To the editor:
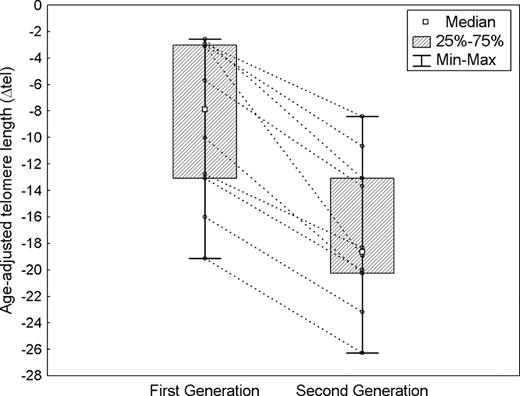
Landgren et al1 have reported the final results of a pivotal population-based case-control study including more than 11 000 patients with myeloproliferative neoplasms (MPNs) and almost 25 000 first-degree relatives. The authors identified a 5- to 7-fold higher risk of MPN among first-degree relatives of patients with MPN. These findings reinforce prior cohort studies2-4 and are in favor of familial clustering in MPN. To evaluate disease anticipation, Landgren et al carried out a survival analysis comparing the occurrence of MPN between the cohort of healthy parents and the cohort of healthy offspring of the affected individuals, with age as the time variable. Unlike our prior observations,3 they did not find disease anticipation. As this phenomenon is usually evaluated within affected members of the same family by studying parent-offspring pairs,5-7 in our prior study we evaluated only families with 2 successive generations of affected individuals.3 Although a register-based study offers a unique opportunity of evaluating a very large number of cases, it may not be suitable to account for the familial links at the individual level, as we did. Furthermore, in the Landgren et al study the curves of MPN risk in offspring and parents largely overlap. This may be explained by the fact that observation time started from birth, while the onset of MPN is extremely rare before 20 years of age. It probably influenced the not-significant log-rank test reported in the paper. After our first report,3 we expanded the study on disease anticipation to a total of 23 families which included 46 patients (20 with polycythemia vera, 19 with essential thrombocythemia, and 7 with primary myelofibrosis). This study was approved by the institutional ethics committee of Pavia and the procedures followed were in accordance with the 1975 Declaration of Helsinki, as revised in 2000. Samples for molecular analysis were obtained after patients provided written informed consent. Applying Wilcoxon matched-pair test, we found that age at diagnosis of MPN was significantly higher in first-generation patients (median 64 years, range 43-78) than in second-generation patients (median 38 years, range 23-57; P < .001). In addition, we studied the telomere length using flow cytometric analysis2 in 10 families with 2-generation pairs. Wilcoxon matched-pair test showed a significantly shortened telomere length in offspring compared with parents (P = .005). Figure 1 shows telomere shortening between the 2 generations and the variation of the length between members of the same family. In conclusion, taking into account for the familial links, our analysis provides evidence of disease anticipation in familial MPN.
Anticipation of disease onset in familial myeloproliferative neoplasms. Age-adjusted telomere length in 10 families with 2-generation pairs. Lines connect 2 members of each family. The average length of telomere in granulocytes was measured by flow cytometry combined with fluorescence in situ hybridization. Telomere length was adjusted for age to avoid the age-effect on telomere shortening. Telomere length was expressed as the difference between the observed length and the age-adjusted normal telomere length predicted from the linear regression line (ΔTEL). Wilcoxon matched pairs test demonstrated that second-generation patients had significantly shorter telomeres than first-generation patients.
Anticipation of disease onset in familial myeloproliferative neoplasms. Age-adjusted telomere length in 10 families with 2-generation pairs. Lines connect 2 members of each family. The average length of telomere in granulocytes was measured by flow cytometry combined with fluorescence in situ hybridization. Telomere length was adjusted for age to avoid the age-effect on telomere shortening. Telomere length was expressed as the difference between the observed length and the age-adjusted normal telomere length predicted from the linear regression line (ΔTEL). Wilcoxon matched pairs test demonstrated that second-generation patients had significantly shorter telomeres than first-generation patients.
Authorship
This study was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan; Fondazione Cariplo, Milan; Fondazione Ferrata Storti, Pavia; Fondazione IRCCS Policlinico San Matteo, Pavia; and Ministry of University and Research, Rome, Italy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisa Rumi, MD, Division of Hematology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: elisarumi@hotmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal