Abstract
A growing body of evidence suggests that peptides containing the Asn-Gly-Arg (NGR) motif can selectively recognize tumor neovasculature and can be used, therefore, for ligand-directed targeted delivery of various drugs and particles to tumors or to other tissues with an angiogenesis component. The neovasculature binding properties of these peptides rely on the interaction with an endothelium-associated form of aminopeptidase N (CD13), an enzyme that has been implicated in angiogenesis and tumor growth. Recent studies have shown that NGR can rapidly convert to isoaspartate-glycine-arginine (isoDGR) by asparagine deamidation, generating αvβ3 ligands capable of affecting endothelial cell functions and tumor growth. This review focuses on structural and functional properties of the NGR motif and its application in drug development for angiogenesis-dependent diseases. Furthermore, we discuss the time-dependent transition of NGR to isoDGR in natural proteins, such as fibronectins, and its potential role of as a “molecular timer” for generating new binding sites for integrins impli-cated in angiogenesis.
Introduction
Phage display libraries are often used for discovering peptide sequences that interact with proteins differentially expressed in normal and pathologic tissues.1,2 Indeed, in vivo panning of peptide-phage libraries in tumor-bearing animal models has proven useful for selecting peptides able to interact with proteins expressed within tumor-associated blood vessels and therefore to home to neoplastic tissues.3 Among the ligands identified so far, cyclic and linear peptides containing the Asn-Gly-Arg (NGR) motif have been exploited for systemic, yet ligand-directed targeted delivery of therapeutic and imaging agents to angiogenic blood vessels, including cytotoxic drugs and cytokines, among other entities (such as viruses and nanoparticles). These findings highlight the value of NGR peptides in drug development. In this review we discuss the biochemical and biologic properties of NGR and NGR-derived compounds. Given that many native proteins contain the sequence NGR, we also address the emerging role of NGR as an unrecognized “molecular timer” due to the time-dependent generation of isoAsp-Gly-Arg (isoDGR), a new integrin-binding motif that regulates a gain-of-function within the extracellular matrix protein fibronectin.4
The discovery of the NGR motif
In vitro panning of several phage libraries against the α5β1 integrin led to the selection of various RGD-containing peptides and also of the peptide NGRAHA.5 These peptides (including NGRHA) inhibited cell attachment mediated by both αvβ3 and αvβ5 integrins. Moreover, 8 NGR-containing peptides were isolated upon screening of cyclic peptide libraries under similar experimental conditions.6 Notably, one selected phage clone displayed the peptide CVLNGRMEC, which is similar to the sequence ALNGREE found within the 9th type III repeat of human fibronectin.7 Further studies based on in vitro selection of libraries on αvβ3, revealed several different peptides containing the NGR motif, such as NGRIPD, TNGRGP, NGRSFR, RSRNGR, NGRNTV.8
In another line of investigation, in vivo phage-display screenings were performed to isolate tumor-homing peptides. Systemic administration of a phage library into nude mice bearing human breast carcinoma xenografts led to selection of a tumor vasculature-homing phage carrying the sequence CNGRCVSGCAGRC.3 Tumor homing was inhibited by co-injection with the CNGRC peptide (NGR-2C) indicating that this short cyclic loop is a functional tumor targeting peptide. Phage displaying the peptides NGRAHA or CVLNGRMEC, previously identified in vitro, also selectively localized to tumors.3
The NGR receptor(s)
In vivo phage display-based studies showed that also the peptide ACDCRGDCFC (RGD-4C), an αvβ3/αvβ5 binding sequence, can bind to tumor neovasculature.3,9 Cross-inhibition experiments of NGR-2C and RGD-4C-phage clones with synthetic RGD-4C and NGR-2C peptides showed that RGD-4C peptide does not compete the homing of NGR-2C-phage to tumors and vice versa.3 This result suggests that NGR-2C and RGD-4C bind to distinct receptors in tumor blood vessels. Studies aimed at elucidating the molecular basis behind NGR tumor-homing properties showed that this motif can specifically bind to cells expressing aminopeptidase N (CD13),10 a membrane-bound metallopeptidase that plays multiple functions as a regulator of various hormones and cytokines, protein degradation, antigen presentation, cell proliferation, cell migration, and angiogenesis.11-13
Remarkably, CD13 is not (or barely) expressed by the endothelium of normal blood vessels but it is up-regulated in angiogenic blood vessels.10,14 Indeed, it has been shown that NGR-containing peptides can target activated endothelial cells and pericytes not only in tumors, but also in other physiologic or pathologic conditions, such as inflammation and retinal disorders. Consistently, Buehler et al recently demonstrated that endothelial CD13 is up-regulated in a murine model of cardiac angiogenesis and that a fluorophore-tagged CNGRC conjugate colocalizes with CD13 and with the endothelial marker CD31 on blood vessels only in angiogenic areas.15 Recent work has also shown that proliferating retinal blood vessels express CD13 and that CNGRC-phage can home to angiogenic retina.16
Many other cell types besides the endothelium of angiogenic blood vessels express CD13 including tumor cells, pericytes, and, in some cases, fibroblasts and smooth muscle cells.12-14,17-19 This peptidase is also expressed by many cells of normal tissues, including myeloid cells, antigen-presenting cells, keratinocytes, mast cells, epithelial cells from renal proximal tubules, small intestine, prostate and bile duct canaliculi.12-14,17,19-21 Given that CD13 is evidently not exclusively expressed in the angiogenic endothelium, the mechanism underlying the neovasculature-homing properties of NGR peptides remains intriguing. Immunohistochemical analysis of CD13 in human tissues showed that differentially immunoreactive forms of CD13 are expressed in tumor-associated blood vessels, myeloid cells and epithelia.14 For instance, the immunoreactivities of 2 anti-CD13 monoclonal antibodies (WM15 and 13C03) with CD13 expressed by tumor endothelia and normal epithelia in renal cell carcinoma sections are markedly different, as WM15 can stain tumor blood vessels and little or not normal kidney epithelia, whereas 13C03 can strongly stain the apical part of epithelial cells of proximal tubules and much less tumor blood vessels.14 The CNGRC peptide, like WM15 can bind CD13-positive blood vessels in tumors, but not epithelia in normal kidney or other CD13-rich tissues.14 It would appear, therefore, that a diverse form of CD13 functionally active in mediating NGR binding is present in the tumor vasculature, but not in other CD13-rich tissues. However, the structural determinants of this selectivity are still unknown.22
CD13 is synthesized as an intracellular precursor of 967 residues and posttranslationally modified in the Golgi to produce a 150 to 240 kDa mature cell surface molecules comprising a short cytoplasmatic N-terminal domain, a single transmembrane part, and an extracellular domain containing the active site.13,23,24 Within the mature glycosylated protein, 25% to 30% of the molecular weight is composed of carbohydrates. Differential utilization of O-glycosylation sites results in at least 5 isoforms that are differentially recognized by antibodies, presumably due to transient masking of protein epitopes.25 Furthermore, binding of CD13 to natural peptide substrates or to antibodies may induce conformational changes and exposure of cryptic epitopes.26 In endothelial cells CD13 specifically interacts with galectin-3, a proangiogenic protein, in a carbohydrate recognition-dependent manner.27 It is possible that the selectivity of NGR peptides for endothelial CD13 is related to differential glycosylation or to conformational changes caused by complex formation with this or other unknown compounds. Selective accessibility due to the structurally abnormal architecture of angiogenic vasculature is also likely to play a role.22 These explanations are not mutually exclusive.
While substantial validation supports the role of CD13 as a critical receptor for NGR in tumor neovasculature, the role of integrins in NGR peptide binding to tumor blood vessels is less clear. Moreover, whether integrins can directly or indirectly contribute to NGR/CD13 interactions is presently unknown. However, recent findings suggest that integrins can play a role in the tumor homing properties of NGR-containing ligand through an unusual mechanism, based on deamidation of the asparagine residue and formation of isoaspartate. This reaction spontaneoulsly converts NGR into isoDGR, a novel cell adhesion motif that efficiently binds αvβ3 and α5β1 integrins.4 Interpretation of the integrin-binding experiments that led to the discovery of NGR peptides should, therefore, be taken with caution. The biochemical mechanism of the transition of NGR to isoDGR and its potential functional implications are discussed in detail later in this review.
Use of NGR peptides for targeted delivery of drugs and particles to neovasculature
Various compounds and particles have been coupled or added synthetically to NGR peptides in an attempt to increase their neovasculature-homing attributes, including cytotoxic drugs, cytokines, antiangiogenic compounds, viral particles, fluorescent compounds, contrast agents, DNA complexes, and other biologic response modifiers.3,15,28-51
The first anticancer drug that has been conjugated to an NGR peptide (CNGRC) is doxorubicin.3 This conjugate shows reduced toxicity and improved efficacy against human cancer xenografts in nude mice, compared with free doxorubicin. Other studies showed that liposomal formulations of doxorubicin can be targeted to tumors by coupling with linear peptides containing the GNGRG sequence.28-30 This formulation caused endothelial and tumor cells apoptosis, and showed antitumor and antimetastatic activity in an orthotopic model of human neuroblastoma.29 CNGRC-targeted proapoptotic peptomimetics such as D(KLAKLAKKLAKLAK) selectively kills angiogenic endothelial cells and has potent anticancer activity in mice.31
Coupling CNGRC to the N-terminus of tumor necrosis factor α (TNF), a cytokine endowed with potent vascular damaging properties and antitumor activity, led to the generation of a new compound, termed NGR-TNF, with improved antitumor activity.32,33 Remarkably, administration of ultra-low doses (picogram range) of NGR-TNF, but not of TNF, exerts synergistic antitumor effects with various chemotherapeutic drugs (such as doxorubicin, melphalan, cisplatin, paclitaxel and gemcitabine), by altering drug-penetration barriers.34-38 This drug is currently tested in Phase II clinical studies. The biologic activity of other cytokines and antiangiogenic molecules, such as IFNγ, IFNα2a, endostatin and tumstatin fragment have been improved by coupling to NGR peptides.41-43,52 Targeted delivery of minute amounts (picogram dose range) of a recombinant IFNγ-CNGRC conjugate (IFNγ-NGR) to tumor vasculature overcomes major counter regulatory mechanisms and delays tumor growth in mice.40
NGR peptides have been used also for gene therapy. Recombinant adeno-associated virus containing linear and cyclic versions of NGR preferentially transduce cells that express CD13.44 Furthermore recombinant adenoviral vectors with NGR introduced in the fiber knob infect human glioma cells (which do not express coxsackievirus and adenovirus receptor) 100 to 1000 times more efficiently than the virus containing wild-type fiber.53 Other studies showed that incorporation of NGR into Moloney murine leukemia virus envelope escort proteins improves retrovirus binding and transduction of endothelial cells.45
A novel system for tumor specific gene delivery mediated by CNGRC-polyethylenimine-DNA polyplex have also been developed.46 Intravenous delivery of this vector carrying a yellow fluorescent protein (YFP)–expressing plasmid showed YFP delivery to both neoplastic and endothelial cells in subcutaneous tumors.
Structure and activity of linear and cyclic NGR peptides
The discovery of linear and cyclic NGR-peptides with different flanking residues, by in vitro and in vivo selection of peptide-phage libraries,3,5,54,55 suggests that the molecular determinants of receptor binding are primarily located within the NGR residues. However, the results of functional studies with different NGR peptides suggest that the residues flanking NGR could also contribute to binding affinity and specificity. For instance, comparison studies of the antitumor properties of recombinant TNF fused with cyclic (CNGRC) and linear (GNGRG) peptides showed that the presence of cysteine residues and disulfide constraint are critical for the targeting efficiency, the activity of GNGRG-TNF being one order of magnitude lower than that of CNGRC-TNF.56 Experiments with recombinant adenoviruses (Ads) incorporating linear (eg, ALNGRMESP, GDGNGRGFG) or cyclic (eg, CNGRCVSGCAGRC, CDCNGRCFC) sequences in the HI loop of adenoviral fiber protein showed that NGR-containing Ads can transduce HEp2-αvβ3 expressing cells more efficiently than wild-type Ad.57 Interestingly, Ads with linear NGR sequences transduce these cells more efficiently than Ads containing cyclic disulfide-linked sequences. However, the same investigators also found that Ads with cyclic peptides transduce more efficiently RD cells that express CD13 and αvβ3 integrin.57 Other investigators showed that a linear LNGRV peptide can inhibit endothelial cell adhesion to ECM components and assumed that this was related to peptide-integrin interactions.58 Head-to-tail cyclization of the LNGRV caused, in this case, only a marginal change in biologic activity.58 Although the role of CD13 and integrins in this and the above studies still remains to be elucidated, these findings highlight the importance of the molecular scaffold in which NGR residues are embedded for receptor affinity and specificity. Possibly, scaffold and molecular constraints affects NGR-peptide/receptor interactions by directly contributing to the binding or by affecting peptide backbone flexibility and spatial distribution of NGR residues. The molecular scaffold could also affect the peptide stability, including the deamidation rate, with important implications in receptor specificity (as discussed in “Indirect interaction of NGR with integrins via isoDGR formation”).
Qualitative and quantitative differences may occur, therefore, in the recognition of receptors in tumor blood vessels when different NGR peptides are coupled to drugs or to particles or are incorporated in protein loops.
Structural similarity between the synthetic NGR peptide and an NGR loop within the 5th fibronectin type I repeat
Does the structure of the NGR motif isolated from phage libraries mimic binding sites in native proteins? Immunogenicity studies of NGR have shown that this motif is poorly, or not at all, immunogenic when flanked by cysteines of by glycines in various conjugates,59 suggesting that such peptides can mimic “self” structures.
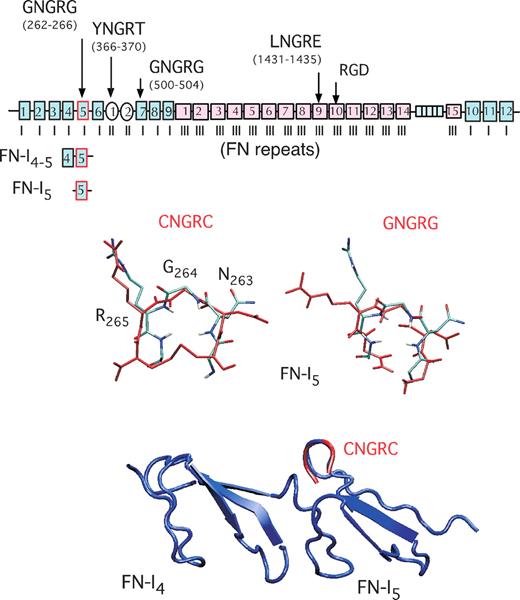
Search for short exact matches of CNGRC or GNGRG with vertebrate proteins in the SWISS-PROT database60 showed that 1 and 17 proteins, respectively, contain these motifs. Interestingly, among such proteins, fibronectins contain 2 conserved GNGRG motifs. Fibronectins (FNs) are large glycoproteins (∼450 kDa) composed of 2 nearly identical disulfide bonded subunits. The molecules are present in most body fluids and in the extracellular matrix of many tissues.61 Each subunit consists of 3 types of repeating homologous modules termed FN-I, FN-II, and FN-III repeats. Interestingly, the 5th type I repeat (FN-I5) contains the CTGNGRGEWKC sequence, which is 100% conserved across species (human, bovine, murine, rat, bird, amphibian, and fish fibronectin).59,61 Consistently, molecular dynamics simulation experiments predict that the most populated structures of CNGRC and GNGRG peptides are highly superimposable to that of the CTGNGRGEWKC hairpin of human FN-I5 (Figure 1).59 This observation may account for the low immunogenicity of NGR peptides and raises the question as to whether this domain of fibronectin can also interact with NGR receptors, as NGR peptides can.
Schematic representation of human fibronectin type I, II, and III modules and superposition of 4th to 5th type I repeats (FN-I4-5) 3-dimensional structure with CNGRC/GNGRG most populated structures. Modules containing NGR and RGD sequences (→). Superposition of the fibronectin (FN)–I4-5-PDB structure (in blue) and GNGRG hairpin of fibronectin (light blue) with the most populated structures of the CNGRC and GNGRG peptides (in red), predicted by molecular dynamics simulation.59 This scheme has been modified from Di Matteo et al.59
Schematic representation of human fibronectin type I, II, and III modules and superposition of 4th to 5th type I repeats (FN-I4-5) 3-dimensional structure with CNGRC/GNGRG most populated structures. Modules containing NGR and RGD sequences (→). Superposition of the fibronectin (FN)–I4-5-PDB structure (in blue) and GNGRG hairpin of fibronectin (light blue) with the most populated structures of the CNGRC and GNGRG peptides (in red), predicted by molecular dynamics simulation.59 This scheme has been modified from Di Matteo et al.59
Biologic function(s) of NGR in peptides and within native proteins
A central question to be addressed is whether the NGR motif is merely a tool for ligand-directed delivery of compounds to angiogenic vasculature or whether it can also exert biologic effects. A second equally important question is whether NGR sequences present in natural proteins such as fibronectin, play an inherent as yet unrecognized physiologic role.
CD13 exists either as a membrane-bound or as a soluble form. Both forms catalyze the removal of N-terminal residues, preferentially neutral, from small peptides.12 Over the past decade, many natural substrates have been discovered for CD13, including vasoactive peptides, neuropeptide hormones, cytokines and immunomodulatory peptides.12,62,63 Remarkably, studies using anti-CD13 monoclonal antibodies indicate that CD13 can undergo regulatory intramolecular alterations that result in exposure of cryptic sites and regulation of enzyme activity.26 Yokoyama et al showed that the activity of aminopeptidase N extracted from HUVEC cells could be partially inhibited by NGR-endostatin, and not by endostatin.42 Other investigators showed that the aminopeptidase activity of HT-80 cell membranes can be partially (42%) inhibited by a doxorubicin-CNGRC conjugate, but not by free doxorubicin or CNGRC peptide.64 These data may suggest that large NGR-containing products and proteins can bind and inhibit the enzymatic activity of CD13 in these cells by steric hindrance or by causing protein conformational changes.
CD13 has been implicated in extracellular matrix degradation, cell migration, angiogenesis and tumor invasion.65-69 Antibodies capable of inhibiting the enzymatic activity of CD13 and bestatin, a chemical inhibitor, can inhibit hypoxia-induced retinal neovascularization.10 CD13 inhibitors can also suppress the growth of tumor xenografts derived from MDA-MB-435 cells that do not express CD13, likely by affecting CD13-positive tumor blood vessels.10 These finding suggest that CD13 plays a crucial role in angiogenesis. According to this view, CD13-null mice showed impaired angiogenesis.68 The role of CD13 may be to facilitate endothelial cell invasion of tissues, which is a critical step in angiogenesis,70 or to modulate the activity of growth factors and cytokines.17
CD13 has also been implicated in signal transduction: ligation of CD13 on monocytes with monoclonal antibodies causes the release of calcium from intracellular stores and influx from the extracellular environment, provokes phosphorylation of mitogen-activated protein kinase 1/2, JNK, and p38, and induce cytokine secretion.71 The signaling activity of CD13 probably depends on the presence of cooperating membrane proteins, as the short cytoplasmatic domain of this ectoenzyme does not contain known signaling motives.72 The possibility that natural and synthetic compounds containing one or more NGR sites could affect CD13 signaling in blood vessels cannot be excluded and this point deserves further investigation.
Indirect interaction of NGR with integrins via isoDGR formation
Several lines of evidence suggest that NGR can somehow interact with integrins. For instance, besides the original works that led to the discovery of NGR from peptide-phage libraries, by panning on α5β1 and αvβ3 integrins, Asokan et al showed that adeno-associated virus type 2 contains an NGR site that is critical for α5β1 binding and viral cell entry.73 Moreover, adenoviruses genetically engineered with linear NGR sequences retarget adenoviruses to αvβ3 integrin expressing cells.57 However, in vitro binding studies with purified αvβ3 integrin and freshly prepared peptides containing the CNGRC or GNGRG sequences showed only a modest interaction (A.C. and F.C., unpublished observations, May 2007). The biologic relevance of direct NGR/integrin interactions remains, therefore, questionable. Nevertheless, recent studies showed that NGR can indeed play a role in integrin binding in an indirect manner, after asparagine deamidation and isoaspartate formation.4
Asparagine deamidation and consequent formation of isoaspartate and aspartate is a nonenzymatic posttranslational modification directly associated with protein aging.74,75 Isoaspartate formation can occur in vivo (eg, in extracellular matrix proteins with slow turnover), and in vitro (during protein isolation and storage).74-76 Isoaspartate formation in extracellular matrix proteins is generally viewed as a degradation reaction, as it is generally associated with “loss-of-function.”76 We have recently shown that spontaneous deamidation of Asn263 of the NGR site of the 5th fibronectin type I repeat (FN-I5; Figure 1) is actually associated with a “gain-of-function,” as deamidated fragments of fibronectin that contain this domain (eg, natural FN-I1-5, recombinant FN-I4-5 and synthetic FN-I5, collectively called “isonectins”) can promote endothelial cell adhesion when they are adsorbed on microtiter plates.4 The FN-I5 fragment can also inhibit cell adhesion to solid-phase vitronectin when added to the liquid phase.4
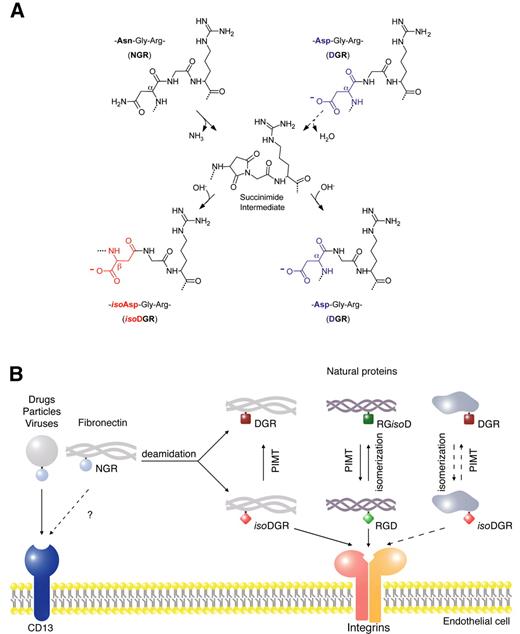
Asn deamidation at NGR sites occurs via succinimide intermediate, which upon hydrolytic cleavage leads to formation of Asp and isoAsp in a 1:3 ratio in L or D configurations (Figure 2A). Studies aimed at identifying the biologically active product after deamidation showed that isoDGR, but not DGR, can promote endothelial cell adhesion and bind purified αvβ3 and α5β1, the latter with lower efficiency.4 Competitive binding experiments showed that isoDGR can inhibit the binding of RGD-containing ligands to both αvβ3 and α5β1, suggesting that the binding site is located within the RGD binding pocket. NMR studies and docking experiments showed that cyclic isoDGR peptides favorably interacts, in an inverted orientation, with the RGD-binding site of αvβ3, recapitulating canonical RGD/αvβ3 contacts and establishing additional polar interactions.77 Conversely, cyclic NGR and DGR peptides cannot establish critical contacts for high affinity binding.77
Formation of isoaspartate in molecules containing NGR, DGR, or RGD motives and schematic representation of the potential effects on receptor binding. (A) NGR transition to isoDGR in conjugates and in natural proteins (eg, fibronectin) can occur by nucleophilic attack of the backbone NH center on the Asn side-chain amide carbonyl, leading to formation of a succinimide intermediate. Hydrolysis of succinimide leads to formation of isoDGR and DGR mixtures, with changes in charge and peptide bond length. Isoaspartate formation can occur, in principle, also at DGR sites in proteins, by aspartate isomerization, although with slower kinetics. These nonenzymatic reactions are thermodynamically spontaneous and the kinetics depend on surrounding sequences and microenvironmental factors. (B) Potential effect of isoaspartate formation in molecules containing NGR, RGD, or DGR on the interaction with endothelial cell membrane receptors. NGR-containing conjugates (drugs, particles, and viruses) can interact with an endothelial form of aminopeptidase N (CD13). Whether NGR sites of certain natural proteins (eg, fibronectin) can also interact with CD13 is unknown. Nonenzymatic formation of isoaspartate at NGR or RGD sites in fibronectin, and potentially in other proteins with low turnover, can generate isoDGR or RGisoD.4 While isoDGR and RGD can interact with integrins on the cells surface (eg, αvβ3), DGR and RGisoD are inactive. Protein-L-isoAsp-O-methyltransferase (PIMT) can enzymatically convert isoDGR to DGR and RGisoD to RGD. Thus, while PIMT may rescue RGD from RGisoD (eg, in aged fibronectin and collagen),4,74,76 this enzyme may regulate in a negative manner the function of isoDGR. NGR sites may work, therefore, as molecular timers for the formation of new integrin binding sites with potential enzymatic regulation. In principle, similar reactions could occur also in proteins containing DGR.
Formation of isoaspartate in molecules containing NGR, DGR, or RGD motives and schematic representation of the potential effects on receptor binding. (A) NGR transition to isoDGR in conjugates and in natural proteins (eg, fibronectin) can occur by nucleophilic attack of the backbone NH center on the Asn side-chain amide carbonyl, leading to formation of a succinimide intermediate. Hydrolysis of succinimide leads to formation of isoDGR and DGR mixtures, with changes in charge and peptide bond length. Isoaspartate formation can occur, in principle, also at DGR sites in proteins, by aspartate isomerization, although with slower kinetics. These nonenzymatic reactions are thermodynamically spontaneous and the kinetics depend on surrounding sequences and microenvironmental factors. (B) Potential effect of isoaspartate formation in molecules containing NGR, RGD, or DGR on the interaction with endothelial cell membrane receptors. NGR-containing conjugates (drugs, particles, and viruses) can interact with an endothelial form of aminopeptidase N (CD13). Whether NGR sites of certain natural proteins (eg, fibronectin) can also interact with CD13 is unknown. Nonenzymatic formation of isoaspartate at NGR or RGD sites in fibronectin, and potentially in other proteins with low turnover, can generate isoDGR or RGisoD.4 While isoDGR and RGD can interact with integrins on the cells surface (eg, αvβ3), DGR and RGisoD are inactive. Protein-L-isoAsp-O-methyltransferase (PIMT) can enzymatically convert isoDGR to DGR and RGisoD to RGD. Thus, while PIMT may rescue RGD from RGisoD (eg, in aged fibronectin and collagen),4,74,76 this enzyme may regulate in a negative manner the function of isoDGR. NGR sites may work, therefore, as molecular timers for the formation of new integrin binding sites with potential enzymatic regulation. In principle, similar reactions could occur also in proteins containing DGR.
Asparagine deamidation can occur with a surprisingly rapid kinetics (half-life 3-4 hours) at GNGRG loop in fibronectin fragments as well as in synthetic or recombinant cyclic CNGRC-containing products.4 However, human plasma fibronectin, freshly isolated, was found to contain only 3% to 4% of deamidated molecules.4 This suggests that the kinetics of deamidation in plasma fibronectin are different from those measured with fragments and peptides. Nevertheless, considering the low turnover of fibronectin after deposition in tissues,78 it is likely that a significant fraction of fibronectin molecules undergoes deamidation reactions at NGR sites in tissue matrix. One interesting possibility is that structural changes caused by fibril formation and proteolytic processing in tissues may play important regulatory roles.
Physiologic relevance of NGR/isoDGR transition in fibronectin
The rapid transition of NGR to isoDGR could have important implications not only for the biochemical and biologic properties of NGR-displaying phage and NGR-conjugates, but also for the physiologic role of fibronectin and fibronectin fragments. Fibronectins are adhesive proteins that mediate a variety of cellular interactions with extracellular matrix and play important roles in hemostasis, thrombosis, inflammation, wound repair, angiogenesis and embryogenesis.61,79 These functions critically depend on the organization of fibronectin dimers into fibrillar networks in tissue matrix. It is well recognized that fibril assembly requires the interaction of fibronectin with α5β1 or αv integrins, via the RGD site in the 10th FN-III repeat.80 By using mice genetically engineered to express a fibronectin variant with nonfunctional RGE (in place of RGD) it has been recently shown that αvβ3 can still assemble fibrils by binding the isoDGR site in FN-I5 repeat.81 This is an unequivocal demonstration that isoDGR plays an important role in fibronectin fibril assembly.
Several investigators implicated αvβ3 also as a receptor for various proteolytic fragments of extracellular matrix proteins that can act as antiangiogenic factors.82-84 Another interesting possibility is that deamidated fibronectin fragments contribute, together with other extracellular matrix protein fragments, to regulate angiogenesis. According to this view, it has been demonstrated that deamidated FN-I5 and a peptide containing the CisoDGRC sequence can inhibit microvascular endothelial cell adhesion and proliferation.4 These products can also inhibit tumor growth when daily administered (intraperitoneally) to mice bearing subcutaneous B16F1 melanomas,4 possibly by specific interactions with integrins implicated in angiogenesis.
Regulation of NGR/isoDGR function
Asn deamidation is a thermodynamically spontaneous reaction independent from enzymatic regulation. However, cells can produce protein-L-isoAsp-O-methyltransferase (PIMT), an enzyme that can convert isoaspartate to aspartate. The release of this enzyme in the microenvironment could represent a mechanism for negative enzymatic regulation of isoDGR in tissues. According to this view, treatment of deamidated FN-I5 (containing isoDGR) with PIMT caused conversion of isoDGR to DGR and loss of in vitro pro-adhesive activity.4 Thus, an “activation-deactivation” mechanism can be envisaged for NGR, based on “spontaneous deamidation-enzymatic isomerization.” While PIMT may be considered a sort of “repairing” enzyme for Asp residues undergoing spontaneous isomerization (eg, to rescue RGD from RGisoD in aged fibronectin and collagen),74,76 it may also be viewed as an enzyme that “deactivate” the function of isoDGR, by converting it to DGR (see Figure 2B for a schematic representation). Of note, increased amounts of extracellular PIMT have been observed in injured tissues and wound healing.85,86
Increased frequency of NGR, DGR, and RGD sequences in proteins involved in cell adhesion
Besides fibronectin, which contains 2 conserved GNGRG in the 5th and 7th type I repeats, and YNGRT and LNGRE sequences in the 1st type II and 9th type III repeats, many other proteins contain NGR motifs. Search for short exact matches of vertebrate proteins with NGR in the SWISS-PROT database showed that 5.02% of proteins contain this motif. This frequency is similar to that of XGR or NXR sequences (6.95 ± 2.9 and 3.98 ± 1.70; respectively, mean ± SD).
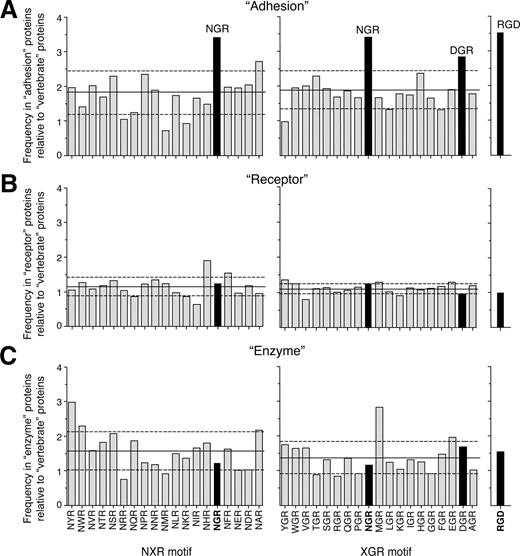
Although it is likely that most of these sites are not properly folded and sufficiently exposed for receptor interaction, it is tempting to speculate that at least some of them might play a role in cell adhesion. This hypothesis is based on the following considerations. First, in vitro selection of peptide-phage libraries on integrins led to the discovery of various NGR peptides with different flanking residues. This suggests that the CNGRC or GNGRG sequence is not a strict requirement for binding. Second, the frequency of NGR in proteins classified with the keyword “adhesion” in the database is higher than the frequency in the total vertebrate proteins (17.23% and 5.02%, respectively). Remarkably, similar frequencies were observed in these categories with RGD (22.28% and 6.35%), a prototypic integrin binding motif. Furthermore, analysis of the relative frequency of NXR or XGR sequences in the “adhesion” category showed that NGR occurs with the highest frequency (Figure 3A). In contrast, the relative frequency of NGR in proteins classified as “receptor” or “enzyme” is similar to that of other NXR or XGR sequences (Figure 3B,C). Interestingly also the frequency of DGR is increased in the “adhesion” category (Figure 3A). Considering that isoDGR can derive from NGR, by Asn deamidation (rapid), or from DGR, by Asp isomerization (slow), via formation of a succinimide intermediate (Figure 2), one interesting possibility is that both NGR and DGR can work as “molecular timers” for generating integrin-binding sites in proteins. Given that the residues surrounding NGR and DGR can also affect the kinetics of these reactions, ranging from hours to years, it is likely that only some NGR or DGR sites in proteins can play a significant role as candidate “molecular timers.”
Relative frequency of NGR in proteins involved in cell adhesion. Frequency of XGR, NXR, and RGD sequence in vertebrate proteins (SWISS-PROT database) classified with the keywords “adhesion” (n = 1010, A), “receptor” (n = 4561, B), and “enzyme” (n = 857, C), relative to the frequency in total vertebrate proteins (n = 39 525). NGR and DGR are the most frequent motives in the “adhesion” category. The analysis was performed using Search Databases for Regular Expression software (Center for Cancer Research, Massachusetts Institute of Technology, Cambridge, MA).87 Solid and dashed horizontal lines represent means and plus or minus SD, respectively.
Relative frequency of NGR in proteins involved in cell adhesion. Frequency of XGR, NXR, and RGD sequence in vertebrate proteins (SWISS-PROT database) classified with the keywords “adhesion” (n = 1010, A), “receptor” (n = 4561, B), and “enzyme” (n = 857, C), relative to the frequency in total vertebrate proteins (n = 39 525). NGR and DGR are the most frequent motives in the “adhesion” category. The analysis was performed using Search Databases for Regular Expression software (Center for Cancer Research, Massachusetts Institute of Technology, Cambridge, MA).87 Solid and dashed horizontal lines represent means and plus or minus SD, respectively.
Conclusions
Cyclic and linear peptides containing NGR have proven useful for ligand-directed targeted delivery of various drugs and particles to angiogenic blood vessels in tumors, highlighting the value of these tools in drug development. However, the recent findings that NGR can rapidly convert to isoDGR by asparagine deamidation, generating αvβ3 ligands, suggest that NGR could represent a molecular timer for the generation of integrin-binding sites in drugs as well as in native proteins. In particular, spontaneous conversion of NGR to isoDGR in fibronectin may represents a novel mechanism for generating αvβ3 binding sites that regulate endothelial cell functions and tumor growth. Generation of isoDGR sites in proteins, by NGR deamidation (or DGR isomerization), may represent a more general mechanism for regulating their function.
Considering that αvβ3 integrin is a good marker of angiogenic blood vessels, natural or synthetic polypeptides containing the isoDGR motif may be exploited, alone or in combination with NGR peptides, as ligands for targeted delivery of drugs, nanoparticles, genes or imaging compounds to angiogenic vasculature in tumors or in other diseases with an angiogenesis component.
Acknowledgment
This work was supported by a grant (46/07/CadC/AC) from the Associazione Italiana Ricerca sul Cancro (AIRC).
Authorship
Contribution: A.C., F.C., W.A., and R.P. worked together planning, drafting, and revising the text and figures.
Conflict-of-interest disclosure: W.A. and R.P. are inventors of a patent on NGR peptides. A.C. and F.C. are inventors of patents on NGR-TNF and isoDGR that have been licensed to Molmed SpA, Milan, Italy. A.C. is a consultant for Molmed SpA.
Correspondence: Angelo Corti, DIBIT-Department of Oncology, San Raffaele Scientific Institute, via Olgettina 58, 20132 Milan, Italy; e-mail: corti.angelo@hsr.it.