To the editor:
Expression of the common class I human leukocyte antigen (HLA) A*01 has recently been shown to increase the risk of developing Epstein-Barr virus (EBV)–positive Hodgkin lymphoma (HL).1 Furthermore, genetic markers closely linked to the HLA-A*01 allele were also shown to be associated with development of acute infectious mononucleosis (IM) upon primary EBV infection.2 It is not surprising that these 2 EBV-associated diseases share the same genetic predisposition because a previous history of IM is a significant risk factor for EBV-associated HL.3
Because HLA-class-I molecules present viral peptides for recognition by CD8+ T cells, and because this T-cell subset is known to play a critical role in the control of EBV infection,4 we investigated the hypothesis that the EBV-specific T-cell response restricted through HLA-A*01 is relatively weak, thereby providing a possible mechanism to explain the link between HLA-A*01 expression and these EBV-associated diseases.
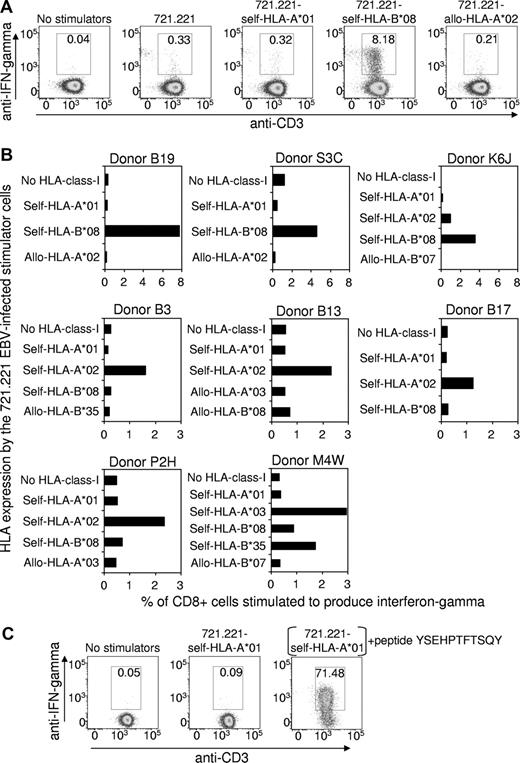
Peripheral blood mononuclear cells from 8 HLA-A*01+, EBV-seropositive healthy subjects were stimulated with an irradiated autologous lymphoblastoid cell line (LCL) to expand the EBV-specific T-cell population. The relative strength of the EBV-specific CD8+ T-cell response, restricted through individual HLA alleles, was then assessed using intracellular cytokine staining (ICS) after a brief period of stimulation with the mutant HLA-class-I–negative 721.221 LCLs5 that had been transfected to expressed only HLA-A*01 or other HLA-class-I alleles. Figure 1A shows representative flow cytometric data for one donor (Donor B19: HLA-A*01, A*03, B*08, B*44), which clearly demonstrates that the EBV-specific T-cell response restricted through HLA-A*01 is negligible, while the response restricted through another self-HLA-allele B*08 is very strong (8.18% of CD8+ cells). As expected, the 721.221 LCLs transfected to express the allogeneic HLA-A*02 allele failed to stimulate a significant response. Data from the other 7 HLA-A*01+ donors are summarized in Figure 1B. Although the overall strength of the EBV-specific CD8+ T-cell response varied between the donors, the data confirmed that the EBV-specific T-cell response restricted through HLA-A*01 is negligible, while responses restricted through at least one other self-HLA allele were significant.
