Abstract
Nucleophosmin (NPM) is frequently overexpressed in leukemias and other tumors. NPM has been reported to suppress oncogene-induced senescence and apoptosis and may represent a therapeutic target for cancer. We fused a NPM-derived peptide to the HIV-TAT (TAT-NPMΔC) and found that the fusion peptide inhibited proliferation and induced apoptotic death of primary fibroblasts and preleukemic stem cells. TAT-NPMΔC down-regulated several NF-κB–controlled survival and inflammatory proteins and suppressed NF-κB–driven reporter gene activities. Using an inflammation-associated leukemia model, we demonstrate that TAT-NPMΔC induced proliferative suppression and apoptosis of preleukemic stem cells and significantly delayed leukemic development in mice. Mechanistically, TAT-NPMΔC associated with wild-type NPM proteins and formed complexes with endogenous NPM and p65 at promoters of several antiapoptotic and inflammatory genes and abrogated their transactivation by NF-κB in leu-kemic cells. Thus, TAT-delivered NPM peptide may provide a novel therapy for inflammation-associated tumors that require NF-κB signaling for survival.
Introduction
Nucleophosmin (NPM) is a multifunctional protein that plays important roles in the regulation of cell proliferation and apoptosis.1 The level of NPM is frequently found to be significantly higher in tumor and proliferating cells than in resting cells.2-6 Normal cells induce genetically encoded programs that prevent deregulated proliferation and thus protect multicellular organisms from cancer progression. Two such programs are apoptosis and senescence that are normally triggered by DNA damage or other stresses. Our recent studies demonstrated that overexpression of NPM suppresses oncogene-induced senescence and apoptosis and accelerated transformation in cells deficient for the Fanconi complementation group C (Fancc) gene and the ataxia telangiectasia mutated (Atm) gene.7 Therefore, NPM could be a potential therapeutic target for neoplastic diseases.
There is increasing interest in employing peptides and proteins as therapeutic compounds for human cancers. For example, several peptides derived from tumor suppressors as well as oncogenes have been developed as antitumorigenic cargoes and proved to be effective in inhibition of tumor cell growth.8-10 Although peptide-based compounds have limitations owing to poor permeability and low selectivity, recent studies have demonstrated that large cargoes, proteins, and peptides can be delivered intracellularly if conjugated to the protein transduction domain (PTD) derived from the HIV-1 TAT protein.11,12 Indeed, rapid and receptor-independent uptake of TAT-conjugated peptides has been demonstrated to occur in many cell types and animals.13-15
In this study, we generated a NPM-derived peptide fused to TAT (TAT-NPMΔC) and demonstrated in an inflammation-associated leukemic mouse model that TAT induced rapid and efficient delivery of the peptide into leukemic cells. TAT-NPMΔC caused growth inhibition and apoptosis of leukemic cells and significantly delayed leukemic development in mice. TAT-NPMΔC associated with endogenous NPM proteins formed complexes with endogenous NPM and the NF-κB subunit p65 and abrogated NF-κB transactivation at promoters of several important antiapoptotic and inflammatory genes controlled by NF-κB in leukemic cells. Thus, the results suggest that the TAT-delivered NPM mutant may provide a novel therapy for tumors that require NF-κB signaling for survival.
Methods
Cloning, expression, and purification of TAT-NPM fusion proteins
The full-length human NPM (GenBank sequence accession number BC009623) and its N-terminally (NPMΔN) and C-terminally (NPMΔC) deleted cDNAs were amplified by polymerase chain reaction (PCR), using Pfu DNA polymerase (Stratagene, La Jolla, CA) and the following primer pairs: 5′-CGGGATCCGGAAGATTCGATGGACATG/5′-CCGCTCGAGAAGAGACTTCCTCCACTG, 5′-CGGGATCCGGAAGAAAAAGCGCCAGTG/5′-CCGCTCGAGAAGAGACTTCCTCCACTG, and 5′-CGGGATCCGGAAGATTC GATGGACATG/5′-CCGCTCGAGTTCATCATCATCCTCTTCA, respectively. The resulting PCR fragments were subcloned into the BamH1 and Xho1 sites of the pTAT-2.1 vector (kindly provided by Steven F. Dowdy, University of California School of Medicine, San Diego, CA) to create pTAT-NPM, pTAT-NPMΔN, and pTAT-NPMΔC, respectively. The TAT-NPM fusion proteins were expressed in Escherichia coli strain BL21 (DE3; Novagen, Madison, WI). Whole cell lysates were obtained by gentle lysis using the BugBuster protein extraction reagent (Novagen). Bacterial debris was pelleted, and the supernatant was subjected to metal-affinity chromatography using an Equilibrate His-Bind Column (Novagen). Urea and salt were removed by gel filtration using a PD-10 Sephadex G-25M column (GE Healthcare, Piscataway, NJ). The TAT protein identity was confirmed by Western blotting.
Determination of TAT-NPM fusion protein uptake and subcellular localization
The purified TAT-NPM fusion proteins were labeled using the EZ-Label fluorescein isothiocyanate (FITC) protein Labeling Kit (Pierce, Rockford, IL) in accordance with manufacturer's instruction. To determine the up-take efficiency of the fusion proteins, 105 HEK293 cells per well were plated in a 12-well plate for 12 to 16 hours. Cells were then incubated with TAT-NPM or TAT-NPMΔC (30 μg/mL each) at the indicated time. The total cell number was determined, and cells were lysed in Tris (tris(hydroxymethyl)aminomethane) [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA (ethylenediaminetetraacetic acid), 1% Triton X-100]. The fluorescence intensity was examined by fluorometer (excitation, 490-500 nm; emission, 515-525 nm). To determine the cellular distribution of the TAT fusions, HEK 293 cells were incubated with 30 μg/mL of the indicated proteins for 30 minutes. Cells were then fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) plus 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL; Sigma-Aldrich, St Louis, MO), and the cellular distribution of the FITC-labeled TAT-fusions was visualized with fluorescence microscope. To determine the cellular localization of TAT-fusions, cells were treated with the TAT fusions (30 μg/mL) for 30 minutes, washed extensively, and incubated in the absence of the TAT fusions for 60 minutes before fixation. The cells were then stained with antibodies anti-His6 tag (Roche Applied Science, Penzbery, Germany; anti-NPM/B23 [clone Fc-61991], Zymed Laboratories/Invitrogen, Carlsbad, CA; or anti-B23 [clone FC82291], Sigma-Aldrich).
Cell proliferation, senescence, and apoptosis assays
Mouse embryo fibroblasts (MEFs) were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) with 10% fetal bovine serum (FBS), 2 mM glutamine, 0.1 mM nonessential amino acids, 55 μM β-mercaptoethanol, and 10 μg/mL gentamycin. Cells were plated in 96-well plates at a density of 2 × 103 per well for overnight. Cells were incubated with the indicated proteins (30 μg/mL) and were changed each day. Cell number was determined at the indicated time points using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche Diagnostics, Indianapolis, IN). For proliferation analysis, cells were treated as above and cultured for 24 hours in normal growth medium supplemented with 10 μM BrdU (Sigma-Aldrich), harvested, and fixed in 70% ethanol. BrdU-labeled cells fixed in 70% ethanol were treated with 2 N HCl (20 minutes at room temperature followed by addition of 2 volumes of 0.1 M sodium borate [pH 8.5]). The cells were incubated with an anti-BrdU mouse monoclonal antibody, washed, and incubated with fluorescein isothiocyanate-conjugated antimouse antibody. Cells were counterstained overnight with 5 μg propidium iodide per milliliter containing 40 μg RNase per milliliter. The stained cells were analyzed by flow cytometry.
Senescence-associated-β-galactosidase (SA-β-gal) activity was determined using an SA-β-gal staining kit from Cell Signaling Technology (Beverly, MA) according to the manufacturer's instruction. Briefly, cells were washed in PBS and fixed in 2% formaldehyde-0.2% glutaraldehyde. Then the cells were washed and incubated at 37°C overnight with fresh senescence-associated β-gal stain solution (1 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside [X-Gal], 40 mM citric acid-sodium phosphate [pH 6.0], 150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide). Senescent cells were identified as blue-stained cells by standard light microscopy, and a total of 500 cells were counted in random fields on a slide to determine the percentage of SA-β-gal–positive cells. To quantify apoptotic cells, we used a phycoerythrin-conjugated antibody to the active form of caspase 3 (Pharmingen, San Diego, CA) in a flow cytometric assay to detect cells in the early stages of apoptosis.
Immunoprecipitation and immunoblotting
The monoclonal NPM antibodies (3F291; Santa Cruz Biotechnology, Santa Cruz, CA) were first conjugated to the M-280 paramagnetic Dynabeads (sheep anti–mouse IgG; Dynal, Lake Success, NY) and incubated with 300- to 500-μg cell extracts (at a ratio of about 100 μg extract proteins to 10 μL anti-NPM beads) for 2 hours at 4°C. Beads were washed 3 times and collected on a magnetic particle concentrator (Dynal) and subjected to immunoblot analysis. For immunoblotting, samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. Immunoblots were incubated with the antibodies specific for cyclins A, B, or E, cdc2 (Oncogene Research Products, Cambridge, MA), Cdk2, 4, or 6 (all from Santa Cruz Biotechnologies), p53, p53ser15, p21 (Cell Signaling Technology, Beverly, MA), or β-actin (Sigma-Aldrich) for 12 to 16 hours at 4°C.
Reporter gene assays
A total of 106 HEK 293 cells were transfected with an κB-site dependent luciferase vector, pGL3-KB-Luc (provided by Albert S. Baldwin, University of North Carolina-Chapel Hill) and plated on a 6-well plate. After 48 hours, cells were incubated with the indicated proteins (30 μg/mL). The cells were harvested 12 hours after treatment, washed with PBS, and luciferase activity was assessed using the dual luciferase assay reporter kit (Promega, Madison, WI).
Establishment of Fancc−/− preleukemic stem cells
Tumor necrosis factor (TNF)–induced Fancc−/− preleukemic stem cells were established as described previously.16 Briefly, low-density bone marrow (BM) mononuclear cells from Fancc−/− mice were depleted of lineage-committed cells. Lin-Sca1+ cells were then purified by staining the Lin− cells with Sca-1-phycoerythrin (PE) antibodies (BD PharMingen, San Jose, CA) followed by cell sorting using a FACSCalibur (BD Biosciences, San Jose, CA). Cells were cultured in IMDM medium containing 100 ng/mL stem cell factor (SCF), 20 ng/mL interleukin-6 (IL-6), 10 ng/mL IL-11, and 50 ng/mL Flt-3 ligand (Flt-3L; Peprotech, Rocky Hill, NJ) with or without 10 ng/mL TNF-α for 30 days.
Clonogenic progenitor assays
Wild-type bone marrow (WT BM) cells or Fancc−/− preleukemic cells were incubated with the indicated proteins for 30 minutes prior to culture in a 35-mm tissue culture dish in 4 mL semisolid medium containing 3 mL MethoCult M 3134 (StemCell Technologies, Vancouver, BC), the indicated proteins at 30 μg/mL, and the following growth factors: 100 ng/mL SCF, 10 ng/mL IL-3, 100 ng/mL granulocyte colony-stimulating factor (G-CSF), and 4 U/mL erythropoietin (Peprotech). On day 10 after plating, erythroid and myeloid colonies were enumerated. Hematopoietic clonal growth results were expressed as means (of triplicate plates) plus or minus SD.
Transduction of TAT-NPM proteins into Fancc−/− leukemic mice
Age-matched congenic B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ; CD45.1+) mice (The Jackson Laboratories, Bar Harbor, ME) were used as transplant recipients. These mice were lethally irradiated (9.5 Gy, 110 cGy/min, 137Cs) and injected intravenously with 106Fancc−/− preleukemic cells (CD45.2+), mixed with 106 competitor cells (BoyJ; CD45.1+). After 10 days, recipient mice were injected intraperitonally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for up to 12 weeks. Survival of recipient mice was quantified by Kaplan-Meier analysis. Donor-derived repopulation in recipients was assessed by the proportion of leukocytes in peripheral blood that expressed the CD45.2 (by staining the cells with CD45.1-PE and CD45.2-APC) marker by flow cytometry. The studies involving the use of mice were conducted in accordance to the guidelines of and approved by the Institutional Animal Care and Use Committee of CCHMC (IACUC protocol # 6C06041; PI: Q. Pang).
Immunohistochemistry and TUNEL labeling
During necropsy, organs were removed, preserved in formalin, and then embedded in paraffin blocks. Paraffin sections were deparaffinized, rehydrated, incubated in 0.1 mM sodium citrate (pH 6.0), washed, and incubated with peroxidase blocking reagent (VectaStain Elite ABC kit; Vector Laboratories, Burlingame, CA). After washing in PBS, the slides were incubated with the primary antibodies Ki67 (NeoMarkers). Following 3 PBS washes, slides were incubated with secondary antibody and then detected with the VectaStain Elite ABC reagents. To detect apoptotic nuclei, formalin-fixed paraffin-embedded sections were analyzed by terminal deoxyribonucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) staining using the in situ cell death detection kit (Roche) following the manufacturer's directions. Slides were counterstained with 0.5% methyl green to visualize cell nuclei.
Determination of NF-κB nuclear translocation and DNA-binding activity
Nuclear protein extracts were prepared from BM cells using a transfactor extraction kit (BD Biosciences). Nuclear extracts were incubated with DNA specific for the NF-κB consensus sequence, and the DNA binding activity of NF-κB was measured using a transfactor kit (BD Biosciences).
Gene expression analysis and chromatin immunoprecipitation assays
Total RNA was prepared with RNeasy kit (Qiagen, Hilden, Germany) following the manufacturer's procedure.
Following treatment with RNase-free DNase, RNA was reverse transcribed using superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed on an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR green PCR master mix (Applied Biosystems) according to the manufacturer's instructions. Samples were normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, and the relative expression levels were determined by the standard curve method. Chromatin immunoprecipitation (ChIP) assays were performed as previously described17 using either anti-6 × His or anti-p65 antibodies (both from Santa Cruz Biotechnology) for immunoprecipitation.
Statistics
Data were analyzed statistically using a 2-tail Student t test or Kaplan-Meier survival analysis. Statistical significance was presumed when P was less than .05.
Results
TAT-NPMΔC induces senescence and apoptosis in primary fibroblasts
Because NPM functions to suppress cellular senescence and apoptosis and is essential for development,7,18 we reasoned that this important molecule may have therapeutic value. We therefore studied NPM transduction by protein transduction domain (PTD). Since we previously showed that a truncated mutant containing the N-terminal 174 aa (NPMΔC) induced apoptosis in unstressed cells as well as in cells treated with genotoxic agents,19 we generated TAT-fusion proteins containing the full-length NPM, NPMΔN containing the C-terminal 108 aa, and NPMΔC. These fusion proteins were expressed in bacteria and purified (Figure 1). To examine whether these TAT fusions were biologically active, we determined their effect on the growth of murine embryonic fibroblasts (MEFs) isolated from WT and Fancc−/− mice. We chose Fancc−/− MEFs because they undergo premature senescence under standard fibroblast culture conditions.7 TAT-NPM suppressed senescence in Fancc−/− MEFs, which was consistent with previous report,7 whereas both TAT-NPMΔN and TAT-NPMΔC completely lost this function (Figure 2A). Furthermore, we found that TAT-NPM and TAT-NPMΔC had opposite effect on cell proliferation. That is, TAT-NPM enhanced proliferation, while TAT-NPMΔC suppressed cell growth (Figure 2B). In addition, TAT-NPMΔC induced apoptosis in WT MEFs and significantly increased apoptotic Fancc−/− cells compared with untreated controls (Figure 2C). In contrast, TAT-NPM and TAT-NPMΔN did not have significant effect on the survival of either WT or Fancc−/− cells (Figure 2C).
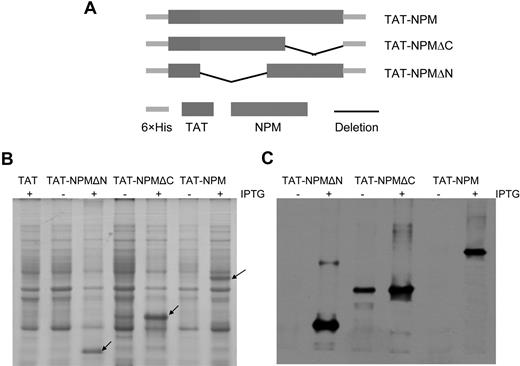
Expression and purification of TAT-NPM fusion proteins. (A) Schematic presentation of TAT-NPM fusions. (B) Expression of TAT-NPM fusions. E coli BL21 cells harboring vector (TAT), TAT-NPMΔN, TAT-NPMΔC, or TAT-NPM were grown in the presence (+) of Isopropyl-β-D-1-thiogalactopyranoside (IPTG) to induce expression of the TAT-fusion proteins. 50 μg cell lysates were analyzed by Coomassie blue staining. Arrows denote the corresponding TAT-fusion proteins. (C) Western blot analysis of the TAT-NPM proteins using an anti-histidine antibody. TAT-NPM fusion proteins were purified by affinity chromatography on a Nickel-sepharose column, followed by gel filtration on a PD-10 column.
Expression and purification of TAT-NPM fusion proteins. (A) Schematic presentation of TAT-NPM fusions. (B) Expression of TAT-NPM fusions. E coli BL21 cells harboring vector (TAT), TAT-NPMΔN, TAT-NPMΔC, or TAT-NPM were grown in the presence (+) of Isopropyl-β-D-1-thiogalactopyranoside (IPTG) to induce expression of the TAT-fusion proteins. 50 μg cell lysates were analyzed by Coomassie blue staining. Arrows denote the corresponding TAT-fusion proteins. (C) Western blot analysis of the TAT-NPM proteins using an anti-histidine antibody. TAT-NPM fusion proteins were purified by affinity chromatography on a Nickel-sepharose column, followed by gel filtration on a PD-10 column.
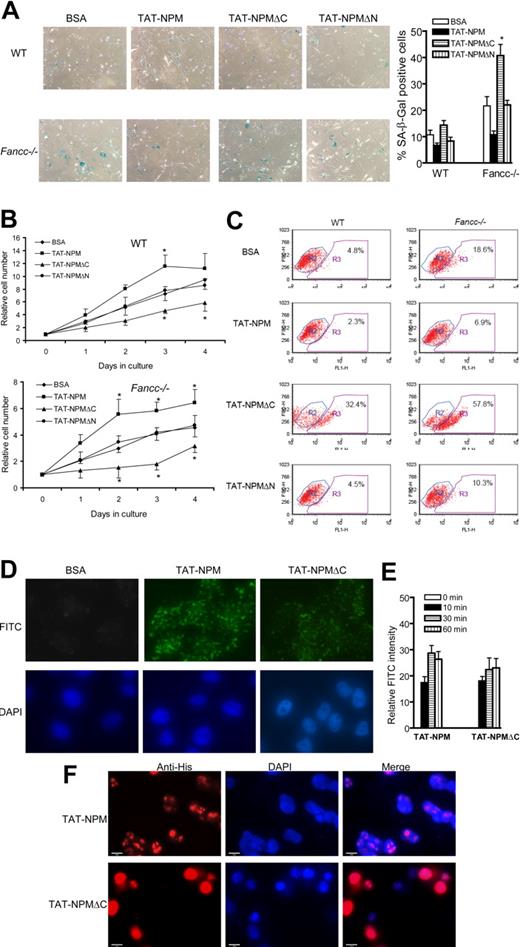
Effect of TAT-NPM fusions on cell proliferation and senescence. (A) TAT-NPMΔC fails to suppress senescence in MEFs. WT or Fancc−/− MEFs at passage 4 were incubated with BSA, TAT-NPM, TAT-NPMΔC, or TAT-NPMΔN (30 μg/mL each) for 6 days, with medium and the proteins changed each day. Cells were then stained for SA-β-gal. The graphs on the right are percentages of the cells stained positive for SA-β-gal quantified by counting a total of 100 cells in random fields on a slide. The data represent the mean plus or minus SD of 3 independent experiments. *Statistical significance between TAT-NPMΔC and BSA samples at P < .05. (B) TAT-NPMΔC inhibits cell proliferation. Cells were plated in 96-well plates at a density of 2 × 103 per well for overnight. Cells were incubated with the indicated proteins (30 μg/mL) and were changed each day. Cell proliferation was determined at the indicated time points. Data represent mean plus or minus SD of 3 experiments. *Statistical significance between TAT-NPM and BSA or between TAT-NPMΔC and BSA samples at P < .05. (C) Cells described in panel A were analyzed for apoptosis, as determined by flow cytometry for the percentage of cells with active caspase 3 (gated in R3). Shown are the representative data of 3 independent experiments with similar results. (D) Uptake of TAT-NPM and TAT-NPMΔC in human cells. HEK293 cells were incubated with 30 μg/mL of the indicated proteins for 30 minutes. After fixation, cells were counterstained with DAPI and the cellular distribution of the FITC-labeled TAT-fusions was visualized with fluorescence microscope. (E) Time course of TAT-NPM protein uptake by human cells. HEK293 cells were incubated with TAT-NPM or TAT-NPMΔC (30 μg/mL each) at the indicated time. Relative FITC intensity was determined by normalizing fluorescence intensity of each treatment with cell numbers. (F) Subcellular localization of the TAT fusions in human cells. HEK293 cells were incubated with TAT-NPM or TAT-NPMΔC (30 μg/mL each) for 30 minutes, washed extensively, and incubated in the absence of the TAT fusions for 60 minutes before fixation. The cells were then stained with anti-His6 antibody and visualized with fluorescence microscope.
Effect of TAT-NPM fusions on cell proliferation and senescence. (A) TAT-NPMΔC fails to suppress senescence in MEFs. WT or Fancc−/− MEFs at passage 4 were incubated with BSA, TAT-NPM, TAT-NPMΔC, or TAT-NPMΔN (30 μg/mL each) for 6 days, with medium and the proteins changed each day. Cells were then stained for SA-β-gal. The graphs on the right are percentages of the cells stained positive for SA-β-gal quantified by counting a total of 100 cells in random fields on a slide. The data represent the mean plus or minus SD of 3 independent experiments. *Statistical significance between TAT-NPMΔC and BSA samples at P < .05. (B) TAT-NPMΔC inhibits cell proliferation. Cells were plated in 96-well plates at a density of 2 × 103 per well for overnight. Cells were incubated with the indicated proteins (30 μg/mL) and were changed each day. Cell proliferation was determined at the indicated time points. Data represent mean plus or minus SD of 3 experiments. *Statistical significance between TAT-NPM and BSA or between TAT-NPMΔC and BSA samples at P < .05. (C) Cells described in panel A were analyzed for apoptosis, as determined by flow cytometry for the percentage of cells with active caspase 3 (gated in R3). Shown are the representative data of 3 independent experiments with similar results. (D) Uptake of TAT-NPM and TAT-NPMΔC in human cells. HEK293 cells were incubated with 30 μg/mL of the indicated proteins for 30 minutes. After fixation, cells were counterstained with DAPI and the cellular distribution of the FITC-labeled TAT-fusions was visualized with fluorescence microscope. (E) Time course of TAT-NPM protein uptake by human cells. HEK293 cells were incubated with TAT-NPM or TAT-NPMΔC (30 μg/mL each) at the indicated time. Relative FITC intensity was determined by normalizing fluorescence intensity of each treatment with cell numbers. (F) Subcellular localization of the TAT fusions in human cells. HEK293 cells were incubated with TAT-NPM or TAT-NPMΔC (30 μg/mL each) for 30 minutes, washed extensively, and incubated in the absence of the TAT fusions for 60 minutes before fixation. The cells were then stained with anti-His6 antibody and visualized with fluorescence microscope.
The differential effects of TAT-NPM and TAT-NPMΔC on cell survival and proliferation was not due to transduction efficiency, as the uptake of these TAT fusions appeared to be similar between TAT-NPM and TAT-NPMΔC (Figure 2D). The intracellular delivery of the TAT-fusions was rapid, as the FITC-labeled fusions could be detected in the treated cells as early as 10 minutes after incubation (Figure 2E). To determine the cellular localization of TAT-NPM fusions, we treated cells with the TAT fusions for 30 minutes, washed the cells extensively, and incubated them in the absence of the TAT fusions for 60 minutes before fixation. We found that while TAT-NPM was localized in the nucleolus, the majority of TAT-NPMΔC was localized in nucleoplasm (Figure 2F).
TAT-NPMΔC down-regulates antiapoptotic and proinflammatory genes
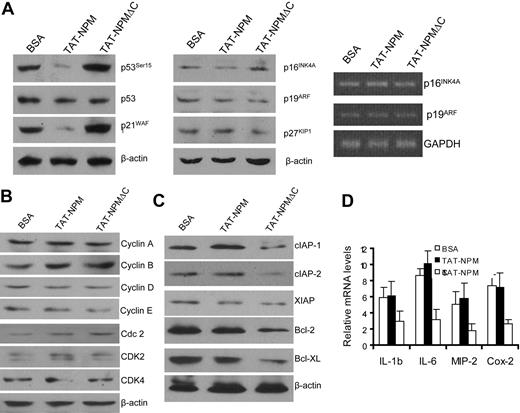
Because TAT-NPMΔC inhibited cell proliferation and induced apoptosis, we wanted to know if the mutant TAT fusion affected the expression or/and activities of factors involved in cell-cycle control and survival signaling. We first examined the expression and activities of the cell-cycle regulators p53, p21WAF1, p16INK4A, and p19Arf, known to be negatively regulated by NPM.7,19-22 While the activity of p53, as reflected by its phosphorylation at Ser15 and p21WAF1 expression, was inhibited in senescent Fancc−/− MEF cells treated with TAT-NPM, TAT-NPMΔC did not reduce the expression of these negative cell-cycle regulators (Figure 3A left panel). The expression of p16INK4A or p19Arf, as determined by Western blot (Figure 3A middle panel) and reverse transcriptase-polymerase chain reaction (RT-PCR) (Figure 3A right panel), was not affected by either TAT-NPM or TAT-NPMΔC. Interestingly, TAT-NPMΔC increased active form of p53 (p53Ser15). The mechanism and functional consequence of its activation of p53 remains to be investigated.
Effect of TAT-NPM proteins on expression of genes controlling cell cycle, apoptosis, and inflammation. (A) Effect on negative regulators of cell-cycle progression. Primary MEFs were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 12 hours, and whole cell extracts or RNA were prepared for analyses of p53, p21Waf1, p16Ink4a, p19Arf, and p27KIP1 by immunoblotting (left and middle panels) or p16Ink4a and p19Arf mRNAs by RT-PCR (right panel). (B) Cells described in panel A were analyzed for expression of positive regulators of cell-cycle progression by immunoblotting. (C) Reduced levels of antiapoptotic proteins in cells treated with TAT-NPMΔC, as examined by immunoblot analysis of cell extracts described in panel A. (D) TAT-NPMΔC suppresses expression of inflammatory genes. Expression of genes encoding inflammatory genes was examined in BSA, TAT-NPM, and TAT-NPMΔC–treated cells stimulated with TNF-α (10 ng/mL) for 30 minutes. Cells were collected, total RNA was prepared, and gene expression was analyzed by real-time PCR and normalized to GAPDH mRNA. Results are means plus or minus SD of 3 independent experiments.
Effect of TAT-NPM proteins on expression of genes controlling cell cycle, apoptosis, and inflammation. (A) Effect on negative regulators of cell-cycle progression. Primary MEFs were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 12 hours, and whole cell extracts or RNA were prepared for analyses of p53, p21Waf1, p16Ink4a, p19Arf, and p27KIP1 by immunoblotting (left and middle panels) or p16Ink4a and p19Arf mRNAs by RT-PCR (right panel). (B) Cells described in panel A were analyzed for expression of positive regulators of cell-cycle progression by immunoblotting. (C) Reduced levels of antiapoptotic proteins in cells treated with TAT-NPMΔC, as examined by immunoblot analysis of cell extracts described in panel A. (D) TAT-NPMΔC suppresses expression of inflammatory genes. Expression of genes encoding inflammatory genes was examined in BSA, TAT-NPM, and TAT-NPMΔC–treated cells stimulated with TNF-α (10 ng/mL) for 30 minutes. Cells were collected, total RNA was prepared, and gene expression was analyzed by real-time PCR and normalized to GAPDH mRNA. Results are means plus or minus SD of 3 independent experiments.
We also examined the expression of the positive cell-cycle regulators including cyclins A, B, D, and E, and cyclin-dependent kinases cdc2 (Cdk1), Cdk2, 4, and 6 in MEF cells. We did not observe significant changes in the expression of these molecules in TAT-NPMΔC–treated cells (Figure 3B). However, we found significantly reduced levels of cellular inhibitor of apoptosis proteins (cIAP)-1, cIAP-2, Bcl-2, and Bcl-XL in TAT-NPMΔC–treated cells compared with cells treated with TAT-NPM (Figure 3C). Surprisingly, the expression of proinflammatory cytokines tumor necrosis factor α (TNF-α), IL-1β, IL-6, macrophage inflammatory protein-2 (MIP-2) and cyclooxygenase 2 (Cox-2) was also reduced by TAT-NPMΔC treatment (Figure 3D).
TAT-NPMΔC inhibits NF-κB transactivation
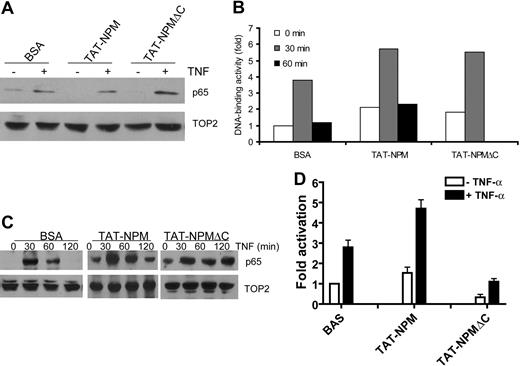
Since the expression of the mentioned inflammatory and antiapoptotic genes is controlled by NF-κB,23 and since NPM can act to activate NF-κB,24 this would suggest that TAT-NPMΔC induces apoptosis through a mechanism involving suppression of NF-κB activation. To test this notion, we examined the effect of TAT-NPMΔC on nuclear translocation and DNA-binding activity of NF-κB in normal lymphoblast cells stimulated with TNF-α. Surprisingly, we found that higher level of NF-κB subunit p65 (RelA) was detected in TAT-NPMΔC–treated cells than those treated with TAT-NPM or bovine serum albumin (BSA; Figure 4A). Consistent with this, significant increase in DNA binding by p65 was observed in TAT-NPMΔC–treated cells, as compared with cells treated with TAT-NPM or BSA (Figure 4B). It is noteworthy that TAT-NPM also enhanced DNA-binding activity of p65, which is consistent with previous observation that NPM activates NF-κB.24 Furthermore, TAT-NPMΔC greatly prolonged the time of TNF-α–induced NF-κB accumulation in nuclei (Figure 4C).
TAT-NPMΔC inhibits NF-κB activation. (A) Effect of TAT-NPMΔC on TNF-α–induced nuclear translocation of NF-κB. HEK293T cells were first treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes then stimulated with TNF-α (10 ng/mL) for 30 minutes. Nuclear localization of the NF-κB subunit p65 in the nuclear extracts was analyzed by immunoblot analysis of the NF-κB subunit p65. (B) Effect of TAT-NPMΔC on TNF-α–induced DNA-binding activity of NF-kB. HEK293T cells were first treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes then stimulated with TNF-α (10 ng/mL) for the indicated time. Nuclear extracts were then prepared and DNA-binding activity of NF-κB was measured by transcription factor enzyme-linked immunosorbent assay (ELISA). Data are presented as fold activation relative to the DNA-binding activity in BSA-treated cells without TNF-α stimulation. (C) TAT-NPMΔC prolongs TNF-α–induced nuclear accumulation of NF-κB. HEK293T cells were first treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes then stimulated with TNF-α (10 ng/mL) for the indicated time. Nuclear localization of the NF-κB subunit p65 in the nuclear extracts was analyzed by immunoblot analysis of the NF-κB subunit p65. (D) TAT-NPMΔC inhibits NF-κB transcriptional activity. HEK293T cells transfected with a 3 × κB-Luc plasmid were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes, then stimulated with TNF-α (10 ng/mL) for another 30 minutes. Results of triplicate experiments are shown with mean and standard deviation.
TAT-NPMΔC inhibits NF-κB activation. (A) Effect of TAT-NPMΔC on TNF-α–induced nuclear translocation of NF-κB. HEK293T cells were first treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes then stimulated with TNF-α (10 ng/mL) for 30 minutes. Nuclear localization of the NF-κB subunit p65 in the nuclear extracts was analyzed by immunoblot analysis of the NF-κB subunit p65. (B) Effect of TAT-NPMΔC on TNF-α–induced DNA-binding activity of NF-kB. HEK293T cells were first treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes then stimulated with TNF-α (10 ng/mL) for the indicated time. Nuclear extracts were then prepared and DNA-binding activity of NF-κB was measured by transcription factor enzyme-linked immunosorbent assay (ELISA). Data are presented as fold activation relative to the DNA-binding activity in BSA-treated cells without TNF-α stimulation. (C) TAT-NPMΔC prolongs TNF-α–induced nuclear accumulation of NF-κB. HEK293T cells were first treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes then stimulated with TNF-α (10 ng/mL) for the indicated time. Nuclear localization of the NF-κB subunit p65 in the nuclear extracts was analyzed by immunoblot analysis of the NF-κB subunit p65. (D) TAT-NPMΔC inhibits NF-κB transcriptional activity. HEK293T cells transfected with a 3 × κB-Luc plasmid were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes, then stimulated with TNF-α (10 ng/mL) for another 30 minutes. Results of triplicate experiments are shown with mean and standard deviation.
We next examined the effect of TAT-NPMΔC on NF-κB transactivation by transient reporter gene assays in HEK293T cells using a luciferase construct with an NF-κB–driven promoter. Cells transduced with TAT-NPM showed enhanced NF-κB luciferase reporter activity induced by TNF-α (Figure 4D). In contrast, TAT-NPMΔC inhibited NF-κB activation induced by TNF-α treatment. Interestingly, the basal activity of the NF-κB luciferase reporter was also significantly reduced in TAT-NPMΔC–treated cells (Figure 4D). Together, these results demonstrate that TAT-NPMΔC acts as a negative regulator of the NF-κB pathway.
Intracellular delivery of TAT-NPMΔC induces apoptosis in Fancc−/− preleukemic stem cells and delays leukemia development in mice
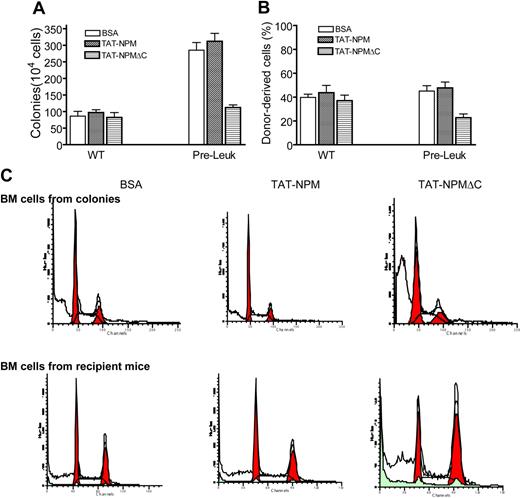
We have recently established a model of TNF-α–promoted leukemogenesis in BM hematopoietic stem cells of mice deficient for the Fanconi group C gene (Fancc). As described previously,16 the TNF-α–induced Fancc−/− preleukemic cells expressed primitive markers Sca-1 and c-kit, underwent clonal proliferation, and gave rise to myeloid leukemia after transplanted into irradiated recipient mice. We also demonstrated that these TNF-α–induced Fancc−/− preleukemic stem cells required NF-κB signaling for survival.16 This inflammation-associated leukemogenic model therefore provided us a unique opportunity to test whether TAT-NPMΔC exerted an inhibitory effect on leukemia development in vivo. First, we examined proliferation and repopulating capacity of TAT-NPMΔC–treated Fancc−/− preleukemic stem cells using clonogenic and bone marrow transplantation assays. We found that TAT-NPMΔC transduction significantly suppressed clonogenic proliferation (Figures 5A, S1A) and hematopoietic repopulating ability (Figures 5B, S1B) of the Fancc−/− preleukemic cells compared with BSA-treated cells. Interestingly, we did not observe further increase in colony-forming and repopulating ability of Fancc−/− pre-leukemic cells treated with TAT-NPM compared with BSA-treated cells (Figures 5, S1). However, we observed a significant increase in apoptosis in colony-forming cells and bone marrow repopulating cells of the TAT-NPMΔC–treated Fancc−/− preleukemic stem cells (Figure 5C).
TAT-NPMΔC suppresses proliferation and induces apoptosis in Fancc−/− preleukemic stem cells. (A) Clonogenic assay. WT BM cells or Fancc−/− preleukemic cells cultured in semi-solid medium containing 30 μg/mL of the indicated proteins were analyzed for colony-forming efficiency of hematopoietic progenitors. Data represents the mean plus or minus SD of 3 experiments. (B) Bone marrow transplantation assay. 106 WT BM cells or Fancc−/− preleukemic cells (CD45.2+) were transplanted, along with 106 competitor cells from B6.BoyJ mice (CD45.1+), into lethally irradiated recipient (B6.BoyJ) mice, which after 10 days were injected intraperitonally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for 4 weeks. Engraftment was evaluated at 4 weeks after transplantation. Data represent mean plus or minus SD of 3 independent experiments, each with 6 animals (total, 18 mice). (C) TAT-NPMΔC induces growth arrest and apoptosis in colony-forming and repopulating Fancc−/− cells. Bone marrow cells from colonies described in panel A or bone marrow cells from recipient mice described in panel B were stained with propidium iodide (PI) followed by analysis for cell-cycle distribution. Shown are representative flow cytometric presentations of 3 colony assays or 3 to 4 recipients in each treatment.
TAT-NPMΔC suppresses proliferation and induces apoptosis in Fancc−/− preleukemic stem cells. (A) Clonogenic assay. WT BM cells or Fancc−/− preleukemic cells cultured in semi-solid medium containing 30 μg/mL of the indicated proteins were analyzed for colony-forming efficiency of hematopoietic progenitors. Data represents the mean plus or minus SD of 3 experiments. (B) Bone marrow transplantation assay. 106 WT BM cells or Fancc−/− preleukemic cells (CD45.2+) were transplanted, along with 106 competitor cells from B6.BoyJ mice (CD45.1+), into lethally irradiated recipient (B6.BoyJ) mice, which after 10 days were injected intraperitonally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for 4 weeks. Engraftment was evaluated at 4 weeks after transplantation. Data represent mean plus or minus SD of 3 independent experiments, each with 6 animals (total, 18 mice). (C) TAT-NPMΔC induces growth arrest and apoptosis in colony-forming and repopulating Fancc−/− cells. Bone marrow cells from colonies described in panel A or bone marrow cells from recipient mice described in panel B were stained with propidium iodide (PI) followed by analysis for cell-cycle distribution. Shown are representative flow cytometric presentations of 3 colony assays or 3 to 4 recipients in each treatment.
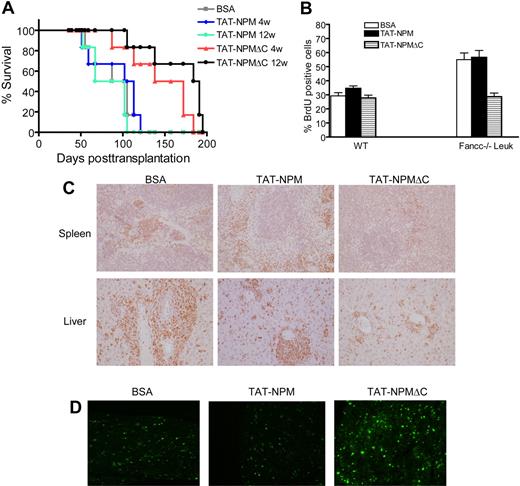
While the data presented above demonstrate that 4-week treatment of the recipient mice with TAT-NPMΔC reduced hematopoietic repopulating ability of the Fancc−/− preleukemic stem cells, it would be of importance to know whether the TAT-fusion was effective in inhibiting leukemia development. We therefore monitored the survival of recipients of Fancc−/− preleukemic cells over 4 and 12 weeks of TAT-NPM or TAT-NPMΔC treatment. Recipient mice injected with BSA were served as controls. To determine whether the TAT-fusions were actually delivered into the bone marrow of the recipient mice as well as the retention kinetics of the TAT-fusions in the recipient mice, we examined the levels of the TAT-fusions in BM cells from the recipient mice at various time points after a single injection. It appeared that TAT-NPM and TAT-NPMΔC were delivered at a similar rate and could be detected in BM cells of the recipient mice for up to 12 hours after injection (Figure S2). Recipients injected with BSA developed acute myeloid leukemia within 150 days (Figure 6A), consistent with previous report.16 The time required for leukemia to develop in recipients treated with TAT-NPM was indistinguishable from that of BSA-treated mice, regardless of the duration of the treatment. However, the occurrence of leukemia was significantly delayed in recipients injected with TAT-NPMΔC fusion, with long-term treatment (12 weeks) showing the better effect than the short-term treatment (4 weeks; Figure 6A). Furthermore, TAT-NPMΔC treatment clearly reduced cell proliferation, as evaluated by BrdU incorporation in the leukemic cells (Figure 6B) and Ki-67 antigen staining in the spleen and liver of the recipient mice (Figure 6C). TUNEL assay showed increased apoptosis in the bone marrow of the recipient mice treated with TAT-NPMΔC compared with BSA- or TAT-NPM–treated recipients (Figure 6D). Collectively, these results demonstrate that TAT-NPMΔC induces apoptosis and proliferative suppression of Fancc−/− leukemic cells and delays leukemia development.
TAT-NPMΔC delays leukemia development. (A) 106Fancc−/− preleukemic cells (along with 106 competitive cells) were injected intrvenously into lethally irradiated recipients, which after 10 days were injected intraperitonally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for up to 12 weeks. Survival of recipient mice was quantified by Kaplan-Meier analysis. Experiments were performed 2 times, each with 3 recipient mice (total, 6 mice per group). (B) 2 × 105 WT BM cells or Fancc−/− leukemic cells were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes. Cells were then washed and cultured for 24 hours in normal growth medium supplemented with 10 μM BrdU, and level of BrdU incorporation was determined by flow cytometry. Data represent mean plus or minus SD of 3 experiments. (C) TAT-NPMΔC inhibits leukemic cell proliferation. Tissue sections of spleen and liver from recipient mice treated with the indicated proteins for 4 weeks were stained with ki-67 and counterstained with hematoxylin and eosin (H&E). Magnification, 40×. (D) TAT-NPMΔC induces apoptosis in leukemic mice. Tissue sections of bone marrow from recipient mice treated with the indicated proteins for 4 weeks were analyzed by TUNEL staining (green). Magnification, 40×.
TAT-NPMΔC delays leukemia development. (A) 106Fancc−/− preleukemic cells (along with 106 competitive cells) were injected intrvenously into lethally irradiated recipients, which after 10 days were injected intraperitonally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for up to 12 weeks. Survival of recipient mice was quantified by Kaplan-Meier analysis. Experiments were performed 2 times, each with 3 recipient mice (total, 6 mice per group). (B) 2 × 105 WT BM cells or Fancc−/− leukemic cells were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes. Cells were then washed and cultured for 24 hours in normal growth medium supplemented with 10 μM BrdU, and level of BrdU incorporation was determined by flow cytometry. Data represent mean plus or minus SD of 3 experiments. (C) TAT-NPMΔC inhibits leukemic cell proliferation. Tissue sections of spleen and liver from recipient mice treated with the indicated proteins for 4 weeks were stained with ki-67 and counterstained with hematoxylin and eosin (H&E). Magnification, 40×. (D) TAT-NPMΔC induces apoptosis in leukemic mice. Tissue sections of bone marrow from recipient mice treated with the indicated proteins for 4 weeks were analyzed by TUNEL staining (green). Magnification, 40×.
TAT-NPMΔC forms complexes with endogenous NPM and p65 at promoters of antiapoptotic and inflammatory genes and abrogates their transactivation by NF-κB in preleukemic cells
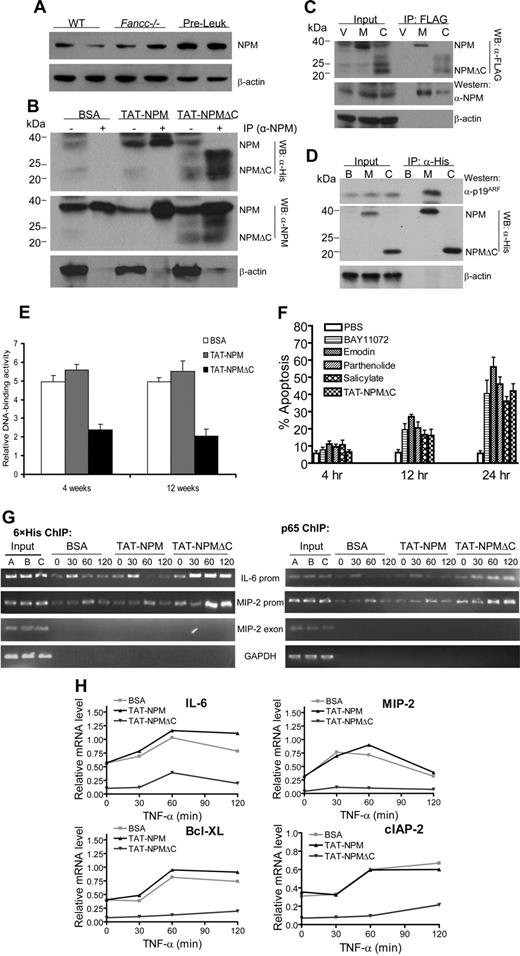
To further investigate the mechanism by which TAT-NPMΔC inhibited NF-κB activation, we first examined the relationship of TAT-NPMΔC and endogenous NPM in Fancc−/− preleukemic cells. We found that NPM was markedly up-regulated in Fancc−/− preleukemic cells compared with bone marrow mononuclear cells isolated from WT or Fancc−/− mice (Figure 7A). This might explain our finding that no further increase in colony-forming and repopulating ability were observed in Fancc−/− preleukemic cells treated with TAT-NPM (Figure 6A,B), as the level of the endogenous NPM with the same functionality as TAT-NPM was already high enough. Shortly (30 minutes) after treatment, both TAT-NPM and TAT-NPMΔC associated with endogenous NPM in nuclei of Fancc−/− preleukemic cells (Figure 7B). This was not surprising because NPMΔC retains the N-terminal oligomerization domain.25 We further confirmed the observed interaction between the NPM fusions and endogenous NPM using a normal lymphoblast cell line that expressed FLAG-NPM or FLAG-NPMΔC (Figure 7C). It has been reported that NPM interacts with p19Arf and regulates the biological function of p19Arf.26,27 We thus determined p19ARF protein levels and interaction between TAT-NPM and p19Arf or between TAT-NPMΔC and p19Arf in Fancc−/− preleukemic cells. Our results show that treatment of the Fancc−/− preleukemic cells with or without the TAT-NPM fusions did not affect p19Arf protein levels and that only the full-length TAT-NPM interacted with p19Arf (Figure 7D).
TAT-NPMΔC forms complexes with endogenous NPM and p65 and abrogates NF-κB activation in Fancc−/− preleukemic cells. (A) Up-regulation of NPM in Fancc−/− preleukemic cells. Whole cell extracts of freshly isolated bone marrow cells from WT and Fancc−/− mice or Fancc−/− preleukemic cells were analyzed for NPM protein levels by immunoblotting. Data with 2 mice from each group are shown. (B) Nuclear association of TAT-NPM fusions with endogenous NPM in Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes. Nuclear extracts were prepared and used for IP with an anti-NPM antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against 6 × histine (top panel), NPM (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lane of each group. (C) Nuclear association of NPMΔC with endogenous NPM in Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were infected with vector (V), WT NPM (M), or NPMΔC (C) retroviruses. Nuclear extracts were prepared and used for IP with an anti-FLAG antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against FLAG (top panel), NPM (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lanes. (D) TAT-NPMΔC does not associate with p19ARF. Fancc−/− preleukemic cells were treated with BSA (B), TAT-NPM (M), or TAT-NPMΔC (C) (30 μg/mL each) for 30 minutes. Whole cell extracts were prepared and used for IP with an anti-His antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against p19ARF (top panel), 6 × histine (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lane of each group. (E) TAT-NPMΔC inhibits NF-kB activity in Fancc−/− leukemic mice. 106Fancc−/− preleukemic cells (along with 106 competitive cells) were injected intravenously into lethally irradiated recipients, which after 10 days were injected intraperitoneally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for 4 and 12 weeks. Nuclear extracts were then prepared from BM cells of the recipient mice 24 hours after the last injection and DNA-binding activity of NF-κB was measured by transcription factor ELISA assays. The results are the means plus or minus SD of 2 independent experiments, each with 3 recipient mice from each group. Statistical significance (P < .05) between BSA or TAT-NPM and TAT-NPMΔC. (F) Kinetics of apoptosis induced by TAT-NPMΔC other NF-κB inhibitors in Fancc−/− leukemic cells. Fancc−/− preleukemic cells were treated with TAT-NPMΔC (30 μg/mL), sodium salicylate (5 mM), emodin 3 μg/mL), parthenolide (2 μM), or BAY11072 (0.2 μM) for the indicated time. Apoptosis was determined by flow cytometry for the percentage of cells with active caspase 3. The results are presented as the means plus or minus SD of 2 experiments. There is no statistically significant difference between TAT-NPMΔC and other tested NF-κB inhibitors. (G) Increased binding of TAT-NPMΔC and p65 to NF-κB–responsive promoters in Fancc−/− preleukemic cells. Chromatin immunoprecipitation assays. Fancc−/− preleukemic cells were treated with TNF-α for the indicated time in the presence of BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each). Recruitment of TAT-NPMΔC or the p65 NF-κB subunit to the IL-6 and MIP-2 promoters was assessed by immunoprecipitation and PCR amplification of the promoter sequences. (H) Down-regulation of proinflammatory and antiapoptotic genes in TAT-NPMΔC–treated Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were treated with TNF-α for the indicated time in the presence of BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each). RNA was isolated, and gene expression was quantified by real-time PCR and normalized to the level of GAPDH mRNA.
TAT-NPMΔC forms complexes with endogenous NPM and p65 and abrogates NF-κB activation in Fancc−/− preleukemic cells. (A) Up-regulation of NPM in Fancc−/− preleukemic cells. Whole cell extracts of freshly isolated bone marrow cells from WT and Fancc−/− mice or Fancc−/− preleukemic cells were analyzed for NPM protein levels by immunoblotting. Data with 2 mice from each group are shown. (B) Nuclear association of TAT-NPM fusions with endogenous NPM in Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were treated with BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each) for 30 minutes. Nuclear extracts were prepared and used for IP with an anti-NPM antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against 6 × histine (top panel), NPM (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lane of each group. (C) Nuclear association of NPMΔC with endogenous NPM in Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were infected with vector (V), WT NPM (M), or NPMΔC (C) retroviruses. Nuclear extracts were prepared and used for IP with an anti-FLAG antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against FLAG (top panel), NPM (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lanes. (D) TAT-NPMΔC does not associate with p19ARF. Fancc−/− preleukemic cells were treated with BSA (B), TAT-NPM (M), or TAT-NPMΔC (C) (30 μg/mL each) for 30 minutes. Whole cell extracts were prepared and used for IP with an anti-His antibody. The immuno-complexes were then analyzed by Western blotting with antibodies against p19ARF (top panel), 6 × histine (middle panel), or actin (bottom panel). Input controls (10%) are shown in left lane of each group. (E) TAT-NPMΔC inhibits NF-kB activity in Fancc−/− leukemic mice. 106Fancc−/− preleukemic cells (along with 106 competitive cells) were injected intravenously into lethally irradiated recipients, which after 10 days were injected intraperitoneally with the indicated proteins (10 mg/kg in 0.5 mL PBS and 10% glycerol) twice a week for 4 and 12 weeks. Nuclear extracts were then prepared from BM cells of the recipient mice 24 hours after the last injection and DNA-binding activity of NF-κB was measured by transcription factor ELISA assays. The results are the means plus or minus SD of 2 independent experiments, each with 3 recipient mice from each group. Statistical significance (P < .05) between BSA or TAT-NPM and TAT-NPMΔC. (F) Kinetics of apoptosis induced by TAT-NPMΔC other NF-κB inhibitors in Fancc−/− leukemic cells. Fancc−/− preleukemic cells were treated with TAT-NPMΔC (30 μg/mL), sodium salicylate (5 mM), emodin 3 μg/mL), parthenolide (2 μM), or BAY11072 (0.2 μM) for the indicated time. Apoptosis was determined by flow cytometry for the percentage of cells with active caspase 3. The results are presented as the means plus or minus SD of 2 experiments. There is no statistically significant difference between TAT-NPMΔC and other tested NF-κB inhibitors. (G) Increased binding of TAT-NPMΔC and p65 to NF-κB–responsive promoters in Fancc−/− preleukemic cells. Chromatin immunoprecipitation assays. Fancc−/− preleukemic cells were treated with TNF-α for the indicated time in the presence of BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each). Recruitment of TAT-NPMΔC or the p65 NF-κB subunit to the IL-6 and MIP-2 promoters was assessed by immunoprecipitation and PCR amplification of the promoter sequences. (H) Down-regulation of proinflammatory and antiapoptotic genes in TAT-NPMΔC–treated Fancc−/− preleukemic cells. Fancc−/− preleukemic cells were treated with TNF-α for the indicated time in the presence of BSA, TAT-NPM, or TAT-NPMΔC (30 μg/mL each). RNA was isolated, and gene expression was quantified by real-time PCR and normalized to the level of GAPDH mRNA.
To study the functional consequence of the interaction between TAT-NPMΔC and endogenous NPM in the context of NF-κB inhibition, we first asked whether TAT-NPMΔC inhibited NF-κB in vivo by determining NF-κB activity in the treated recipient mice. As shown in Figure 7E, treatment of TAT-NPMΔC for 4 and 12 weeks dramatically reduced DNA-binding activity of NF-κB in the recipient mice. In addition, treatment with TAT-NPMΔC induced apoptosis in Fancc−/− leukemic cells at both kinetics and magnitude that are comparable to other NF-κB inhibitors such as sodium salicylate, emodin, parthenolide, BAY11072 (Figure 7F).
Next, we used ChIP assay to determine whether in vivo association of TAT-NPMΔC with endogenous NPM affected the binding of NF-κB to the promoters of IL-6 and MIP-2 genes. After 30 minutes of TNF-α stimulation, the association of TAT-NPMΔC and p65 with the kB-responsive elements at the IL-6 promoter was increased in TAT-NPMΔC–treated Fancc−/− preleukemic cells compared with BSA or TAT-NPM–treated cells (Figure 7G). Similarly, the association TAT-NPMΔC and p65 with the MIP-2 promoter was also increased in TAT-NPMΔC–treated cells. Importantly, both 6 × His and p65 ChIPs showed prolonged residence of TAT-NPMΔC and p65 at NF-κB–responsive promoters of the IL-6 and MIP-2 genes in TAT-NPMΔC–treated Fancc−/− pre-leukemic cells (Figure 7G). This may explain the prolonged TNF-α–induced NF-κB accumulation in nuclei of TAT-NPMΔC–treated cells (Figure 4C). Consistent with its inhibitory effect on NF-κB activation, TAT-NPMΔC greatly down-regulated expression of proinflammatory and antiapoptotic genes in Fancc−/− preleukemic cells, such as IL-6, COX2, Bcl-XL, and cIAP-1 known to be the targets of NF-κB (Figure 7H). Therefore, TAT-NPMΔC appears to inhibit NF-κB activation by binding to p65-associated endogenous NPM and thus converting NF-κB to a transcriptionally inactive or repressive state.
Discussion
Given that NPM is frequently up-regulated in actively proliferating cells and human cancers,1-6 it is of great interest to investigate the therapeutic value by targeting the overexpressed NPM in tumor cells. In this paper, we present a novel protein therapy targeting the overexpressed NPM in inflammation-associated leukemia. We have used the TAT protein transduction domain to rapidly and efficiently deliver a polypeptide derived from the N-terminus of the NPM protein into preleukemic cells and tissues of leukemic mice. Using an inflammation-associated leukemogenic model we recently established, we demonstrated that the TAT fusion peptide (TAT-NPMΔC) induced proliferative suppression and apoptosis of preleukemic cells and significantly delayed leukemic development in mice. Furthermore, we found that the TAT-NPMΔC fusion formed complexes with endogenous NPM and specifically interfered with NF-κB function in leukemic cells. Thus, our results suggest that TAT-delivered NPM peptide may provide a novel therapy targeting inflammation-associated tumors that require NF-κB signaling for survival.
TAT-NPMΔC appears to be very potent in repressing NF-κB transactivation of numerous inflammatory and antiapoptotic genes, which may explain our observations that TAT-NPMΔC could effectively act to induce apoptosis in leukemic cells and delay the development of inflammation-associated leukemia in mice. NPM has been identified as an NF-κB coactivator.24 The results of the present study clearly demonstrate TAT-NPMΔC–mediated repression of the expression of inflammatory and antiapoptotic genes that is dependent on NF-κB. The results also reveal the underlying mechanism of inhibition. That is, TAT-NPMΔC forms a tertiary complex with the endogenous NPM and NF-κB, thus converting NF-κB to a transcriptionally inactive state that becomes trapped at κB element-containing promoters. This notion is supported by the finding that the inhibition of NF-κB transactivation by TAT-NPMΔC is accompanied by a paradoxical increase in the nuclear accumulation and the DNA-binding activity of NF-κB. This mechanism of action is in contrast with that of the classic NF-κB inhibitors, which act to prevent NF-κB nuclear translocation by inhibiting the phosphorylation and degradation of IκBα.28,29 Persistent NF-κB activation is commonly observed in inflammatory diseases and malignancies,30 and overexpression of NPM is often found in human cancers.1-6 In this context, use of the TAT-delivered NPMΔC to not only convert NF-κB to inactive state but also sequester the overexpressed endogenous NPM to biologically nonfunctional protein complexes may prove to be therapeutically attractive.
In addition to inhibiting NF-κB, TAT-NPMΔC appears to be able to activate p53 in cultured primary cells. The precise mechanism by which TAT-NPMΔC induces the activation of endogenous p53 in the steady state remains to be further elucidated; however, our results indicate that treatment of presenescent cells with TAT-NPMΔC strongly enhanced phosphorylation of p53 at the Ser15 residue and subsequently induced p53 target p21WAF1/CIP1 (Figure 4). Phosphorylation at the Ser15 residue of p53 is critical for p53-dependent transactivation.31 The ability of TAT-NPMΔC to augment p53 activity can explain, at least in part, its ability to induce apoptosis and to accelerate senescence in unstressed cells (Figure 2). Loss of p53 functions has been implicated in more than 50% of human cancers due to inactivating mutations.32 Therefore, reactivation of p53 functions or inactivation of p53 negative regulators has long been considered as an effective therapy for cancers. The data provided here reveal that the TAT-delivered NPMΔC causes a significant increase of the active form of p53 and thus suggest that TAT-NPMΔC might be able to reactivate the p53-mediated apoptotic pathway in tumor cells. In this context, TAT-NPMΔC could be used as a novel therapeutic agent in combination with DNA damage drugs for cancer prevention and treatments.
The dual effect of TAT-NPMΔC as a NF-κB inhibitor and a p53 activator raises an important question of whether the effects of TAT-NPMΔC on NF-κB inhibition and p53 activation are interrelated. The observation that TAT-NPMΔC exerted its negative effect on NF-κB by forming an inactive complex with the endogenous NPM and NF-κB excludes the possibility that would place TAT-NPMΔC–induced p53 activation upstream of NF-κB inhibition, as TAT-NPMΔC appears to exert its negative effect directly on NF-κB. Mechanisms of mutual negative regulation of NF-κB and p53 have been reported.33 Recently, it has been shown that inhibition of NF-κB by small molecules reactivates p53 in renal cell carcinoma,34 suggesting that there are unidentified regulatory factors that provide interactions between NF-κB and p53 signaling pathway. Another possibility is that TAT-NPMΔC could act on p53 in parallel with NF-κB inhibition. The exact mechanism remains to be determined.
In conclusion, we have demonstrated the efficiency and biological activity of intracellular delivery of a mutant NPM peptide using TAT PTD, thereby providing a novel protein therapy targeting the overexpressed NPM in inflammation-associated leukemia. TAT-NPMΔC may also be applicable for other tumors and disease conditions that require NF-κB signaling for survival.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Manuel Buchwald (Hospital for Sick Children, University of Toronto, ON) for the Fancc+/− mice, Dr Steven F Dowdy (University of California, San Diego, CA) for the pTAT-2.1 vector, Dr Albert S. Baldwin (University of North Carolina-Chapel Hill) for the pGL3-KB-Luc plasmids, Drs Xiaoling Zhang, Zsuzsanna Adam, and Yuhui Qiu (Cincinnati Children's Hospital Medical Center) for technical assistance, Jeff Bailey and Victoria Summey for bone marrow transplantation, and the Vector Core of the Cincinnati Children's Research Foundation (Cincinnati Children's Hospital Medical Center) for the preparation of retroviruses.
This work was supported by a Leukemia Research Foundation grant and National Institutes of Health grants R01 CA109641 and R01 HL076712. Q.P. is supported by a Leukemia & Lymphoma Society Scholar award.
National Institutes of Health
Authorship
Contribution: Y.Z. and W.D. performed research, analyzed data, and wrote the paper; T.K. performed research and analyzed data; G.C.B. designed research; and Q.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qishen Pang, Division of Experimental Hematology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Avenue, Cincinnati, OH 45229; e-mail: qishen.pang@cchmc.org.
References
Author notes
*Y.Z. and W.D. contributed equally to this work.