Abstract
Leukocyte-derived microparticles (MPs) are markers of cardiovascular diseases and contribute to pathogenesis by their interaction with various cell types. The presence and activation state of a multifunctional leukocyte receptor, integrin αMβ2 (CD11b/18), on MPs derived from human neutrophils (PMNs) were examined. αMβ2 expression was significantly enhanced on MPs derived from stimulated compared with resting PMNs. Furthermore, αMβ2 on MPs from stimulated but not resting PMNs was in an activated conformation because it was capable of binding activation-specific monoclonal antibodies (CBRM1/5 and mAb24) and soluble fibrinogen. MPs expressing active αMβ2 interacted with and were potent activators of resting platelets as assessed by induction of P-selectin expression and activation of αIIbβ3. With the use of function-blocking antibodies and MPs obtained from αM−/−-deficient mice, we found that engagement of GPIbα on platelets by αMβ2 on MPs plays a pivotal role in MP binding. Platelet activation by MPs occurs by a pathway dependent on Akt phosphorylation. PSGL-1/P-selectin interaction also is involved in the conjugation of MPs to platelets, and the combination of blocking reagents to both αMβ2/GPIbα and to PSGL-1/P-selectin completely abrogates MP-induced platelet activation. Thus, cooperation of these 2 receptor/counterreceptor systems regulates the prothrombotic properties of PMN-derived MPs.
Introduction
Leukocyte-platelet cross-talk plays multiple and important roles in inflammatory and thrombotic responses.1 Activated platelets and platelet-released mediators, such as PDGF and PAF, activate leukocytes enhancing their responses such as adhesion, chemotaxis, phagocytosis, and superoxide generation.2-5 Conversely, activated leukocytes induce platelet activation as evidenced by increased platelet P-selectin expression.6 In vivo studies show that leukocytes and platelets colocalize within atherosclerotic and restenotic lesions, at sites of hemorrhage and ischemia-reperfusion injury.7-10 Indeed, increased levels of leukocyte-platelet aggregates are found in patients with diabetes, acute coronary syndromes, trauma, and sepsis.11-13
Numerous studies have analyzed the receptors involved in leukocyte-platelet interactions. P-selectin on activated platelets binds PSGL-1 on leukocytes14,15 to support their rolling on adherent platelets and to activate leukocyte integrin αMβ2, which mediates firm leukocyte adhesion.16-18 Several αMβ2 counterreceptors on platelets have been proposed, including Fg-bound αIIbβ3,19 GPIbα,20,21 and JAM-3.22 Recognition of these counterreceptors requires that αMβ2 be in an activated conformation. αMβ2 can be activated by stimulation of leukocytes by agonists and can be monitored with monoclonal antibodies (mAbs), CBRM1/5 and mAb24, which react selectively with the activated conformation.
Microparticles (MPs) are released by membrane blebbing from cells undergoing activation or apoptosis. MPs are usually defined by 2 criteria: size (< 1 μm) and surface expression of negatively charged phosphatidylserine (reviewed in Boulanger et al,23 Morel et al,24 Martinez et al,25 and Distler et al26 ). MPs are not simply the markers of cell activation or damage but may exert pleiotropic effects on blood and vascular cells. MPs of platelet and endothelial origin have procoagulant and proinflammatory activities (reviewed in Boulanger et al,23 Morel et al,24 Martinez et al,25 and Distler et al26 ) and are markers of acute coronary syndromes.27,28 The functions of leukocyte-derived MPs are less clear. MPs shed by PMNs behave as inflammatory mediators and activate endothelial cells29,30 but also exert antiinflammatory effects on macrophages.31 One of the most important features of leukocyte-derived MPs is the expression of tissue factor32 and negatively charged phospholipids, suggesting that they may contribute to blood coagulation. Indeed, in vivo studies have shown that leukocyte MPs are captured by activated platelets within thrombi by a P-selectin/PSGL-1–dependent mechanism that leads to accumulation of tissue factor and ultimately enhanced fibrin deposition.33,34 Although the interactions of leukocyte-derived MPs with activated platelets have been well documented, additional mechanisms in which leukocyte MPs may activate resting platelets, when platelet P-selectin expression is low, have not been examined. In this study, we show that MPs released from stimulated PMNs contain αMβ2 in a functionally active conformation in which it can mediate their interaction with resting platelets. As a consequence, platelets become activated, increase their P-selectin expression, and perpetuate thrombus formation.
Methods
Monoclonal antibodies and reagents
Lerner Research Institute's internal animal care and use committee review approved this study. Mouse phycoerythrin (PE)-labeled anti–human P-selectin, anti–human αM (ICRF44), Fluorescein isothiocyanate (FITC)–labeled anti–human CD66b, FITC-labeled anti–mouse P-selectin, PE-labeled anti–mouse αIIb, FITC-conjugated PAC-1 antibody,35 and mouse FITC- and PE-labeled isotype control antibodies were from BD PharMingen (San Diego, CA). PE-conjugated anti–human αM activation epitope, CBRM1/5, was from eBioscience (San Diego, CA),36 and anti-αM mAb24 was a gift from Dr Nancy Hogg (London Research Institute, London, United Kingdom).37 CBRM1/5, mAb24, and ICRF44 were biotinylated using a protein biotinylation kit from Pierce (Rockford, IL). PE-conjugated anti–mouse αM and anti–human PSGL-1 were from Calbiochem (San Diego, CA). Mouse anti–human αIIb was from Chemicon International (Temecula, CA). Alexa488-labeled anti–mouse Gr-1 was from Serotec (Oxford, United Kingdom). Function-blocking monoclonal antibodies (mAbs) used were as follows: anti–human GPIbα (VM16d), Accurate Chemical (Westbury, NY)20 ; anti–human PSGL-1 (PL1), Abcam (Cambridge, United Kingdom)38 ; anti–human P-selectin (WAPS12.2),39 anti–human αM (44a, 904), anti–human β2 (IB4)40 (ATCC, Manassas, VA). Fg was from Enzyme Research Laboratories (South Bend, IN), and NIF was from Corvas International (San Diego, CA). Flow Cytometry Size Calibration and AlexaFluor Protein Labeling kits were from Invitrogen (Carlsbad, CA). PMA, lipopolysaccharide (LPS), platelet activating factor (PAF), thrombin, and Akt inhibitor II were from Calbiochem (San Diego, CA). ADP was from Sigma-Aldrich (St Louis, MO). Immunopure F(ab′)2 goat anti–mouse IgG was from Pierce.
Neutrophil and platelet preparations
Cleveland Clinic Institutional Review Board approval and informed consent were obtained from all blood donors and in accordance to the Declaration of Helsinki. PMNs were isolated as described.41 Platelets were isolated by differential centrifugation followed by gel filtration on Sepharose 2B-CL.
αM−/−-Deficient mice were backcrossed for more than 12 generations into a C57BL/J6 background.42 To isolate mouse platelets and PMNs, blood was collected from hearts of anesthetized mice using acid-citrate-dextrose plus 1 μg/mL prostaglandin E1 as an anticoagulant and diluted 1:1 with Tyrode buffer (137 mM NaCl, 12 mM NaHCO3, 2.55 mM KCl, 4.4 mM MgCl2, pH 7.2) before centrifugation at 100g. Leukocytes and platelets were obtained from blood pooled from 5 to 7 mice.
PMN MPs
All buffers used during MP preparation and fluorescence-activated cell sorting (FACS) were filtered (0.2 μm). Freshly isolated PMNs were resuspended at 3 × 106 cells/mL in HBSS buffer (pH 7.4) and stimulated in the presence or absence of Ca2+/Mg2+ (1 mM each), PMA (50 nM), PAF (500 nM), or LPS (5 μg/mL) for 15 to 30 minutes at 37°C. PMNs were removed by centrifugation (4000g, 20 minutes), and MPs were concentrated from PMN supernatants and washed using Centricon Plus-20 centrifugal filters (5000 MW cut-off, Millipore, Billerica, MA). Protein concentrations in MP preparations were measured using Bio-Rad DC Protein Assays (Hercules, CA). On FACS confirming MP size and CD66b expression, they were stored in aliquots at −80°C until use. A single freeze/thaw cycle did not alter the activity of MPs. MPs from mouse PMNs were obtained as described above with the exception that MPs were recovered from PMN supernatants by ultracentrifugation at 100 000g for 1 hour, washed with HBSS buffer, and tested for Gr-1 expression, a mouse PMN marker. MPs derived from resting MPs are defined in this study as “resting” MPs, and MPs derived from PMA or PAF-stimulated PMNs are designated “PMA” or “PAF” MPs.
MP identification and characterization
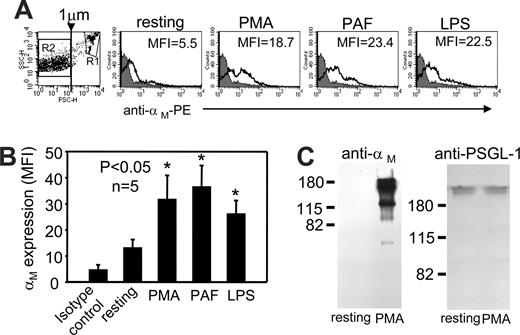
Isolated human PMNs were either resting or agonist-stimulated as described in “PMN MPs” in the presence of Alexa488-labeled human Fg (20 μg/mL), FITC-labeled anti-CD66b, anti–αM-PE (ICRF44), anti–αM-PE (CBRM1/5), mAb24, or PE- or FITC-labeled isotype control antibodies. Samples were analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ). MPs were identified on the basis of forward-scatter (FSC) and side-scatter (SSC). FSC and SSC channels were set at logarithmic gains to analyze particles of various sizes, and the MP gate R2 (ie, ≤ 1 μm; Figure 1A) was defined using 1-μm calibration beads. When PMNs were separated from MPs, events representing PMNs were present in gate R1 (Figure 1A), whereas MPs recovered by ultracentrifugation were detected in R2. Events falling in the R2 gate were positive for CD66b, confirming their identity as PMN-derived MPs.43 When expression levels of total and active αMβ2 on MPs were measured, 10 000 events from gate R2 were analyzed under low flow to reduce background signals.
αMβ2 is present on the surface of PMN-derived MPs. (A,B) Human PMNs were resting or stimulated with PMA (50 nM), PAF (500 nM), or LPS (5 μg/mL) in HBSS buffer containing Ca2+/Mg2+ ions (1 mM each) and PE-conjugated anti-αM mAb (clone ICRF44, black, open histograms) or PE-labeled mouse isotype control (IgG1) antibody (gray-filled histograms) for 30 minutes at 37°C. Samples were fixed with 1% paraformaldehyde and analyzed by FACS. The histogram and graph data are mean fluorescent intensities (MFIs) (± SEM) of 10 000 events from gate R2 representing MPs (Figure 1A dot plot). The data are representative of 5 experiments performed with PMNs from 5 different blood donors. (C) Protein concentration in samples of MPs derived from resting or PMA-stimulated PMNs was determined, and equal amounts of protein were resolved on 9% (left) or 6% (right) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and immunoblotted with mouse anti–human αM or PSGL-1 to confirm equal loading of resting and PMA MPs. The band patterns are representative of 3 Western blots from 3 different MPs preparations.
αMβ2 is present on the surface of PMN-derived MPs. (A,B) Human PMNs were resting or stimulated with PMA (50 nM), PAF (500 nM), or LPS (5 μg/mL) in HBSS buffer containing Ca2+/Mg2+ ions (1 mM each) and PE-conjugated anti-αM mAb (clone ICRF44, black, open histograms) or PE-labeled mouse isotype control (IgG1) antibody (gray-filled histograms) for 30 minutes at 37°C. Samples were fixed with 1% paraformaldehyde and analyzed by FACS. The histogram and graph data are mean fluorescent intensities (MFIs) (± SEM) of 10 000 events from gate R2 representing MPs (Figure 1A dot plot). The data are representative of 5 experiments performed with PMNs from 5 different blood donors. (C) Protein concentration in samples of MPs derived from resting or PMA-stimulated PMNs was determined, and equal amounts of protein were resolved on 9% (left) or 6% (right) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and immunoblotted with mouse anti–human αM or PSGL-1 to confirm equal loading of resting and PMA MPs. The band patterns are representative of 3 Western blots from 3 different MPs preparations.
MPs binding to platelets
Human and mouse MPs (200 μg) were labeled with Alexa488 using the AlexaFluor Protein Labeling Kit. When mean fluorescence intensities (MFIs) of conjugated MPs were compared by FACS, they were similar for resting and PMA MPs and also for WT and αM−/− MPs. Human or mouse platelets were incubated in Tyrode buffer plus 1 mM CaCl2 with increasing concentrations (0-50 μg/mL) of Alexa488-labeled human or mouse MPs derived from resting or PMA-stimulated PMNs. Incubation mixtures also contained PE-conjugated antibodies to human or mouse integrin αIIb (10 μg/mL) for platelet identification. After incubation for 0 to 90 minutes at 37°C, MP binding was measured immediately by FACS, and the Alexa488 MFI per 10 000 αIIb-positive events was recorded. In inhibition experiments, platelets and MPs were pretreated with respective function-blocking reagents for 20 minutes at 37°C before they were combined.
Platelet activation assays
Human platelets (6 × 106 in 150 μL) were incubated with increasing concentrations (0-100 μg/mL) of resting, PMA, or PAF MPs in Tyrode plus 1 mM CaCl2 and PE-conjugated anti–human P-selectin mAb or FITC-conjugated PAC-1 antibody for 0 to 60 minutes at 37°C. Platelets were also stimulated with thrombin (1 U/mL), ADP (10 μM), or collagen (40 μg/mL). Mouse WT platelets were incubated with 50 μg/mL MPs generated from resting or PMA-stimulated WT or αM−/− PMNs for 45 minutes at 37°C in the Tyrode buffer containing 1 mM CaCl2 and FITC-conjugated anti–mouse P-selectin. At selected time points, platelets were fixed with 1% paraformaldehyde and analyzed by FACS.
Akt activation
Human platelets were resuspended in Tyrode plus 1 mM CaCl2 (4 × 107 platelets in 200 μL) in the presence of increasing concentrations (0-100 μg/mL) of resting or PMA MPs for 30 minutes at 37°C and then lysed with 4× Laemmli buffer containing a cocktail of protease inhibitors (Roche, Indianapolis, IN) and 1 mM Na3VO4. Lysates were analyzed by Western blots using Abs to total or serine 473-phosphorylated Akt (Cell Signaling Technology, Beverly, MA). Mouse WT platelets were incubated with MPs (0-25 μg/mL) obtained from resting or PMA-stimulated mouse WT or αM−/− PMNs for 30 minutes at 37°C and analyzed for Akt activation as described in this paragraph for human platelet experiments.
Data analysis
Data are expressed as means plus or minus SEMs. A 2-tailed Student t test was performed with the use of Sigma-Plot software program (SPSS, Chicago, IL) to determine significance at P less than .05.
Results
PMN-derived MPs contain αMβ2 in an active conformation
Integrin αMβ2 is one of the major receptors mediating leukocyte-platelet interactions, which are involved in thrombus formation and the initiation and progression of atherosclerosis44 and restenosis.45 We sought to examine whether αMβ2 is present on PMN-derived MPs. Although the protein markers on MP surfaces and on parent cells are likely to overlap, expression on MPs will vary depending on agonists inducing their formation (reviewed in Boulanger et al,23 Morel et al,24 and Martinez et al,25 ). Thus, to generate MPs, freshly isolated human PMNs were stimulated with PMA, PAF, or LPS, and a PE-conjugated mAb to an activation-independent epitope within the integrin αM subunit (ICRF44) was used to monitor αMβ2 expression by FACS. PMN-derived MPs were identified based on their size, less than or equal to 1 μm.23-26 The MP gate, R2, in Figure 1A has been defined using 1-μm calibration beads. In addition, events falling into R2 were positive for annexinV and CD66b binding, a PMN-specific marker (data not shown), confirming their identity as PMN-derived MPs.43 The R1 gate includes PMNs, which were also CD66b+. In each sample, surface αM expression levels were analyzed in 10 000 MPs within the R2 gate. As shown in representative histograms in Figure 1A and summarized from 5 experiments in Figure 1B, αM expression was detected on MP populations. MPs derived from unstimulated PMNs showed low expression of the αM subunit compared with isotype control samples. These levels increased by 1.8- to 2.5-fold when MPs were released from PMNs stimulated with PMA, PAF, or LPS (P < .05; n = 5; Figure 1B). In addition, equal amounts of total protein from MPs were immunoblotted under nonreducing conditions with anti-αM (clone 44a) and anti–PSGL-1 mAbs. As shown on Figure 1C (left panel), the anti-αM blot showed a major 170-kDa band of the αM subunit as well as lesser amounts of 2 other bands (120-kDa and 50-kDa bands), which may be proteolytic products of αM. The αM subunit was found in MPs derived from PMA-stimulated PMNs, whereas it was not detected in MPs from resting cells, even with prolonged exposure of the Western blots. Serving as a loading control, PSGL-1 expression levels were similar in MPs from resting and PMA-stimulated PMNs (right panel).
Two different mAbs recognizing distinct activation-dependent epitopes within the αM subunit were used to examine the αMβ2 activation status on PMN-derived MPs: CBRM1/5 (Figure 2A,C) and mAb24 (Figure 2B,C).36,37 CBRM1/5 mAb only interacts with the I-domain of active αM integrin subunit, whereas mAb24 binds to a Mg2+- and activation-dependent epitope within the αM integrin subunit.36,37 To evaluate the extent of integrin activation on MPs, we determined the ratio of Alexa488-CBRM1/5 or Alexa488-mAb24 binding, which interact only with activated αMβ2, to ICRF44-PE binding, which measures total surface expression of αMβ2 (isotype control mAb for each was subtracted, and various reactions were all performed in the same FACS tube). MPs derived from resting PMNs contained no detectable CBRM1/5 or mAb24 binding; ie, the MFI values of samples stained with these mAbs were the same as those of isotype control mAbs. Thus, CBRM1/5/ICRF44 or mAb24/ICRF44 ratios were equal to 0 (Figure 2C) and did not increase even when biotinylated CBRM1/5 or mAb24 were used to amplify the signal (staining of MPs derived from PMA-stimulated PMNs were enhanced approximately 6-fold when biotinylated CBRM1/5 or mAb24 were used). In contrast, αMβ2 on MPs from PMA- or PAF-stimulated PMNs was in an active conformation as shown by positive staining with both CBRM1/5 and mAb24 mAbs (0.64-0.74 ± 0.08 and 0.34-0.4 ± 0.04, respectively). However, the extent of integrin activation on MPs strongly depended on the agonist used to induce their generation. PMA was the most potent stimulator of not only MP generation but also αMβ2 activation (Figure 2), based on the number of MPs per 1000 PMNs (data not shown). LPS, at concentrations as high as 5 μg/mL, was the weakest stimulator tested, and αMβ2 activation compared with the resting MPs could only be detected with mAb24 (0.22 ± 0.09; P < .05; n = 5), whereas results of CBRM1/5 stainings were statistically insignificant. On the basis of specificity of the mAbs, the differences in reactivity of LPS-MPs could be due to increased expression of active αL or αX, detected by mAb24, rather than the αM integrin subunit. One of the features of active integrins is their ability to bind soluble ligands. As shown in Figure 2D, MPs derived from PMA-stimulated PMNs bound soluble Alexa488-labeled human Fg in the αMβ2-specific manner; this interaction was abrogated by function-blocking mAbs to the αM (44a) and β2 subunits (IB4) but not by the control anti–MHC-1 mAb (W6/32). In contrast, Fg binding to MPs generated by resting PMNs, was low and αMβ2 independent. These data are consistent with our results showing no active αMβ2 (negative for CBRM1/5 and mAb24 binding) on resting MPs and indicate that active and functional αMβ2 is present on MPs generated from stimulated PMNs.