The EBV-specific CD8+ T-cell response restricted through individual HLA-class I alleles. (A) An EBV-specific T-cell culture was raised from the peripheral blood lymphocytes of the HLA-A*01+, EBV-seropositive healthy donor B19 (HLA-A*01, A*03, B*08, B*44) by stimulation (responder-stimulator ratio of 10:1) with an irradiated autologous LCL, generated earlier by infection of B lymphocytes with the B95-8 strain of EBV. After 3 days of culture, the tissue culture medium (10% fetal bovine serum/RPMI 1640) was supplemented with recombinant interleukin-2 (20 units/mL), and on day 10 an ICS assay was performed to assess the number of EBV-specific T cells restricted through individual HLA alleles. This involved flow cytometric detection of interferon-γ–producing T cells after overnight incubation at 37°C in medium supplemented with brefeldin A (BD Pharmingen) and with the HLA-class-I–negative 721.221 cell line that had been transfected to express a single HLA-class-I allele. These cell lines were established as follows: cDNA for each HLA allele was amplified using sequence-specific primers and cloned into the expression vector pEGFP-N1 (Clontech, Palo Alto, CA), which contains a green fluorescent protein (GFP) tag and an antibiotic resistance gene to allow for positive selection. The HLA-EGFP-N1 expression constructs were transfected into 721.221 cells using the Bio-Rad Gene Pulser, and transfectants were cultured in the presence of G418 antibiotic (800 μg/mL) for 3 weeks. GFP-positive cells were sorted using a FACSVantage, and the purified cells were maintained in the presence of G418 (800 μg/mL). HLA class I expression on the transfectants was confirmed using an anti–HLA-class-I monoclonal antibody (W6/32) and flow cytometry. The antibody staining for the ICS flow cytometry analysis involved incubation of the responder-stimulator cell mixtures at 4°C for 30 minutes with peridinin chlorophyll protein–conjugated anti-CD8, and allophycocyanin-conjugated anti-CD3. Cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) at 4°C for 20 minutes, washed and incubated with phycoerythrin (PE)–conjugated anti-interferon-γ (BD Pharmingen) at 4°C for 30 minutes. Gated CD8+ cells are shown, and the numbers on the figure show the percentage of CD8+ cells coexpressing interferon-γ and CD3. (B) EBV-specific T-cell cultures were raised from another 7 HLA-A*01+, EBV-seropositive healthy donors and were analyzed, as described, to assess the number of EBV-specific T cells restricted through individual HLA alleles. Data are expressed as the percentage of CD8+ cells stimulated to produce interferon-γ. Note that Donors B19, S3C and K6J responded more strongly to some stimulator cells compared with the other 5 donors and so the graph scales have been adjusted accordingly. The HLA-A/B types of the donors are as follows: Donor P2H: A*01, A*02, B*08, B*44; Donor K6J: A*01, A*02, B*08, B*57; Donor B3: A*01, A*02, B*08, B*40; Donor B13: A*01, A*02, B*27, B*57; Donor B17: A*01, A*02, B*07, B*08; Donor S3C: A*01, B*08; M4W: A*01, A*03, B*08, B*35. (C) Control experiment demonstrating that the 721.221 cell line transfected with HLA-A*01 does express functional HLA molecules on the cell surface. A CD8+ T-cell clone was raised from a healthy HLA-A*01+ human cytomegalovirus–exposed donor by in vitro stimulation with the YSEHPTFTSQY peptide from the pp65 antigen which has been shown to be immunogenic in the context of HLA-A*01. An ICS assay, performed as described, showed that the clone failed to be stimulated by the HLA-A*01-transfected 721.221 cells unless these cells were preincubated with the YSEHPTFTSQY peptide (1 μg/mL) and washed 5 times.
The EBV-specific CD8+ T-cell response restricted through individual HLA-class I alleles. (A) An EBV-specific T-cell culture was raised from the peripheral blood lymphocytes of the HLA-A*01+, EBV-seropositive healthy donor B19 (HLA-A*01, A*03, B*08, B*44) by stimulation (responder-stimulator ratio of 10:1) with an irradiated autologous LCL, generated earlier by infection of B lymphocytes with the B95-8 strain of EBV. After 3 days of culture, the tissue culture medium (10% fetal bovine serum/RPMI 1640) was supplemented with recombinant interleukin-2 (20 units/mL), and on day 10 an ICS assay was performed to assess the number of EBV-specific T cells restricted through individual HLA alleles. This involved flow cytometric detection of interferon-γ–producing T cells after overnight incubation at 37°C in medium supplemented with brefeldin A (BD Pharmingen) and with the HLA-class-I–negative 721.221 cell line that had been transfected to express a single HLA-class-I allele. These cell lines were established as follows: cDNA for each HLA allele was amplified using sequence-specific primers and cloned into the expression vector pEGFP-N1 (Clontech, Palo Alto, CA), which contains a green fluorescent protein (GFP) tag and an antibiotic resistance gene to allow for positive selection. The HLA-EGFP-N1 expression constructs were transfected into 721.221 cells using the Bio-Rad Gene Pulser, and transfectants were cultured in the presence of G418 antibiotic (800 μg/mL) for 3 weeks. GFP-positive cells were sorted using a FACSVantage, and the purified cells were maintained in the presence of G418 (800 μg/mL). HLA class I expression on the transfectants was confirmed using an anti–HLA-class-I monoclonal antibody (W6/32) and flow cytometry. The antibody staining for the ICS flow cytometry analysis involved incubation of the responder-stimulator cell mixtures at 4°C for 30 minutes with peridinin chlorophyll protein–conjugated anti-CD8, and allophycocyanin-conjugated anti-CD3. Cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) at 4°C for 20 minutes, washed and incubated with phycoerythrin (PE)–conjugated anti-interferon-γ (BD Pharmingen) at 4°C for 30 minutes. Gated CD8+ cells are shown, and the numbers on the figure show the percentage of CD8+ cells coexpressing interferon-γ and CD3. (B) EBV-specific T-cell cultures were raised from another 7 HLA-A*01+, EBV-seropositive healthy donors and were analyzed, as described, to assess the number of EBV-specific T cells restricted through individual HLA alleles. Data are expressed as the percentage of CD8+ cells stimulated to produce interferon-γ. Note that Donors B19, S3C and K6J responded more strongly to some stimulator cells compared with the other 5 donors and so the graph scales have been adjusted accordingly. The HLA-A/B types of the donors are as follows: Donor P2H: A*01, A*02, B*08, B*44; Donor K6J: A*01, A*02, B*08, B*57; Donor B3: A*01, A*02, B*08, B*40; Donor B13: A*01, A*02, B*27, B*57; Donor B17: A*01, A*02, B*07, B*08; Donor S3C: A*01, B*08; M4W: A*01, A*03, B*08, B*35. (C) Control experiment demonstrating that the 721.221 cell line transfected with HLA-A*01 does express functional HLA molecules on the cell surface. A CD8+ T-cell clone was raised from a healthy HLA-A*01+ human cytomegalovirus–exposed donor by in vitro stimulation with the YSEHPTFTSQY peptide from the pp65 antigen which has been shown to be immunogenic in the context of HLA-A*01. An ICS assay, performed as described, showed that the clone failed to be stimulated by the HLA-A*01-transfected 721.221 cells unless these cells were preincubated with the YSEHPTFTSQY peptide (1 μg/mL) and washed 5 times.
As a positive control, to confirm that the HLA-A*01–transfected 721.221 cell line expressed this HLA molecule on the cell surface, a T-cell clone specific for the HLA-A*01–binding human cytomegalovirus epitope YSEHPTFTSQY6 was tested for recognition of 721.221-HLA-A*01 cells that had been preincubated with the YSEHPTFTSQY synthetic peptide and washed extensively. As shown in Figure 1C, these peptide-treated cells stimulated 71.5% of the T cells, confirming expression of functional HLA-A*01 molecules on the surface of the 721.221-HLA-A*01 cells.
These data provide strong evidence that HLA-A*01 fails to present highly immunogenic peptides from the EBV latent antigens expressed by LCLs. Although we are not aware of any previous studies that have specifically examined the HLA-A*01-restricted, EBV-specific response, it is notable that no T-cell epitopes that bind to this HLA allele have yet been mapped to any EBV antigens. Thus T-cell control over the proliferation of EBV+ B cells and the malignant, EBV+ Hodgkin Reed Sternberg cells may be relatively inefficient in HLA-A*01+ individuals, particularly those homozygous for this allele, thereby contributing to an increased risk of acute infectious mononucleosis and EBV+ Hodgkin lymphoma.
Authorship
Contribution: R.M.B. performed research, analyzed data, and wrote the paper; and S.R.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott Burrows, Queensland Institute of Medical Research and School of Medicine, University of Queensland, Herston, Brisbane, Australia; e-mail: scott.burrows@qimr.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal