In this issue of Blood, Sarasquete and colleagues report that the rs1934951 polymorphism mapped within the cytochrome P450-2C polypeptide 8 gene (CYP2C8) is associated with increased risk for the development of ONJ in myeloma patients who receive intravenous BPs.
Osteonecrosis of the jaw (ONJ) is a potentially serious complication of bisphosphonate therapy (BPs), characterized by the development of exposed bone in the oral cavity with no healing of the soft tissue for more than 8 weeks of follow-up. Pathologically, the lesions are characterized by necrotic and acellular bone associated with a quiescent bone surface.1 The cause of ONJ in myeloma is uncertain and likely multifactorial. The incidence of ONJ is higher after the use of the more potent intravenous BPs and when invasive dental procedures are performed. The risk for ONJ increases with BP treatment duration and has been reported to be 5% to 15% at 4 years.2 Guidelines for the prevention, diagnosis, and management of ONJ have been proposed by both the American Society for Clinical Oncology and the Mayo Clinic.3,4 It seems that appropriate preventive measures, such as a detailed assessment of dental status by experienced specialists and avoidance of invasive dental procedures during BP therapy, have the potential to reduce the risk of ONJ.5 Furthermore, myeloma patients who develop ONJ after dental procedures are less likely to have recurrence or nonhealing lesions after BP reinitiation following ONJ healing compared with those who develop spontaneous ONJ lesions.6 As BPs remain the cornerstone for the management of myeloma bone disease, it is important to identify patients at a higher risk for development of ONJ.
Genetic variants are associated with disease susceptibility. The recently developed single nucleotide polymorphism (SNP) array enables simultaneous detection of a large number of DNA polymorphic loci in a simple way. Using this technology Sarasquete et al evaluated half a million SNPs in 2 groups of myeloma patients who received the same induction therapy. The first group included 22 patients who developed ONJ and the second included 65 patients who did not develop ONJ (control group). All patients received BP therapy, either pamidronate (16 ONJ cases and 57 controls) or zoledronic acid (6 ONJ cases and 8 controls) for 2 years. In the studied cohort of patients, the incidence of ONJ was 5.6% at 5 years.
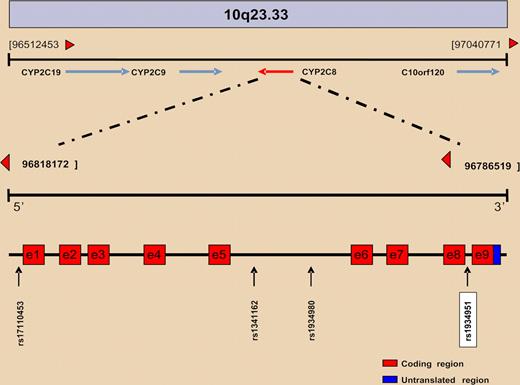
The initial analysis discovered 4 SNPs that could possibly be associated with the presence of ONJ: rs1934951, rs1934980, rs1341162, and rs17110453 (see figure). All of them mapped within CYP2C8, which spans a 31Kb region sited within a cluster of cytochrome P450 genes on chromosome 10q23. However, only the SNP rs1934951, which is located at intron 8, was significantly associated with ONJ, having an odds ratio of 12.75. More importantly, individuals homozygous for the T allele were exclusively found within the ONJ group.
Overview of the physical and genetic structure of the 10q23 region where the CYP2C family of genes is located. CYP2C8 gene structure and SNP positions are represented at bottom.
Overview of the physical and genetic structure of the 10q23 region where the CYP2C family of genes is located. CYP2C8 gene structure and SNP positions are represented at bottom.
CYP2C enzymes account for about 20% of the total CYP in liver microsomes and are involved in the metabolism of about 20% of clinically used drugs. All members of the CYP2C gene subfamily, namely CYP2C8, CYP2C9, CYP2C18, and CYP2C19, are polymorphic. CYP2C8 plays a significant role in the metabolism of the anticancer drug paclitaxel, the antimalarial drug amodiaquine, and the hypoglycaemic agent troglitazone, as well as amiodarone, verapamil, and ibuprofen. Polymorphisms of CYP2C8 are associated with side effects of the aforementioned drugs.7 The CYP2C8*3 and CYP2C9*2 are the major variant alleles in whites. However, it is difficult to find a biological link between the rs1934951 polymorphism of the CYP2C8 and the development of ONJ in myeloma patients who receive BPs, as CYP2C8 is not involved in the metabolism of BPs and the rs1934951 polymorphism is placed in an intronic region, which theoretically should not imply alterations at the protein level. Possible explanations for this finding is that CYP2C8 is also involved in the initiation of the 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoAR) pathway, a biological key metabolic cascade for cholesterol synthesis, which can be inhibited by BPs, and also that CYP2C8 metabolizes arachidonic acid to epoxyeicosatrienoic acids, which play a key role in the regulation of vascular tone and cardiovascular homeostasis.8 However, these hypotheses have yet to be proven.
The value of the study by Sarasquete et al is that, for the first time, a genetic variable is associated with the development of ONJ. The confirmation of these results may lead to the recognition of a subset of myeloma patients who are predisposed to develop ONJ after intravenous BPs. For these patients, a different approach for bone maintenance or BP administration may need to be considered.
Conflict-of-interest disclosure: The authors have received honoraria for participating on Novartis advisory boards. ■