphoma cell line (—), at the E:T ratios shown. The calculated specific cytolysis values are displayed as percentages. (A) The CD20-specific cytotoxicity of the reinfused T cells. Data shown represent the mean combined data from all treated patients (± 1 SD). Triplicate assays were performed for each patient. (B) CD20 expression of the target cell lines of EL4, EL4-CD20, and Daudi, as determined by flow cytometry using PE-labeled mouse anti–human CD20 Ab.
Cytotoxicity of modified T cells. Standard chromium release assays were performed using preinfusion-modified T cells at 8 to 12 days after restimulation, using the following MHC-mismatched target cells: EL4-CD20, a murine T-cell lymphoma line transfected to express the human CD20 molecule (----), the parental EL4 nontransfected CD20− line (…), and the Daudi CD20+ Burkitt lymphoma cell line (—), at the E:T ratios shown. The calculated specific cytolysis values are displayed as percentages. (A) The CD20-specific cytotoxicity of the reinfused T cells. Data shown represent the mean combined data from all treated patients (± 1 SD). Triplicate assays were performed for each patient. (B) CD20 expression of the target cell lines of EL4, EL4-CD20, and Daudi, as determined by flow cytometry using PE-labeled mouse anti–human CD20 Ab.
Adoptive therapy with CD20-specific T cells is safe and well tolerated
Seven patients received a total of 20 T-cell infusions. No grade 3 or 4 toxicities were seen, and no adverse events attributable to the T-cell infusions themselves were observed. Grade 2 toxicities associated with subcutaneous IL-2 injections included a flulike syndrome (1 of 4 patients), fever (1 of 4 patients), and skin reactions at injection site (2 of 4 patients) and resolved after the cessation of IL-2. Grade 1 toxicities attributable to IL-2 included chills, myalgias, dyspnea, dysgeusia, malaise or fatigue, diaphoresis, and injection site reactions (Table 4).
Adverse events possibly or probably related to the treatment regimen
| Patient* . | Grade 1 . | Grade 2 . |
|---|---|---|
| A | None | None |
| B | None | None |
| D | None | None |
| F | Chills, myalgias, and shortness of breath (all during IL-2 treatment) | Injection site skin reaction (during IL-2 treatment) |
| G | None | Injection site skin reaction (during IL-2 treatment) |
| H | Dysgeusia, fatigue, diaphoresis, and injection site skin reactions (all during IL-2 treatment) | Flulike syndrome (during IL-2 treatment) |
| I | Malaise/fatigue, chills, injection site reactions, and dyspnea (all during IL-2 treatment) | Fever (during IL-2 treatment) |
| Patient* . | Grade 1 . | Grade 2 . |
|---|---|---|
| A | None | None |
| B | None | None |
| D | None | None |
| F | Chills, myalgias, and shortness of breath (all during IL-2 treatment) | Injection site skin reaction (during IL-2 treatment) |
| G | None | Injection site skin reaction (during IL-2 treatment) |
| H | Dysgeusia, fatigue, diaphoresis, and injection site skin reactions (all during IL-2 treatment) | Flulike syndrome (during IL-2 treatment) |
| I | Malaise/fatigue, chills, injection site reactions, and dyspnea (all during IL-2 treatment) | Fever (during IL-2 treatment) |
None of the patients experienced any grade 3 or grade 4 events.
Patients C and E are omitted because they did not receive T-cell infusions.
In vivo persistence of modified T cells
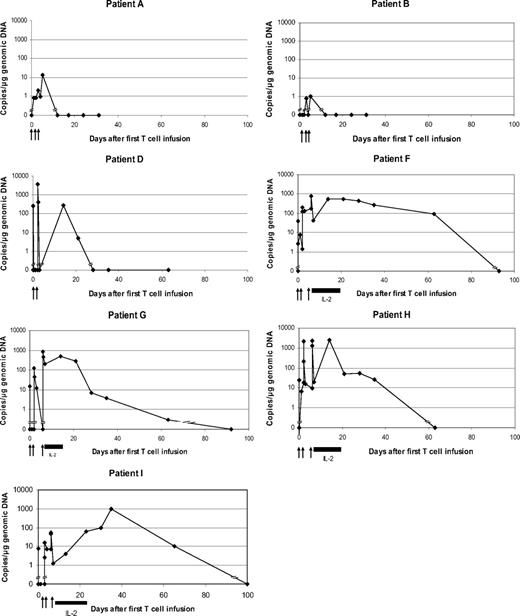
We measured the in vivo persistence of modified T cells using quantitative real-time PCR of DNA from patient PBMCs collected at serial time points after T-cell infusion. Modified T cells were detectable by PCR for 5 to 21 days in the first 3 patients receiving T cells without adjuvant IL-2. In contrast, modified T cells were detectable for 5 to 9 weeks in the last 4 patients, who received T cells produced in bulk culture and 14 days of subcutaneous IL-2 (Figure 7).
In vivo persistence of modified T cells. Genomic DNA was isolated from patient PBMCs collected at serial time points after T-cell infusions and used for quantitative real-time PCR using one primer within the human CD3ζ gene and the other from the adjacent CD4 transmembrane region in the scFvFc:ζ plasmid. The copy number of scFvFc:ζ-specific DNA based on quantitative reverse transcription-PCR results for all treated patients is shown. Arrows denote T-cell infusions, and horizontal black bars indicate the period of subcutaneous IL-2 injections for patients F, G, H, and I. Modified T cells were detectable for 12, 5, 21, 63, 63, 35, and 65 days, respectively, in the 7 patients.
In vivo persistence of modified T cells. Genomic DNA was isolated from patient PBMCs collected at serial time points after T-cell infusions and used for quantitative real-time PCR using one primer within the human CD3ζ gene and the other from the adjacent CD4 transmembrane region in the scFvFc:ζ plasmid. The copy number of scFvFc:ζ-specific DNA based on quantitative reverse transcription-PCR results for all treated patients is shown. Arrows denote T-cell infusions, and horizontal black bars indicate the period of subcutaneous IL-2 injections for patients F, G, H, and I. Modified T cells were detectable for 12, 5, 21, 63, 63, 35, and 65 days, respectively, in the 7 patients.
Modified T cells were also detectable by PCR in the bone marrow 24 hours after the final T-cell infusion in patients A, D, F, and G (lymphoma cells were detectable in the marrow by flow cytometry only in patients G and H at this time point). Of the 7 treated patients, only patients B and G had accessible lymph nodes for biopsy after T-cell infusions. The lymph node from patient B showed only fibroadipose tissue, and patient G's lymph node showed tumor, but no modified T cells were detectable.
Modified T cells were not immunogenic
The introduction of foreign transgenes in therapeutic vectors has resulted in immune responses against modified T cells in previous gene therapy clinical trials.27,30 Given the significant immunosuppression present in patients with lymphoma, we hypothesized that the transgenic cells in this trial might elicit lower immune response rates, and we used 3 assays to test this hypothesis. To detect cellular immune responses, 51Cr-release assays were performed with serially collected patient PBMCs that had been coincubated with irradiated T cells expressing the scFvFc:ζ and neoR gene products (Figure S1A), using transfected and nontransfected T cells as target cells. In 2 patients the antigen responsiveness of recovered T cells was confirmed using allogeneic LCL as target cells; in both cases a cytotoxic response was elicited at all time points tested (Figure S1B). For humoral immune responses, patient serum collected at various time points after T-cell infusion was assessed in an ELISA assay in which the Leu-16 murine anti–human CD20 Ab served as an internal control (Figure S1C). Although it is theoretically possible that immune responses against other cTCR epitopes (eg, junctional regions) could be missed with this assay, the Leu-16 murine domain is expected to be the dominant immunogenic cTCR epitope because all the other domains are of human derivation. The absence of anti-cTCR Ab in patient sera at serial time points was confirmed by flow cytometry (Figure S2). No humoral or cellular immune responses were observed with these assays in any of the 7 patients who received infusions of genetically modified T cells. Two patients, however, were subsequently found to be seropositive for HAMA, 3 and 12 months after T-cell infusions, respectively. One of those patients had been previously treated with a radioiodinated murine monoclonal Ab (tositumomab).
Effect of adoptive T-cell therapy on native lymphocyte subsets
Anti-CD20 Ab therapy substantially depletes normal CD20+ B cells,31 and adoptive anti-CD20 T-cell therapy was therefore also expected to reduce circulating B-cell numbers. We found, however, that, although B-cell counts as measured by flow cytometry fluctuated during the period of T-cell infusions, the number of CD20+ B cells remained stable or slightly increased in the months after treatment in all patients (Table S2). Other lymphocyte subsets (CD3+, CD4+, CD8+, CD56+, and FoxP3+ cells) showed similar vacillations before and after T-cell infusions, but no consistent trends were noted (Table S3).
Responses to T-cell therapy
Two of the 7 patients achieved a complete response to cytoreductive chemotherapy administered before the T-cell infusions and remained disease-free 3 months and 13 months after T-cell infusions (Table 5). Another patient attained an objective partial response lasting 3 months after treatment with T-cell infusions plus IL-2. Four patients exhibited stable disease for 3, 5, 6, and 12 months. One of these patients showed decreased uptake of fluorodeoxyglucose, a marker for viable tumor metabolism, on positron emission tomographic scanning 3 months after T-cell infusions.
Clinical responses
| Patient* . | Response to cytoreductive chemotherapy . | Response to T-cell Infusions† . | Duration of response after T-cell infusions, mo‡ . |
|---|---|---|---|
| A | CR | NED | 13 |
| B | PR | SD | 3 |
| D | SD | SD | 12 |
| F | CR | NED | 3 |
| G | Did not receive chemotherapy | SD (with FDG response on PET scan) | 5 |
| H | PR | PR§ | 3 |
| I | PR | SD | 6 |
| Patient* . | Response to cytoreductive chemotherapy . | Response to T-cell Infusions† . | Duration of response after T-cell infusions, mo‡ . |
|---|---|---|---|
| A | CR | NED | 13 |
| B | PR | SD | 3 |
| D | SD | SD | 12 |
| F | CR | NED | 3 |
| G | Did not receive chemotherapy | SD (with FDG response on PET scan) | 5 |
| H | PR | PR§ | 3 |
| I | PR | SD | 6 |
CR indicates complete response; NED, no evidence of disease; PR, partial response; SD, stable disease; FDG, fluorodeoxyglucose; and PET, positron emission tomography.
Patients C and E are omitted because they did not receive T-cell infusions.
Response assessment was performed compared with the restaging imaging after cytoreductive chemotherapy.
All patients progressed after the times listed.
Patient H achieved an additional objective PR after T-cell infusions.
Discussion
MCL and indolent NHL almost invariably relapse after standard therapy despite complete clinical remissions, suggesting the existence of a minimal residual disease state in which a small number of cells resistant to chemotherapy, radiation, or Ab therapy persist and eventually lead to recurrent lymphoma. These cells appear to be susceptible to cellular immune responses, however, because patients undergoing nonmyeloablative allogeneic SCT or DLI can achieve long-term disease-free remissions, suggesting cure.11 However, the high morbidity and mortality rates associated with these treatments,9,32 primarily resulting from graft-versus-host disease, indicate a need for less-toxic forms of cellular therapy. One such strategy is infusion of ex vivo–expanded autologous T cells modified to recognize lymphoma-associated surface antigens.
We initiated a proof-of-concept clinical trial to test the safety and feasibility of this approach and found that autologous PBMCs from patients with lymphoma could be reliably modified to express a CD20-specific cTCR, expanded ex vivo, and reinfused with minimal toxicities. The process of generating therapeutic numbers of genetically modified anti-CD20 T cells by limiting dilution proved laborious, however, and the target cell dose was reached in only 1 of 5 patients. In contrast, expansion of transfected cells produced in bulk cultures was reliable, more efficient, and may also facilitate longer in vivo persistence in patients receiving such T cells requiring a shorter ex vivo expansion period. All patients tolerated the T-cell infusions well, with no adverse events attributable to T cells, and only grade 1 to 2 toxicities associated with IL-2 injections.
Adoptively transferred T cells presumably must survive in vivo for many weeks to effect a complete clinical response, and establishing memory cell responses may be required to produce durable remissions. We found that the administration of low-dose IL-2 after T-cell infusions prolonged in vivo persistence of modified T cells, consistent with previous observations.33 Immunophenotypic differences between T cells produced by limiting dilution and those produced in bulk cultures were minimal, suggesting this was probably not the dominant reason for longer survival of the T cells from bulk cultures, although the shorter culture time may have contributed by decreasing replicative senescence. These questions cannot be directly assessed from our data because the change in culture methods was coincident with initiation of adjuvant IL-2 treatment. The administration of lymphodepleting chemotherapy before adoptive T-cell transfer is another factor potentially affecting in vivo persistence by enhancement of homeostatic proliferation by IL-7 and IL-15 up-regulation,34,35 depletion of Treg cells,36 and suppression of host immune responses to modified cells. Recent T-cell trials for melanoma incorporating aggressive lymphodepletion regimens have shown long periods of T-cell survival in some patients.37,38 In the present trial, few compelling conclusions can be drawn as to the potential contribution of lymphodepletion to T-cell persistence because of the limited number of patients, the variability in chemotherapy regimens used, and the required 4-week interval between chemotherapy and T-cell infusions.
Recent data by Berger et al39 indicate that CD8+ T cells derived from central memory cells (TCM) persist much longer in vivo than those derived from effector memory cells, raising the question of whether the longer persistence of T cells produced in bulk cultures in this study could be due to the presence of TCM-derived cells among the infused T cells. Although this is theoretically possible, the low transfection efficiency by electroporation (0.002%-0.004%)24 and the small number of clones infused make it unlikely that any infused cells were TCM derived. It is much more likely that the difference in persistence in our study was due primarily to the IL-2 injections. Nevertheless, it may be important in future clinical trials to select, modify, and expand CD62L+ cells to establish a population of persisting TCM.
The expansion of genetically modified T cells produced by limiting dilution confers the theoretical advantage of permitting selection of cells with an in vitro cytotoxicity that exceeds the mean of a population of unselected cells in bulk culture. We observed, however, that the highest lytic activity and best proliferative capacity usually did not coincide in the same clonal population, and, consequently, expansion of the most cytolytic T cells was problematic. We believe, therefore, that the more favorable growth kinetics and apparently longer in vivo persistence of T cells produced in bulk cultures outweighs any minor differences in cytotoxicity. T cells from all patients showed CD20-specific cytotoxicity despite the low levels of cTCR they expressed.
Efficacy was not a primary end point of this proof-of-concept study, but clearly that is the long-term goal. Objective clinical responses were modest, with one unequivocal partial remission among the 5 patients with evaluable disease at the time of T-cell infusions. There are many possible explanations for the limited therapeutic antitumor activity in this trial, including insufficient numbers of surviving modified T cells, CD20 antigen competition from native B cells, ineffective localization of T cells to tumor sites, inadequate cTCR surface expression leading to poor killing, and lack of costimulatory signaling from the cTCR construct. The suboptimal in vitro cytotoxicity of these cells suggests that the latter 2 factors were probably at least partially responsible. We plan to address some of these pitfalls in a subsequent trial by using T cells engineered with a “second-generation” anti-CD20 cTCR that includes CD28 and CD137 costimulatory domains and an SP163 translational enhancer. T cells transfected with this second-generation cTCR have shown superior proliferation, surface expression, and cytotoxicity in preclinical studies.25
Another possible explanation for the modest responses is competitive inhibition of cTCR-antigen binding by residual anti-CD20 Ab present in the serum of patients previously treated with rituximab, ibritumomab, or tositumomab. However, none of the treated patients (with the exception of patient I, who received 131I-tositumomab 4 months before T-cell infusions) had been exposed to anti-CD20 Ab within 1 year of T-cell infusions. Therefore, with the exception of this patient, it is unlikely that significant circulating levels of anti-CD20 Ab were present at the time of adoptive T-cell transfer. It is somewhat surprising that significant changes in circulating B-cell levels were not seen in this study, in view of the well-documented B-cell depletion associated with rituximab treatment.31,40 We do not know whether this reflects a resistance of normal resting B cells in vivo to the cytolytic activity of the cTCR+ effector cells or a demonstration that adoptive T-cell immunotherapy with the “first-generation” plasmid as implemented in this trial has insufficient in vivo activity. This issue may be more clearly resolved after testing T cells expressing cTCR with the augmented second-generation plasmid described earlier.
In summary, adoptive immunotherapy with anti-CD20 cTCR-bearing T cells is a safe, feasible, and well-tolerated treatment for patients with relapsed or refractory indolent NHL, although clinical responses were modest. Expansion of transfected T cells produced in bulk cultures proved to be more efficient for achieving therapeutic numbers than generating T cells by limiting dilution. Furthermore, in vivo persistence of T cells was longer in patients receiving T cells produced by bulk cultures and low-dose IL-2 than in patients receiving T cells produced by limiting dilution and administered without subcutaneous IL-2. These results are encouraging but identify areas for improvement to be addressed in future adoptive T-cell therapy trials.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The plasmid used in these studies was produced by the National Gene Vector Laboratory at the City of Hope, Duarte, CA.
This work was supported by National Institutes of Health (NIH) grants R01 CA92302 and R21 CA-117131-01A (O.W.P.), Lymphoma Research Foundation grant MCLI-07-012 (O.W.P.) and Career Development Award (B.G.T.), American Society of Clinical Oncology Young Investigator Award (B.G.T.), and by gifts from David and Patricia Giuliani, Geary and Mary Britton-Simmons, the Hext Family Foundation, and the Edson Foundation. A portion of this work was conducted through the Clinical Research Center Facility at the University of Washington and supported by NIH grant M01-RR-00 037.
National Institutes of Health
Authorship
Contribution: B.G.T. enrolled patients, collected and analyzed data, and wrote the paper; M.C.J. designed the plasmid used to modify the T cells and developed the treatment protocol; J.W. performed PCR, cytotoxicity, and immune response assays; E.Y.C. enrolled patients and collected and analyzed data; B.L.W. and H.A.G. performed flow cytometry and TCR spectratyping experiments and contributed to writing the paper; X.Q. performed flow cytometry and humoral immune response assays; S.E.J. analyzed data and contributed to the design of experiments; A.R. and S.J.F. contributed to trial design; A.K.G. and J.M.P. enrolled patients and contributed to the interpretation of data; C.G.L. supervised T-cell transfection and expansion; P.D.G. and S.R.R. contributed to the design of the trial and experiments, provided the TM-LCL cell line, and analyzed data; and O.W.P. contributed to the conception, design, analysis, and interpretation of the clinical trial and experiments, wrote the protocol, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian Till, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D3-190, Seattle, WA 98109; e-mail: tillb@fhcrc.org.