αMβ2 Present on PMN-derived MPs is in active conformation and functional. (A-C) The samples were prepared from human PMNs and analyzed as described in Figure 1. The mAbs used were directed to activation-dependent epitopes within the αM subunit: CBRM1/5-PE (A), mAb 24-Alexa488 (B) (black, open histograms), or mouse antibody isotype controls (PE-IgG1) and Alexa488-IgG1 (gray-filled histograms). (C) Alternatively, the samples were stained in the same FACS tube with mixtures of CBRM1/5-Alexa488 plus ICRF44-PE or mAb24-Alexa488 plusICRF44-PE mAbs, and ratios of binding of the αMβ2 activation-specific mAbs to total αMβ2 (measured with ICRF44) were calculated. (D) Human PMNs were pretreated with function-blocking mAbs to the αM (44a), the β2 (IB4), or MHC-1 (W6/32) mAbs (10 μg/mL) for 15 minutes at 37°C and then either left resting or stimulated with PMA (50 nM) in the absence or presence of Alexa488-labeled human Fg (20 μg/mL) for 30 minutes at 37°C. The samples were fixed, and 10 000 events falling into gate R2 (Figure 1A dot plot) were analyzed. The data are MFI (± SEM) and are representative of 5 independent experiments.
αMβ2 Present on PMN-derived MPs is in active conformation and functional. (A-C) The samples were prepared from human PMNs and analyzed as described in Figure 1. The mAbs used were directed to activation-dependent epitopes within the αM subunit: CBRM1/5-PE (A), mAb 24-Alexa488 (B) (black, open histograms), or mouse antibody isotype controls (PE-IgG1) and Alexa488-IgG1 (gray-filled histograms). (C) Alternatively, the samples were stained in the same FACS tube with mixtures of CBRM1/5-Alexa488 plus ICRF44-PE or mAb24-Alexa488 plusICRF44-PE mAbs, and ratios of binding of the αMβ2 activation-specific mAbs to total αMβ2 (measured with ICRF44) were calculated. (D) Human PMNs were pretreated with function-blocking mAbs to the αM (44a), the β2 (IB4), or MHC-1 (W6/32) mAbs (10 μg/mL) for 15 minutes at 37°C and then either left resting or stimulated with PMA (50 nM) in the absence or presence of Alexa488-labeled human Fg (20 μg/mL) for 30 minutes at 37°C. The samples were fixed, and 10 000 events falling into gate R2 (Figure 1A dot plot) were analyzed. The data are MFI (± SEM) and are representative of 5 independent experiments.
αMβ2 and PSGL1 cooperate to mediate MP-platelet interaction
We examined whether PMN-derived MPs may interact with platelets by αMβ2. MPs were labeled with Alexa488 (the extent of labeling of resting and PMA MPs was similar by FACS). Resting human platelets were incubated with increasing concentrations of Alexa488-conjugated resting or PMA MPs for 0 to 90 minutes, followed by FACS. Because platelets and MPs were of similar size, to identify platelets in the mixtures, a PE-conjugated platelet-specific marker, integrin αIIb or PE-labeled-isotype control mAb, was added to the platelets together with MPs, and 10 000 αIIb-positive events were analyzed for Alexa488 positivity (MPs binding). In control samples, MPs were negative when stained with the anti-αIIb mAb. The αIIb-PE–positive platelets bound the Alexa488-labeled PMA MPs in a dose-dependent manner (Figure 3A), and the shifts of the αIIb-positive events into the double-positive (αIIb/Alexa488-MP) region can be readily appreciated. Both resting and PMA MPs associated with resting platelets in dose- and time-dependent manners (Figure 3B), and maximum binding of both types of MPs was observed at 45 minutes. When the binding of resting versus PMA MPs was compared, significantly more PMA MPs associated with platelets, suggesting that more platelet binding sites could be engaged by PMA MPs.
PMN-derived MPs interact with platelets in time-dependent and saturable manner involving αMβ2 and PSGL-1 receptors. (A,B) Gel-filtered human platelets were incubated with increasing concentrations (0-50 μg/mL) of Alexa488-labeled MPs derived from resting or PMA-stimulated human PMNs for 0 to 90 minutes at 37°C. To identify platelets the samples in addition to MPs contained PE-labeled anti–human αIIb mAbs or isotype control mouse antibody (IgG1). On incubation, MP binding was measured by FACS, and 10 000 of αIIb-positive events (which accounted for 98% of total events) were analyzed in every sample. (C) Gel-filtered human platelets were preincubated in the absence or presence of function-blocking mAbs to GPIbα, P-selectin, or the β3 integrin subunit (10 μg/mL), whereas MPs (25 μg/mL final concentration) were pretreated with NIF (20 nM) or anti-αM mAb (44a; 10 μg/mL) for 20 minutes at 37°C. Next, the cells and MPs were combined and incubated for 45 minutes at 37°C in the presence of anti–αIIb-PE mAb and analyzed by FACS as described in panels A and B. The data are expressed as MFI (± SEM) from 4 experiments using platelets from 4 different blood donors and MPs from 2 preparations.
PMN-derived MPs interact with platelets in time-dependent and saturable manner involving αMβ2 and PSGL-1 receptors. (A,B) Gel-filtered human platelets were incubated with increasing concentrations (0-50 μg/mL) of Alexa488-labeled MPs derived from resting or PMA-stimulated human PMNs for 0 to 90 minutes at 37°C. To identify platelets the samples in addition to MPs contained PE-labeled anti–human αIIb mAbs or isotype control mouse antibody (IgG1). On incubation, MP binding was measured by FACS, and 10 000 of αIIb-positive events (which accounted for 98% of total events) were analyzed in every sample. (C) Gel-filtered human platelets were preincubated in the absence or presence of function-blocking mAbs to GPIbα, P-selectin, or the β3 integrin subunit (10 μg/mL), whereas MPs (25 μg/mL final concentration) were pretreated with NIF (20 nM) or anti-αM mAb (44a; 10 μg/mL) for 20 minutes at 37°C. Next, the cells and MPs were combined and incubated for 45 minutes at 37°C in the presence of anti–αIIb-PE mAb and analyzed by FACS as described in panels A and B. The data are expressed as MFI (± SEM) from 4 experiments using platelets from 4 different blood donors and MPs from 2 preparations.
Several PMN receptors have been implicated in formation of PMN-platelet conjugates,14,15,19-22 and their involvement in MP-platelet complexes were tested with the following blocking reagents: WAPS12.2 to P-selectin,39 which blocks platelet association with leukocytes; PL1 to PSGL-1,38 25E11 to the integrin β3 subunit,46 VM16d to GPIbα, which blocks its interaction with VWF and αMβ220; mAbs to the αM or β2 subunit40 ; and neutrophil inhibitory factor (NIF), a high-affinity ligand of αMβ2, known to compete with most αMβ2 ligands and to block the αMβ2-dependent PMN functions.47 None of the reagents tested reduced interaction of resting MPs with platelets in a statistically significant manner (data not shown). In contrast, the interaction of PMA MPs with platelets (Figure 3C) was attenuated by 30% to 40% with the anti–P-selectin mAb, NIF, and anti-GPIbα mAb (P < .05; n = 8). The anti-αM and anti-β2 function-blocking mAbs were as effective as NIF and the anti-GPIbα mAb (data not shown). Interestingly, when anti–P-selectin mAb was added together with NIF or mAb to GPIbα, inhibition of the PMA MP interaction with platelets was almost complete, suggesting that both P-selectin/PSGL-1 and αMβ2/GPIbα are involved in the interaction (P < .01; n = 8). These data are consistent with the presence of PSGL-1 on the surface of resting and PMA MPs (see Figure 1C). However, simultaneous blockade of GPIbα and αMβ2 did not have an additive effect, supporting the notion that these 2 receptors mediate the same platelet-PMA MP interaction. In contrast, the function-blocking mAb to the β3 integrin did not alter PMA MP–platelet interactions. Together, our results indicate that enhanced association of PMA MPs with platelets entails cooperativity between P-selectin/PSGL-1 and αMβ2/GPIbα receptors.
PMN-derived MPs activate platelets in αMβ2-dependent manner
Platelet activation in the presence of increasing concentrations of MPs was measured with P-selectin expression as a platelet activation marker.48 PMA and PAF MPs induced platelet activation in a dose-dependent manner (Figure 4A,B). In contrast, resting and LPS MPs did not activate platelets, consistent with the absence or extremely low levels of activated αMβ2 on their surface. PMA MPs were more potent activators than were the same number of PAF MPs. To exclude that PMA or PAF within the MP preparations might be responsible for platelet activation, the MPs were extensively washed with HBSS buffer using the Centricon filter unit. In addition, we tested P-selectin expression on platelets treated directly with PMA or PAF. With the same concentrations of these agonists as used to stimulate PMNs, P-selectin expression induced by PAF was negligible and by PMA was low and did not exceed 20% to 25% of that observed with PMA MPs when they were added at saturating protein concentrations (50-100 μg/mL; data not shown). Thus, platelet activation was MP specific and was not caused by agonist contamination of the MPs. Next, the efficiency of platelet activation by MPs was compared with known platelet agonists. PMA MPs but not resting MPs induced platelet P-selectin expression in dose- and time-dependent manners (Figure 4C). Notably, the PMA MPs, at 50 μg/mL (which can be obtained from 150 to 200 × 106 PMNs), were almost as potent as thrombin at 1 U/mL in activating platelets. However, stimulation by thrombin was more rapid (t1/2 of maximal response by thrombin was approximately 1.8 minutes compared with t1/2 of approximately 18 minutes for PMA MPs in inducing P-selectin). PMA MPs were more potent stimulators than was ADP (10 μM) under the conditions. Another hallmark of platelet stimulation is activation of αIIbβ3, which can be analyzed by PAC-1 mAb binding. MPs derived from resting PMNs only weakly stimulated PAC-1 binding at 1 hour (MFI = 35.4 ± 8) compared with resting platelets without MPs present (MFI = 20.4 ± 4) and an isotype mAb control (MFI = 8.2 ± 2) (Figure 4D). αIIbβ3 activation was slightly greater in the presence of PAF MPs (MFI = 44.8 ± 10) and substantially greater in the presence of PMA MPs (MFI = 83.5 ± 15). Enhancement of P-selectin expression by PMA MPs was reduced (approximately 50%) by the reagents blocking αMβ2, its counterreceptor GPIbα and P-selectin (P < .05; n = 4) and was completely abrogated by combination of the P-selectin and αMβ2/GPIbα blocking reagents (P < .01; n = 4; Figure 4E). However, when blocking reagents to αMβ2 and GPIbα were added together, their effect was not additive, confirming that these receptors affect platelet activation by engaging each other. In control samples, the anti–P-selectin blocking mAb did not compete with the PE-conjugated mAb to this receptor.
MPs derived from PMA-stimulated PMNs activate platelets. (A,B) Gel-filtered human platelets (6 × 106 in 150 μL) were incubated with increasing concentrations (0-100 μg/mL) of MPs as indicated in Tyrode buffer containing 1 mM Ca2+ and PE-conjugated mAb to human P-selectin or its isotype control (IgG1-PE) for 1 hour at 37°C. After incubation, the platelets were fixed and analyzed by FACS. (C) The experiments were performed as described in panels A and B, but in addition to resting or PMA MPs, platelets were stimulated with human thrombin (1 U/mL), ADP (10 μM) for 0 to 60 minutes at 37°C. (D) Human platelets were incubated with MPs (50 μg/mL) and PAC-1-FITC mAb (filled histograms) or FITC-labeled mouse isotype control (IgM; open histogram) as described above. PAC-1 binding was analyzed by FACS. (E) Gel-filtered human platelets were preincubated in the absence or presence of function-blocking mAbs to GPIbα or P-selectin or both or normal mouse IgG (10 μg/mL), whereas MPs (50 μg/mL, final concentration) were pretreated with NIF (20 nM) or anti-αM mAb (44a; 10 μg/mL) for 20 minutes at 37°C. Next, the cells and MPs were combined in the presence of PE-conjugated mAb to human P-selectin or its isotype control and incubated for 30 minutes at 37°C. The data are expressed as MFI (± SEM) from 3 separate experiments using platelets from 4 different blood donors and MPs from 3 preparations.
MPs derived from PMA-stimulated PMNs activate platelets. (A,B) Gel-filtered human platelets (6 × 106 in 150 μL) were incubated with increasing concentrations (0-100 μg/mL) of MPs as indicated in Tyrode buffer containing 1 mM Ca2+ and PE-conjugated mAb to human P-selectin or its isotype control (IgG1-PE) for 1 hour at 37°C. After incubation, the platelets were fixed and analyzed by FACS. (C) The experiments were performed as described in panels A and B, but in addition to resting or PMA MPs, platelets were stimulated with human thrombin (1 U/mL), ADP (10 μM) for 0 to 60 minutes at 37°C. (D) Human platelets were incubated with MPs (50 μg/mL) and PAC-1-FITC mAb (filled histograms) or FITC-labeled mouse isotype control (IgM; open histogram) as described above. PAC-1 binding was analyzed by FACS. (E) Gel-filtered human platelets were preincubated in the absence or presence of function-blocking mAbs to GPIbα or P-selectin or both or normal mouse IgG (10 μg/mL), whereas MPs (50 μg/mL, final concentration) were pretreated with NIF (20 nM) or anti-αM mAb (44a; 10 μg/mL) for 20 minutes at 37°C. Next, the cells and MPs were combined in the presence of PE-conjugated mAb to human P-selectin or its isotype control and incubated for 30 minutes at 37°C. The data are expressed as MFI (± SEM) from 3 separate experiments using platelets from 4 different blood donors and MPs from 3 preparations.
Akt activation by engagement of GPIbα with MP αMβ2 is crucial for platelet stimulation
Akt is important in platelet activation and function,49,50 and we sought to establish whether this kinase is activated in platelets by PMN-derived MPs. PMA MPs induced Akt activation in a dose-dependent manner, as assessed with Ab recognizing the activation-dependent phosphorylation of Akt (Figure 5A). In contrast, MPs derived from resting PMNs did not activate Akt. Importantly, MP-mediated Akt activation markedly depended on the interaction of MP αMβ2 with its platelet counterreceptor(s) because NIF significantly reduced (by approximately 60%-70%) Akt activation (Figure 5B left). The reaction was partially attenuated by a function-blocking mAb to platelet P-selectin (Figure 5B left). When platelets were treated with function-blocking mAb to GPIbα (VM16d), the extent of Akt inhibition was the same as induced by NIF, suggesting that GPIbα is a major αMβ2 counterreceptor on platelets for Akt activation and that this interaction is upstream of Akt activation (Figure 5B right). When reagents blocking both P-selectin/PSGL-1 and GPIbα/αMβ2 pathways were combined, Akt activation in platelets was completely blocked, indicating cooperation between the 2 systems. Control normal mouse IgG failed to alter Akt activation indicative of specificity. To further confirm that engagement of GPIbα is upstream of Akt activation in platelets, this receptor was crosslinked with mAb VM16d. In the presence of mAbs to GPIbα or F(ab′)2 fragments of goat anti–mouse IgG [F(ab′)2GAM IgG] added separately, no Akt activation was detected (Figure 5C). In contrast, when platelets were preincubated with the GPIbα mAb and then treated with F(ab′)2GAM IgG to crosslink the receptors, robust Akt activation was observed (Figure 5C), which was similar to that induced by PMA MPs.
αMβ2- And GPIbα-mediated Akt phosphorylation is crucial for platelet activation. (A) Gel-filtered human platelets (4 × 107 in 200 μL) were incubated in Tyrode buffer containing 1 mM Ca2+ with increasing concentrations (0-100 μg/mL) of resting or PMA MPs for 30 minutes at 37°C and lysed by the addition of 4× Laemmli buffer. Lysates were resolved on 10% SDS-PAGE and immunoblotted with Abs to activated Akt (PSer473; top) or to total Akt (bottom). (B) The experiments were performed as described in panel A, but platelets and MPs (final concentration, 50 μg/mL) were pretreated with respective blocking reagents for 20 minutes at 37°C and then combined. The observed Akt activation status is representative of 3 independent experiments. (C) Human platelets (2 × 107 in 150 μL) were incubated with or without GPIbα mAb (VM16d; 10 μg/mL) for 15 minutes at room temperature followed by incubation in the presence or absence of F(ab′)2 fragments of goat anti–mouse IgG (20 μg/mL) for 15 minutes at 37°C. Lysates were analyzed by Western blots using biotinylated Abs to activated or total Akt and streptavidin-HRPO conjugate. (D,E) Human platelets were pretreated with increasing concentrations (0-100 μM) of Akt inhibitor II for 20 minutes at 37°C followed by incubation in the presence or absence of PMA MPs (25 μg/mL) and anti–P-selectin-PE (D,E, left panels), PAC-1-FITC (D,E, right panels) or mouse isotype control antibodies for 1 hour at 37°C. After incubation, platelets were fixed and analyzed by FACS. The data are MFI (± SEM) and are representative from 2 independent experiments.
αMβ2- And GPIbα-mediated Akt phosphorylation is crucial for platelet activation. (A) Gel-filtered human platelets (4 × 107 in 200 μL) were incubated in Tyrode buffer containing 1 mM Ca2+ with increasing concentrations (0-100 μg/mL) of resting or PMA MPs for 30 minutes at 37°C and lysed by the addition of 4× Laemmli buffer. Lysates were resolved on 10% SDS-PAGE and immunoblotted with Abs to activated Akt (PSer473; top) or to total Akt (bottom). (B) The experiments were performed as described in panel A, but platelets and MPs (final concentration, 50 μg/mL) were pretreated with respective blocking reagents for 20 minutes at 37°C and then combined. The observed Akt activation status is representative of 3 independent experiments. (C) Human platelets (2 × 107 in 150 μL) were incubated with or without GPIbα mAb (VM16d; 10 μg/mL) for 15 minutes at room temperature followed by incubation in the presence or absence of F(ab′)2 fragments of goat anti–mouse IgG (20 μg/mL) for 15 minutes at 37°C. Lysates were analyzed by Western blots using biotinylated Abs to activated or total Akt and streptavidin-HRPO conjugate. (D,E) Human platelets were pretreated with increasing concentrations (0-100 μM) of Akt inhibitor II for 20 minutes at 37°C followed by incubation in the presence or absence of PMA MPs (25 μg/mL) and anti–P-selectin-PE (D,E, left panels), PAC-1-FITC (D,E, right panels) or mouse isotype control antibodies for 1 hour at 37°C. After incubation, platelets were fixed and analyzed by FACS. The data are MFI (± SEM) and are representative from 2 independent experiments.
To corroborate the importance of MP-induced Akt phosphorylation in platelet activation, P-selectin expression and αIIbβ3 activation (PAC-1 binding) on platelets were incubated with PMA MPs in the presence of increasing concentrations of Akt-specific inhibitor II, which prevents generation of PIP3 and competes with PIP3 binding to Akt.51 Representative FACS histograms of P-selectin expression (Figure 5D, left) and PAC-1 binding (Figure 5D right) and the graphs summarizing the quantified data (Figure 5E) indicate that both platelet activation markers are down-regulated by the inhibitor in a dose-dependent manner. However, platelet secretion (P-selectin expression) was approximately 10-fold more sensitive (concentration that inhibits response by 50% [IC50] = 6 μM) than was integrin αIIbβ3 activation (PAC-1 binding) (IC50 = 60 μM) to the inhibitor. Specificity of the Akt inhibitor used is suggested by the observation that it did not (even at 100 μM) reduce activation of other kinases, such as src and lyn, or signaling proteins, such as S6 ribosomal protein or HSP 27 in platelets despite complete inhibition of Akt phosphorylation (data not shown). However, we cannot exclude nonspecific effects of the inhibitor at the higher dose.
Verification of the pivotal role of αMβ2 in MP-dependent platelet activation
We examined the binding of MPs derived from resting or PMA-stimulated WT and αM−/− PMNs to WT mouse platelets. MPs derived from resting PMNs bound poorly to platelets, and no difference between WT and αM−/− MPs was observed. However, binding of MPs derived from PMA-stimulated PMNs was markedly enhanced and dose dependent. However, the interaction of αM−/− MPs with platelets was reduced by approximately 50% compared with WT MPs (Figure 6A). These observations were corroborated in experiments using function-blocking mAb to mouse αM subunit (M1/70).10 This mAb inhibited the interaction of WT PMA MPs with mouse platelets to the level observed with the αM−/− PMA MPs, and they failed to reduce the binding of the αM−/− PMA MPs to platelets (data not shown).
αM−/− MPs have an impaired ability to interact and activate platelets. (A) Gel-filtered mouse WT platelets were incubated with increasing concentrations (0-50 μg/mL) of Alexa488-labeled MPs derived from resting or PMA-stimulated WT or αM−/− PMNs for 1 hour at 37°C. MPs binding was analyzed by FACS as described in Figure 3A with one exception that PE-labeled rat anti–mouse αIIb antibody was used to identify platelets. The data are MFI (± SEM) from 3 experiments. (B) Gel-filtered mouse WT platelets (4 × 107 in 200 μL) were incubated in Tyrode buffer containing 1 mM Ca2+ in the absence or presence of MPs derived from resting (25 μg/mL each) or PMA-stimulated (5 and 25 μg/mL) mouse WT or αM−/− PMNs for 30 minutes at 37°C. After this incubation, platelets were lysed and Akt activation was analyzed as described in Figure 5A. (C,D) Mouse WT platelets were incubated in the absence or presence of WT or αM−/− MPs as indicated (at 50 μg/mL each) in Tyrode buffer containing 1 mM Ca2+ and FITC-conjugated mAb to mouse P-selectin or its isotype control (IgG1) for 45 minutes at 37°C. The data represent MFI (± SEM) from 3 independent experiments.
αM−/− MPs have an impaired ability to interact and activate platelets. (A) Gel-filtered mouse WT platelets were incubated with increasing concentrations (0-50 μg/mL) of Alexa488-labeled MPs derived from resting or PMA-stimulated WT or αM−/− PMNs for 1 hour at 37°C. MPs binding was analyzed by FACS as described in Figure 3A with one exception that PE-labeled rat anti–mouse αIIb antibody was used to identify platelets. The data are MFI (± SEM) from 3 experiments. (B) Gel-filtered mouse WT platelets (4 × 107 in 200 μL) were incubated in Tyrode buffer containing 1 mM Ca2+ in the absence or presence of MPs derived from resting (25 μg/mL each) or PMA-stimulated (5 and 25 μg/mL) mouse WT or αM−/− PMNs for 30 minutes at 37°C. After this incubation, platelets were lysed and Akt activation was analyzed as described in Figure 5A. (C,D) Mouse WT platelets were incubated in the absence or presence of WT or αM−/− MPs as indicated (at 50 μg/mL each) in Tyrode buffer containing 1 mM Ca2+ and FITC-conjugated mAb to mouse P-selectin or its isotype control (IgG1) for 45 minutes at 37°C. The data represent MFI (± SEM) from 3 independent experiments.
In addition, activation of Akt in mouse platelets by WT and αM−/− MPs was compared. MPs (25 μg/mL) derived from resting WT or αM−/− PMNs failed to induce Akt phosphorylation in platelets (Figure 6B). Higher MP concentrations were also ineffective (not shown). In contrast, MPs derived from PMA-stimulated WT MPs induced Akt activation in a dose-dependent manner, whereas Akt phosphorylation was almost absent in the presence of PMA αM−/− MPs (Figure 6B). Next, platelet activation in the presence of WT or αM−/− MPs was examined. Figure 6C shows a representative histogram of P-selectin expression on platelets incubated with WT or αM−/− MPs derived from PMA-stimulated PMNs. In this experiment, platelet activation was markedly decreased in the presence of αM−/− MPs compared with WT MPs. P-selectin expression on platelets incubated with MPs from resting WT or αM−/− PMNs was low and similar to that on platelets without MPs present (Figure 6D). MPs derived from PMA-stimulated WT MPs enhanced P-selectin levels by 4-fold, whereas the αM−/− MPs failed to do so. Together, these results indicate the importance of αMβ2 not only in MP binding to platelets but also in MP-induced platelet activation.
Discussion
In this study, we have shown that MPs derived from stimulated PMNs contain activated and functional integrin αMβ2 on their surface. This conclusion is based on the expression of 2 distinct αMβ2 activation-dependent epitopes on MPs and αMβ2-mediated binding of a soluble Fg by the MPs. Although the presence of αMβ2 and other integrins on PMN-derived MPs has been previously reported,43 to our knowledge this study presents the first evidence to ascribe functional attributes to the MP-associated integrin. On activation, leukocyte integrins, including αMβ2, accumulate within lipid rafts,52-54 and numerous studies indicate that MPs bleb from such rafts. Thus, there is a clear pathway for activated αMβ2 to accumulate within MPs. The inability to detect active αMβ2 on resting MPs is in accord with literature indicating that MP content reflects composition of plasma membrane of the parental cells, and only low levels of inactive αMβ2 are present on resting PMNs.23-25,55 The surface density of αM on MPs derived from stimulated PMNs appears to be quite high. When surface densities were calculated for PMA MPs versus stimulated PMNs using the FACS signals and an average MP radius of 0.5 μm versus 7.5 μm for PMNs, there are approximately 9.5 αM MFI units/μm2 on PMA-MPs compared with approximately 0.8 αM MFI units/μm2 on PMNs. Thus, although average αM MFI values on MPs (per 1 MP) are 10- to 20-fold lower than on PMNs (per 1 PMN), the MP surface (diameter = 1 μm) is 225-fold smaller than the PMN surface (diameter = 15 μm), suggesting an approximate 10-fold enrichment of αM density on the MP versus the PMN surface. Preferential release of MPs containing active αMβ2 may be one mechanism for down-regulating the proinflammatory status of activated leukocytes, together with shedding56 and internalization of the integrin as well as apoptosis of the cells. By virtue of its capacity to bind numerous ligands, including ICAM-1,57 GPIbα,20,21 JAM-3,22 fibronectin,58 and Fg,59 active αMβ2 on MPs may mediate their interaction with endothelial cells, platelets, and extracellular matrix. MPs also were shown to interact with plasminogen and to form plasmin on their surface,60 and activated αMβ2 can bind plasminogen and urokinase.41,61 αMβ2 also binds metalloproteinases 2 and 9,62 suggesting that the active integrin on MP may regulate proteolysis.
In addition to these projected functions, our study shows directly that activated αMβ2 on MPs mediates their interaction with resting platelets most likely by engagement of GPIbα. This interaction results in Akt activation and enhances P-selectin expression. The importance of αMβ2 in MP interaction with platelets and MP-dependent platelet activation was corroborated using several independent approaches: blocking αMβ2 functions with NIF, mAbs to both integrin subunits, and using αM−/− MPs. These approaches attenuated both MP-platelet interaction and Akt activation. Although the activation-dependent CBRM1/5 and mAb24 epitopes reside within the αM integrin subunit, it is not surprising that αMβ2 functions could be blocked by mAbs to both subunits because engagement of both subunits is necessary for the binding of most ligands.63 In addition, the anti-β2 mAb (IB4) does not directly compete with ligand binding, but exerts its effects through an allosteric mechanism leading to integrin deactivation.64 Furthermore, under the conditions used, among various platelet counterreceptors of αMβ2,19-22 GPIbα appears to be prominent. MP binding to platelets and MP-induced platelet activation were reduced by mAbs to GPIbα and to the region of the αMI-domain, which engages GPIbα.20,21,65 These latter reagents reduced MP-platelet interactions by approximately 50%, attesting to the prominent role of this receptor-counterreceptor pairing in conjugation of MP to the cells. No effect of the function-blocking mAb to the integrin β3 subunit was observed, which is in agreement with the data showing that platelet-leukocyte interactions are not reduced by αIIbβ3 antagonists and β3-deficient platelets.66
Notably, not only GPIbα/αMβ2 but also P-selectin/PSGL-1–blocking reagents inhibited MP-platelet interaction, and the combination of reagents targeting both systems completely inhibited MP-platelet conjugation. The way in which these 2 receptor-counterreceptor systems cooperate remains to be resolved, particularly because P-selectin levels on resting platelets are extremely low. One possible scenario is that activated αMβ2 on MP binds to GPIbα, which is abundant on resting platelets. Engagement of GPIbα induces weak αIIbβ3 activation and redistributes some P-selectin to platelet surface. These events are in accord with numerous studies showing the importance of GPIbα in platelet activation.67,68 Now enhanced, but still low levels of P-selectin on platelets may be able in interact more efficiently with MP expressing PSGL-1 to further enhance P-selectin expression and platelet activation. Hence, there may be a positive amplification loop to enhance platelet activation initiated by activated αMβ2 on MPs. A similar amplification loop exists for sulfatides, which interact with P-selectin on activated platelets to enhance further platelet activation and P-selectin expression.69 This interaction pathway also recapitulates the cooperativity between P-selectin and αMβ2 in mediating PMN-endothelial cell conjugation; engagement of PSGL-1 on PMNs with P-selectin on endothelial cells results in PMN rolling and αMβ2 activation (reviewed in McEver and Cummings70 ). However, because blockade of αMβ2/GPIbα results in only approximately 50% reduction of platelet activation, a second independent pathway for MP activation of platelets is also likely to exist and could involve platelet agonists, which are reported to be present in PMN-derived MPs.71
Studies in vitro and in vivo show that platelet P-selectin is a key receptor mediating association of activated platelets with leukocytes. Its interaction with leukocyte PSGL-1 supports leukocyte rolling and induces αMβ2 activation, enabling leukocyte adhesion to activated platelets.14-17 P-selectin also promotes rapid recruitment of leukocyte-derived MPs into platelet-rich growing thrombi.33 Both activated leukocytes and MPs of leukocyte origin expose active tissue factor on their surface,32,34 and platelet P-selectin contributes to accumulation of tissue factor within thrombus leading to enhanced coagulation and thrombus propagation. In addition, P-selectin stabilizes platelet aggregates and is enriched at cell-cell contacts of stable platelet aggregates playing a role in thrombus formation.72 Thus, MPs derived from stimulated PMNs enhance coagulation not only because they bear tissue factor (not shown) but also because they can activate platelets and enhance platelet P-selectin expression.
Our data indicate that MPs released by stimulated PMNs activate Akt downstream of GPIbα engagement with αMβ2. Akt phosphorylation was critical in MP-dependent platelet activation because both αIIbβ3 activation and P-selectin cell-surface expression were attenuated by an Akt-specific inhibitor. These data are consistent with studies indicating that PI3 kinase, which is upstream of Akt, colocalizes with GPIbα and is activated downstream of this receptor73 and that Akt activation is downstream of GPIbα engagement with von Willebrand factor.74 In addition, Chen et al50 and Woulfe et al49 showed impaired platelet functions in Akt1−/− and Akt2−/− mice. When the efficiency of PMN-derived MPs to activate platelets was compared with other platelet agonists, MPs could induce a response as extensive as that induced by thrombin but was much slower than thrombin in attaining this activated state.
Taken together, this is the first study showing the capability of PMN-derived MPs, which express active and functional integrin αMβ2, to activate platelets by engagement of platelet GPIbα. These novel MP-platelet interactions may lead to hyperactive platelets and contribute to atherosclerosis, chronic prothrombotic states, and other cardiovascular diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants H1 P50 HL 081011 and HL17964.
National Institutes of Health
Authorship
Contribution: E.P. designed and performed research and wrote the manuscript; N.M.W. performed research, analyzed data, and drafted the manuscript; D.S. performed research; C.M.B. provided αM−/− mice, interpreted data, and critically reviewed the manuscript; D.A.S. performed statistical analysis; D.I.S. interpreted the data and provided significant critique; E.F.P. designed the research and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward F. Plow, Department of Molecular Cardiology, NB50, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195; e-mail: plowe@ccf.org.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal