Abstract
A total of 152 patients with myelodysplastic syndrome (MDS) receiving a first stem cell transplant had marrow cells prospectively analyzed to calculate the flow cytometric scoring system (FCSS) score. The FCSS scores were retrospectively compared with patient outcomes in both univariate and multivariate models. The cumulative incidence of posttransplantation relapse at 3 years was 15%, 10%, and 36% for patients with mild, moderate, and severe FCSS scores, respectively, with the hazard for relapse of 2.8 (P = .02) for severe scores in comparison to patients with mild or normal FCSS scores. In multivariate analyses, the FCSS score was associated with relapse even after accounting for International Prognostic Scoring System (IPSS) score or for marrow myeloblast percentage. Among patients with intermediate-1 risk by IPSS, severe FCSS scores were associated with an increased hazard of relapse (3.8; P = .02) compared with patients with normal/mild/moderate FCSS scores. Among patients with less than 5% marrow myeloblasts, myeloblast dyspoiesis was associated with an increased hazard of relapse (3.7; P = .02). This analysis confirmed that FCSS scores are predictive of posttransplantation outcomes in patients with MDS even after adjusting for risk factors such as marrow myeloblast percentage and IPSS score.
Introduction
Various patient and disease characteristics are associated with the prognosis in myelodysplastic syndromes (MDSs). The most commonly used prognostic classification system is the International Prognostic Scoring System (IPSS),1 based on the presence of cytopenias, cytogenetic abnormalities, and bone marrow blast percentage. Recently, the World Health Organization (WHO) classification-based prognostic scoring system (WPSS), which considers the WHO classification,2 patient karyotypes, and red blood cell transfusion requirements, was published.3 The chief advantage of the WPSS is that it provides a real-time assessment of prognosis as opposed to the IPSS, which is applicable at the time of diagnosis. The application of the IPSS has been extended from providing prognostic implication to therapeutic decision analysis.4 We have previously shown in patients with MDS who underwent allogeneic hematopoietic cell transplantation (HCT) that the severity of pretransplantation dyspoiesis and immunophenotypic aberrancy in marrow cells, as determined by a flow cytometric scoring system (FCSS), correlated with the probability of disease relapse after HCT.5 The FCSS score distinguished risk groups above and beyond the impact of the IPSS score. This initial finding has since been corroborated by other investigators in a small nontransplantation cohort.6 In that study, the FCSS was also associated with transfusion dependence, disease progression, and WPSS. Here we applied the FCSS to a large new cohort of patients with MDS (not included in the initial cohort) to validate the predictive capacity of FCSS scores in the setting of HCT.
Methods
Patient, disease, and transplantation characteristics
The FCSS, as previously described,5 was prospectively collected before HCT on 152 patients with MDS. Patients included in this analysis received an allogeneic HCT between January 2001 and April 2005. Characteristics are summarized in Table 1. The diagnosis and classification of MDS were based on French-American-British and WHO criteria.2,7 Bone marrow myeloblast percentages were calculated on the basis of morphologic analysis, carried out as part of the standard pretransplantation workup, and occurred independently from the flow cytometric analysis. Cytogenetic studies were performed by a centralized laboratory on direct and on 24- to 48-hour unstimulated bone marrow cultures. All patients included in this analysis had 20 metaphases evaluable. Patients who had received chemotherapy before flow cytometric analysis and patients who received HCT after nonmyeloablative conditioning were excluded.8 The median age of patients was 51 years, and the median follow-up after HCT was 2.4 years among surviving patients. Given that this was indeed a transplantation cohort, it is not surprising that a minority of patients fit within the low-risk group by IPSS. Approximately half of the patients included in this cohort had demonstrable cytogenetic abnormalities, which corresponds to previous findings in an unselected population of patients with MDS.9 Most patients were conditioned with a regimen of targeted busulfan and cyclophosphamide as described.10 Granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from allogeneic donors served as the source of stem cells in the majority of the patients.11 Most patients received methotrexate and cyclosporine as prophylaxis for graft-versus-host disease (GVHD). All patients were enrolled in Fred Hutchinson Cancer Research Center Institutional Review Board–approved protocols active at time of enrollment and provided informed consent for data collection in accordance with the Declaration of Helsinki.
Patient, disease, and transplantation characteristics
| Characteristic . | Total . | Normal/mild . | Moderate . | Severe . |
|---|---|---|---|---|
| No. of patients | 152 | 47 | 48 | 57 |
| Age, y [median (range)] | 51 (1-66) | 46 (1-65) | 49 (3-65) | 47 (3-66) |
| Sex, M/F, no. of patients | 89/63 | 24/23 | 32/16 | 33/24 |
| Follow-up, y [median (range)] | 2.4 (1-5) | 2.3 (1-5) | 2.2 (1-5) | 2.6 (1-5) |
| Time from diagnosis to transplantation, d [median (range)] | 252 (17-4755) | 362 (17-4380) | 212 (49-1601) | 233 (84-4755) |
| WHO stage, no. (%) of patients | ||||
| RA | 24 (16) | 15 (32) | 7 (15) | 2 (4) |
| RARS/RCMD-RS | 7 (5) | 4 (9) | 3 (6) | 0 (0) |
| RCMD | 24 (16) | 10 (21) | 7 (15) | 7 (12) |
| MDS-U | 10 (7) | 5 (11) | 4 (8) | 1 (2) |
| 5q- | 6 (4) | 4 (9) | 2 (4) | 0 |
| RAEB-1 | 31 (20) | 5 (11) | 7 (15) | 19 (33) |
| RAEB-2 | 23 (15) | 1 (2) | 9 (19) | 13 (23) |
| tAML | 27 (18) | 3 (6) | 9 (19) | 15 (26) |
| IPSS risk group, no. (%) of patients | ||||
| Low | 9 (6) | 8 (17) | 1 (2) | 0 (0) |
| Intermediate-1 | 78 (51) | 28 (60) | 28 (58) | 22 (39) |
| Intermediate-2 | 39 (26) | 9 (19) | 13 (27) | 17 (30) |
| High | 26 (17) | 2 (4) | 6 (13) | 18 (31) |
| Cytogenetic risk group, no. (%) of patients | ||||
| Good | 66 (43) | 22 (47) | 23 (48) | 21 (37) |
| Intermediate | 46 (30) | 13 (28) | 16 (33) | 17 (30) |
| Poor | 40 (27) | 12 (25) | 9 (19) | 19 (33) |
| GVHD prophylaxis | ||||
| MTX/CSP | 122 (80) | 41 (87) | 36 (75) | 45 (79) |
| MTX/FK506 | 17 (11) | 5 (11) | 7 (15) | 5 (9) |
| CSP/MMF | 4 (3) | 0 | 2 (4) | 2 (4) |
| MTX/CSP/rapamycin | 7 (5) | 1 (2) | 2 (4) | 4 (6) |
| CSP/rapamycin | 2 (1) | 0 | 1 (2) | 1 (2) |
| Related donor | 65 (43) | 21 (45) | 22 (46) | 22 (39) |
| HLA-identical sibling | 62 (41) | 19 (41) | 22 (46) | 21 (37) |
| HLA-matched family member | 3 (2) | 2 (4) | 0 | 1 (2) |
| Unrelated donor | 87 (57) | 26 (55) | 26 (54) | 35 (61) |
| HLA-matched | 60 | 17 (36) | 19 (40) | 24 (42) |
| HLA-mismatched | 27 | 9 (19) | 7 (14) | 11 (19) |
| Source of stem cells | ||||
| Peripheral blood | 129 (85) | 38 (81) | 43 (90) | 48 (84) |
| Marrow | 21 (14) | 9 (19) | 4 (8) | 8 (14) |
| Cord blood | 2 (1) | 0 | 1 (2) | 1 (2) |
| Conditioning regimen | ||||
| tBuCy | 120 (79) | 37 (79) | 36 (75) | 47 (83) |
| BuFlu | 27 (18) | 9 (19) | 10 (21) | 8 (14) |
| CyTBI | 5 (3) | 1 (2) | 2 (4) | 2 (4) |
| Abnormal blast category | ||||
| Normal blasts ≤ 5% | 59 (39) | 42 (90) | 16 (33) | 1 (2) |
| Normal blasts > 5% | 2 (1) | 2 (4) | 0 | 0 |
| Abnormal blasts ≤ 5% | 31 (20) | 2 (4) | 16 (33) | 13 (23) |
| Abnormal blasts > 5% | 60 (40) | 1 (2) | 16 (33) | 43 (75) |
| BM myeloblast dyspoiesis | ||||
| Phenotypically normal | 61 (40) | 44 (94) | 16 (33) | 1 (2) |
| ≤ 5% abnormal | 31 (20) | 2 (4) | 16 (33) | 13 (23) |
| 5%-10% abnormal | 26 (17) | 1 (2) | 11 (23) | 14 (25) |
| 10%-20% abnormal | 27 (18) | 0 | 5 (10) | 7 (12) |
| > 20% abnormal | 7 (5) | 0 | 0 |
| Characteristic . | Total . | Normal/mild . | Moderate . | Severe . |
|---|---|---|---|---|
| No. of patients | 152 | 47 | 48 | 57 |
| Age, y [median (range)] | 51 (1-66) | 46 (1-65) | 49 (3-65) | 47 (3-66) |
| Sex, M/F, no. of patients | 89/63 | 24/23 | 32/16 | 33/24 |
| Follow-up, y [median (range)] | 2.4 (1-5) | 2.3 (1-5) | 2.2 (1-5) | 2.6 (1-5) |
| Time from diagnosis to transplantation, d [median (range)] | 252 (17-4755) | 362 (17-4380) | 212 (49-1601) | 233 (84-4755) |
| WHO stage, no. (%) of patients | ||||
| RA | 24 (16) | 15 (32) | 7 (15) | 2 (4) |
| RARS/RCMD-RS | 7 (5) | 4 (9) | 3 (6) | 0 (0) |
| RCMD | 24 (16) | 10 (21) | 7 (15) | 7 (12) |
| MDS-U | 10 (7) | 5 (11) | 4 (8) | 1 (2) |
| 5q- | 6 (4) | 4 (9) | 2 (4) | 0 |
| RAEB-1 | 31 (20) | 5 (11) | 7 (15) | 19 (33) |
| RAEB-2 | 23 (15) | 1 (2) | 9 (19) | 13 (23) |
| tAML | 27 (18) | 3 (6) | 9 (19) | 15 (26) |
| IPSS risk group, no. (%) of patients | ||||
| Low | 9 (6) | 8 (17) | 1 (2) | 0 (0) |
| Intermediate-1 | 78 (51) | 28 (60) | 28 (58) | 22 (39) |
| Intermediate-2 | 39 (26) | 9 (19) | 13 (27) | 17 (30) |
| High | 26 (17) | 2 (4) | 6 (13) | 18 (31) |
| Cytogenetic risk group, no. (%) of patients | ||||
| Good | 66 (43) | 22 (47) | 23 (48) | 21 (37) |
| Intermediate | 46 (30) | 13 (28) | 16 (33) | 17 (30) |
| Poor | 40 (27) | 12 (25) | 9 (19) | 19 (33) |
| GVHD prophylaxis | ||||
| MTX/CSP | 122 (80) | 41 (87) | 36 (75) | 45 (79) |
| MTX/FK506 | 17 (11) | 5 (11) | 7 (15) | 5 (9) |
| CSP/MMF | 4 (3) | 0 | 2 (4) | 2 (4) |
| MTX/CSP/rapamycin | 7 (5) | 1 (2) | 2 (4) | 4 (6) |
| CSP/rapamycin | 2 (1) | 0 | 1 (2) | 1 (2) |
| Related donor | 65 (43) | 21 (45) | 22 (46) | 22 (39) |
| HLA-identical sibling | 62 (41) | 19 (41) | 22 (46) | 21 (37) |
| HLA-matched family member | 3 (2) | 2 (4) | 0 | 1 (2) |
| Unrelated donor | 87 (57) | 26 (55) | 26 (54) | 35 (61) |
| HLA-matched | 60 | 17 (36) | 19 (40) | 24 (42) |
| HLA-mismatched | 27 | 9 (19) | 7 (14) | 11 (19) |
| Source of stem cells | ||||
| Peripheral blood | 129 (85) | 38 (81) | 43 (90) | 48 (84) |
| Marrow | 21 (14) | 9 (19) | 4 (8) | 8 (14) |
| Cord blood | 2 (1) | 0 | 1 (2) | 1 (2) |
| Conditioning regimen | ||||
| tBuCy | 120 (79) | 37 (79) | 36 (75) | 47 (83) |
| BuFlu | 27 (18) | 9 (19) | 10 (21) | 8 (14) |
| CyTBI | 5 (3) | 1 (2) | 2 (4) | 2 (4) |
| Abnormal blast category | ||||
| Normal blasts ≤ 5% | 59 (39) | 42 (90) | 16 (33) | 1 (2) |
| Normal blasts > 5% | 2 (1) | 2 (4) | 0 | 0 |
| Abnormal blasts ≤ 5% | 31 (20) | 2 (4) | 16 (33) | 13 (23) |
| Abnormal blasts > 5% | 60 (40) | 1 (2) | 16 (33) | 43 (75) |
| BM myeloblast dyspoiesis | ||||
| Phenotypically normal | 61 (40) | 44 (94) | 16 (33) | 1 (2) |
| ≤ 5% abnormal | 31 (20) | 2 (4) | 16 (33) | 13 (23) |
| 5%-10% abnormal | 26 (17) | 1 (2) | 11 (23) | 14 (25) |
| 10%-20% abnormal | 27 (18) | 0 | 5 (10) | 7 (12) |
| > 20% abnormal | 7 (5) | 0 | 0 |
Bu indicates busulfan; CSP, cyclosporine; Cy, cyclophosphamide; FK506, tacrolimus; Flu, fludarabine; MMF, mycophenolate mofetil; MTX, methotrexate; RA, refractory anemia; RARS/RCMD-RS, refractory anemia with ringed sideroblasts/refractory cytopenia with multilineage dysplasia and ringed sideroblasts; RCMD, refractory anemia multilineage dysplasia; MDS-U, myelodysplastic syndrome, unclassified; RAEB, refractory anemia with excess blasts; tAML, acute myeloid leukemia with multilineage dysplasia tranformed from a previous diagnosis of myelodysplastic syndrome; and TBI, total body irradiation.
Flow cytometry
Flow cytometric analyses were performed as previously described.5 Briefly, 3-color flow cytometry was performed on total nonerythroid bone marrow cells after lysis of erythroid cells with NH4Cl.12 A standardized panel of monoclonal antibodies was used to characterize populations of myeloid and monocytoid lineage (Table 2). Acquisition of flow data was performed on a FACS Calibur (BD Biosciences, San Jose, CA). Data analysis was performed using Winlist software (Verity Software House, Topsham, ME). Daily instrument quality controls were performed.13 Cell populations of interest were separated electronically using Boolean logic gating schemes.14 Cells were defined as having abnormalities when the characteristics under consideration were at least 0.5 decades disparate from corresponding cells from healthy donors on a 4-decade log scale. Flow cytometric analysis was performed on bone marrow aspirates obtained within 14 days before conditioning for HCT. The final FCSS was based on the independent analysis of data by 2 investigators (D.A.W., M.R.L.) who were blinded as to MDS category and transplantation outcomes.
Antibody panel
| . | FITC . | PE . | PerCP . |
|---|---|---|---|
| 1 | HLA-CR | CD11b | CD45 |
| 2 | CD5 | CD19 | CD45 |
| 3 | CD38 | CD56 | CD45 |
| 4 | CD16 | CD13 | CD45 |
| 5 | CD15 | CD34 | CD45 |
| 6 | CD14 | CD33 | CD45 |
| 7 | CD7 | CD56 | CD45 |
| 8 | HLA-DR | CD34 | CD45 |
| . | FITC . | PE . | PerCP . |
|---|---|---|---|
| 1 | HLA-CR | CD11b | CD45 |
| 2 | CD5 | CD19 | CD45 |
| 3 | CD38 | CD56 | CD45 |
| 4 | CD16 | CD13 | CD45 |
| 5 | CD15 | CD34 | CD45 |
| 6 | CD14 | CD33 | CD45 |
| 7 | CD7 | CD56 | CD45 |
| 8 | HLA-DR | CD34 | CD45 |
FITC indicates fluorescein isothio cyanate; PE, phycoerythrin; and PerCP, peridinin chlorophyll protein.
As in the initial report, based on the total flow score patients were grouped into the following categories: score 0 or 1, normal/mild; 2 or 3, moderate; 4 or 9, severe. The analysis of dyspoiesis in myeloid blasts included the following: presence/absence and intensities of myeloid and lymphoid antigens. The quantity of myeloblasts was based on analysis of multiple antigens, and the presence of dyspoiesis was used to group patients into 5 categories: phenotypically normal myeloblasts, abnormal myeloblasts less than 5%, abnormal myeloblasts 5% to 10%, abnormal myeloblasts 10% to 20%, and abnormal myeloblasts more than or equal to 20%. Of the 91 patients who had phenotypic aberrancies detected by flow cytometry, 36 had one abnormality, 37 had 2 abnormalities, 13 had 3 abnormalities, and 4 had 4 abnormalities. The myeloid abnormalities detected in this validation cohort are summarized in Table 3.
Percentage of myeloid abnormalities detected by flow cytometry, N = 165
| Abnormality . | n (%) . |
|---|---|
| Lack of HLA-DR | 5 (3) |
| Decreased CD45 expression | 58 (35) |
| Lack or decreased CD34 | 13 (8) |
| Lack or decreased CD13 | 15 (9) |
| Lack or decreased CD33 | 21 (13) |
| Overexpression of CD34 | 14 (9) |
| Overexpression of CD13 | 9 (5) |
| Overexpression of CD33 | 3 (2) |
| Expression of CD56 | 7 (4) |
| Expression of lymphoid antigens | 16 (10) |
| Expression of CD11b | 4 (2) |
| Abnormality . | n (%) . |
|---|---|
| Lack of HLA-DR | 5 (3) |
| Decreased CD45 expression | 58 (35) |
| Lack or decreased CD34 | 13 (8) |
| Lack or decreased CD13 | 15 (9) |
| Lack or decreased CD33 | 21 (13) |
| Overexpression of CD34 | 14 (9) |
| Overexpression of CD13 | 9 (5) |
| Overexpression of CD33 | 3 (2) |
| Expression of CD56 | 7 (4) |
| Expression of lymphoid antigens | 16 (10) |
| Expression of CD11b | 4 (2) |
Definitions of end points
All patients had post-HCT marrow evaluations scheduled on days 28, 56, and 84 (± 3 days), and 1 year after HCT, and subsequently as clinically indicated. Relapse was defined as posttransplantation reappearance of dysplastic cells by flow cytometry,15 morphologic evidence of dysplastic myeloblasts, or the reappearance of clonal cytogenetic abnormalities identified before transplantation.16 In patients with morphologic, hematologic, or cytogenetic evidence of relapse, relapse rather than graft rejection was considered to be the cause of failed engraftment. All surviving patients were asked to return at 1 year to the Fred Hutchinson Cancer Research Center for assessment of GVHD and relapse.
Statistical analysis
Correlations between outcomes were evaluated using Spearman rank correlation coefficients (ρ). Overall survival and relapse-free survival were estimated using the Kaplan-Meier method.17 Probability of relapse was calculated using cumulative incidence estimates, treating death without relapse as a competing risk event.18 Unadjusted and adjusted hazard ratios (HRs) were evaluated using proportional hazards regression models.19 Time to the event of interest (death, relapse, or first of either) was counted from the date of HCT to the earliest event of interest, competing risk event, or date of last contact (censoring time). P values for comparison of time to event outcomes were based on Wald or likelihood ratio statistics from the proportional hazards model. All statistical analyses were performed with Stata software (version 10.0, StataCorp LP, College Station, TX). A 2-sided alpha level of 0.05 was considered statistically significant. Data collected through December 2006 were used in analyses.
Results
FCSS compared with IPSS and marrow myeloblast dyspoiesis
Similar to the initial report, the IPSS correlated significantly with the FCSS (Spearman ρ = 0.45, P < .001), primarily because of strong agreement between low and high scores of the 2 scoring systems. There was greatest heterogeneity of the FCSS among patients with intermediate-1 and intermediate-2 risk MDS by IPSS (Table 1). Similarly, marrow myeloblast dyspoiesis was correlated with the FCSS (ρ = 0.82, P < .001; Table 1).
FCSS and posttransplantation outcome(s)
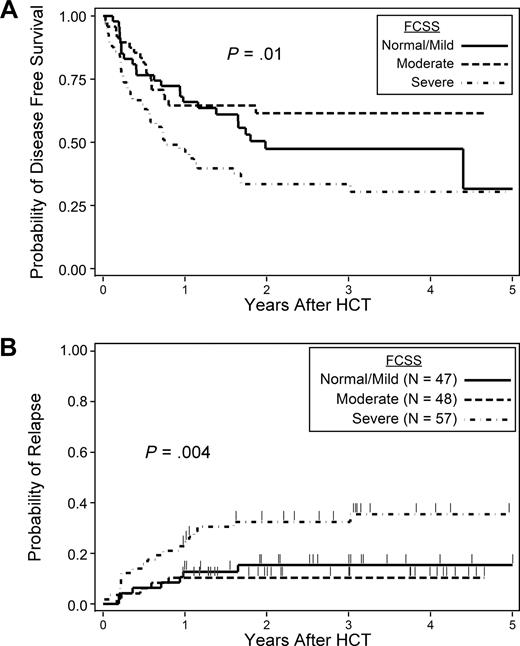
High FCSS scores were associated with an increased hazard of posttransplantation relapse (HR = 2.8, 95% confidence interval [CI], 1.2-6.7, P = .02) for severe versus normal/mild subjects. Differences in overall survival and relapse-free survival (RFS) were not statistically significant, although results suggested some impact on RFS (severe vs normal/mild HR = 1.6, 95% CI, 1.0-2.7, P = .06; Table 4). The probabilities of RFS at 2 years among patients with normal/mild, moderate, and severe FCSS scores were 50%, 62%, and 34%, respectively (P = .01, overall likelihood ratio test; Figure 1A). The cumulative incidences of posttransplantation relapse at 2 years among patients with normal/mild, moderate, and severe FCSS were 15%, 10%, and 32%, respectively (P = .004, overall LR test; Figure 1B).
Univariate analyses
| . | Death . | Relapse . | Death or relapse* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P† . | P‡ . | HR (95% CI) . | P† . | P‡ . | HR (95% CI) . | P† . | P‡ . | |
| FCSS | |||||||||
| Normal/mild† | 1 | NA | .13 | 1 | NA | .004 | 1 | NA | .01 |
| Moderate | 0.8 (0.4-1.6) | .58 | 0.7 (0.2-2.2) | .56 | 0.7 (0.4-1.4) | .33 | |||
| Severe | 1.4 (0.8-2.5) | .18 | 2.8 (1.2-6.7) | .02 | 1.6 (1.0-2.7) | .06 | |||
| IPSS | |||||||||
| Low | 0.5 (0.1-1.9) | .27 | .45 | 0.7 (0.1-5.1) | .69 | .06 | 0.6 (0.2-2.0) | .42 | .17 |
| Intermediate-1§ | 1 | NA | 1 | NA | 1 | NA | |||
| Intermediate-2 | 1.2 (0.7-2.1) | .44 | 1.5 (0.6-3.7) | .37 | 1.2 (0.7-2.1) | .47 | |||
| High | 1.1 (0.6-2.2) | .67 | 3.2 (1.4-7.4) | .007 | 1.7 (1-3) | .05 | |||
| Bone marrow myeloblast dyspoiesis | |||||||||
| Phenotypically normal§ | 1 | NA | .35 | 1 | NA | .008 | 1 | NA | .17 |
| ≤ 5% abnormal | 1.2 (0.6-2.3) | .64 | 3.1 (1.1-9.0) | .04 | 1.3 (0.7-2.5) | .35 | |||
| 5%-10% abnormal | 1.8 (1.0-3.5) | .07 | 1.2 (0.6-7.5) | .25 | 1.7 (0.9-3.3) | .09 | |||
| > 10% abnormal | 1.3 (0.7-2.4) | .4 | 4.8 (1.8-12.6) | .002 | 1.8 (1.0-3.1) | .05 | |||
| . | Death . | Relapse . | Death or relapse* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P† . | P‡ . | HR (95% CI) . | P† . | P‡ . | HR (95% CI) . | P† . | P‡ . | |
| FCSS | |||||||||
| Normal/mild† | 1 | NA | .13 | 1 | NA | .004 | 1 | NA | .01 |
| Moderate | 0.8 (0.4-1.6) | .58 | 0.7 (0.2-2.2) | .56 | 0.7 (0.4-1.4) | .33 | |||
| Severe | 1.4 (0.8-2.5) | .18 | 2.8 (1.2-6.7) | .02 | 1.6 (1.0-2.7) | .06 | |||
| IPSS | |||||||||
| Low | 0.5 (0.1-1.9) | .27 | .45 | 0.7 (0.1-5.1) | .69 | .06 | 0.6 (0.2-2.0) | .42 | .17 |
| Intermediate-1§ | 1 | NA | 1 | NA | 1 | NA | |||
| Intermediate-2 | 1.2 (0.7-2.1) | .44 | 1.5 (0.6-3.7) | .37 | 1.2 (0.7-2.1) | .47 | |||
| High | 1.1 (0.6-2.2) | .67 | 3.2 (1.4-7.4) | .007 | 1.7 (1-3) | .05 | |||
| Bone marrow myeloblast dyspoiesis | |||||||||
| Phenotypically normal§ | 1 | NA | .35 | 1 | NA | .008 | 1 | NA | .17 |
| ≤ 5% abnormal | 1.2 (0.6-2.3) | .64 | 3.1 (1.1-9.0) | .04 | 1.3 (0.7-2.5) | .35 | |||
| 5%-10% abnormal | 1.8 (1.0-3.5) | .07 | 1.2 (0.6-7.5) | .25 | 1.7 (0.9-3.3) | .09 | |||
| > 10% abnormal | 1.3 (0.7-2.4) | .4 | 4.8 (1.8-12.6) | .002 | 1.8 (1.0-3.1) | .05 | |||
HR indicates hazard rate ratio; CI, confidence interval; and NA, not applicable.
Inverse of disease-free survival.
Wald test.
Likelihood ratio test for multilevel risk factor.
Referent category.
Outcome probabilities. Probability of disease-free survival by FCSS (A) and probability of relapse by FCSS (B).
Outcome probabilities. Probability of disease-free survival by FCSS (A) and probability of relapse by FCSS (B).
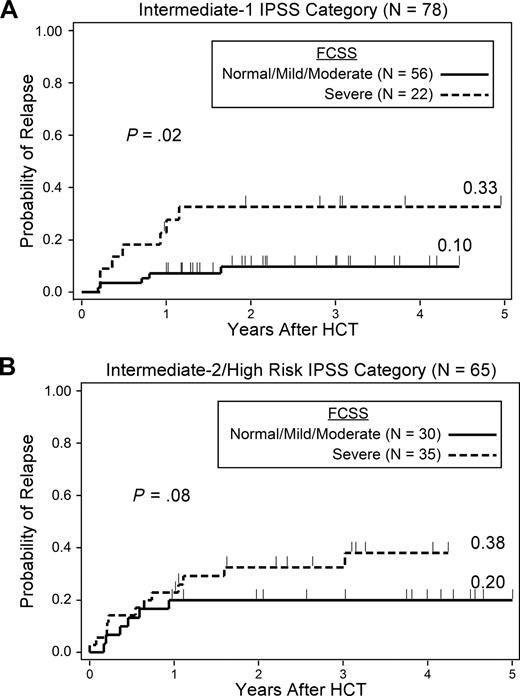
Stratified analyses were performed to evaluate whether FCSS scores provided additional predictive power within categories of IPSS and myeloblast percentage scores that would be considered low risk by currently accepted criteria (Table 5). There were insufficient numbers of patients with low-risk disease by IPSS to evaluate the impact of FCSS scores among these patients. Among patients with intermediate-1 risk disease, a severe FCSS score was associated with an increased hazard of relapse (HR = 3.8, 95% CI, 1.2-12.0, P = .02) compared with the combined group of normal/mild/moderate FCSS subjects. The cumulative incidence of relapse at 2 years among these intermediate-1 risk disease patients was 10% and 33% for patients with normal/mild/moderate and severe FCSS, respectively (Figure 2A). Patients with intermediate-2 and high-risk disease were examined as a single group because of the small number of patients with high-risk disease. Among patients with intermediate-2 or high-risk disease, the FCSS was not associated with a significantly increased hazard of relapse, although there appeared to be a trend (HR = 2.3, 95% CI, 0.9-6.2, P = .08). The cumulative incidence of relapse at 2 years for intermediate-2 or high-risk disease patients with normal/mild/moderate versus severe FCSS was 20% and 38%, respectively (Figure 2B). In the intermediate-2 or high-risk subjects, the hazard of death or relapse was significantly worse for patients with severe FCSS (HR = 2.0, 95% CI, 1.0-3.8, P = .04) compared with patients with normal/mild/moderate FCSS. There was no significant difference in the hazard of death between subjects with normal/mild/moderate and severe FCSS in either the intermediate-1 or intermediate-2 risk groups.
Stratified analyses
| . | Death . | Relapse . | Death or relapse* . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P† . | HR (95% CI) . | P† . | HR (95% CI) . | P† . | |
| IPSS Intermediate-1 | ||||||
| Normal/mild/moderate‡ | 1 | NA | 1 | NA | 1 | NA |
| Severe | 1.3 (0.6-2.6) | .5 | 3.8 (1.2-12.0) | .02 | 1.6 (0.8-3.0) | .19 |
| IPSS Intermediate-2/high | ||||||
| Normal/mild/moderate‡ | 1 | NA | 1 | NA | 1 | NA |
| Severe | 1.7 (0.9-3.5) | .12 | 2.3 (0.9-6.2) | .08 | 2.0 (1.0-3.8) | .04 |
| Marrow myeloblast percentage < 5% | ||||||
| Normal myeloblasts‡ | 1 | NA | 1 | NA | 1 | NA |
| Abnormal myeloblasts | 1.2 (0.6-2.3) | .63 | 3.7 (1.2-11.3) | .02 | 1.4 (0.7-2.5) | .34 |
| . | Death . | Relapse . | Death or relapse* . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P† . | HR (95% CI) . | P† . | HR (95% CI) . | P† . | |
| IPSS Intermediate-1 | ||||||
| Normal/mild/moderate‡ | 1 | NA | 1 | NA | 1 | NA |
| Severe | 1.3 (0.6-2.6) | .5 | 3.8 (1.2-12.0) | .02 | 1.6 (0.8-3.0) | .19 |
| IPSS Intermediate-2/high | ||||||
| Normal/mild/moderate‡ | 1 | NA | 1 | NA | 1 | NA |
| Severe | 1.7 (0.9-3.5) | .12 | 2.3 (0.9-6.2) | .08 | 2.0 (1.0-3.8) | .04 |
| Marrow myeloblast percentage < 5% | ||||||
| Normal myeloblasts‡ | 1 | NA | 1 | NA | 1 | NA |
| Abnormal myeloblasts | 1.2 (0.6-2.3) | .63 | 3.7 (1.2-11.3) | .02 | 1.4 (0.7-2.5) | .34 |
HR indicates hazard rate ratio; CI, confidence interval; and NA, not applicable.
Inverse of disease-free survival.
Likelihood ratio test.
Referent category.
Probability of relapse by FCSS. (A) Patients with intermediate-1 risk disease. (B) Patients with intermediate-2 risk disease.
Probability of relapse by FCSS. (A) Patients with intermediate-1 risk disease. (B) Patients with intermediate-2 risk disease.
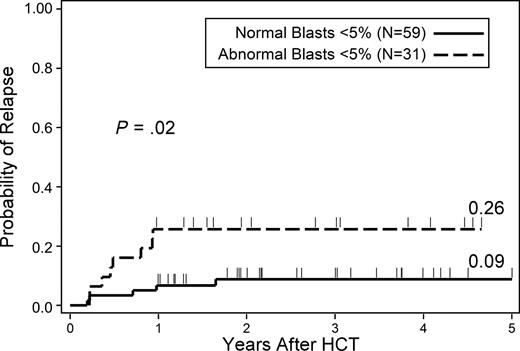
We also examined transplantation outcomes in patients with less than 5% marrow myeloblasts who did or did not have flow cytometric evidence of phenotypic abnormalities (Table 5). There was an increased hazard of relapse among patients with myeloblast dyspoiesis compared with those without myeloblast dyspoiesis (HR = 3.7, 95% CI, 1.2-11.3, P = .02). The cumulative incidence of relapse at 2 years was 9% for less than 5% normal myeloid blasts and 26% for patients with less than 5% abnormal myeloid blasts (Figure 3). There were no observed differences in survival or RFS for these groups.
Probability of relapse in patients with less than 5% marrow myeloblasts that did or did not have evidence of myeloid dyspoiesis as assessed by flow cytometry.
Probability of relapse in patients with less than 5% marrow myeloblasts that did or did not have evidence of myeloid dyspoiesis as assessed by flow cytometry.
Discussion
The primary purpose of this prospective analysis was to validate that a flow cytometry–based scoring system, FCSS, was useful as a predictor for posttransplantation outcomes in patients with MDS. We showed previously that FCSS scores were associated with the hazard of posttransplantation relapse independent of the IPSS scores.5 Since that initial publication, another center has presented data in small cohorts of MDS patients, which suggest that the FCSS is a useful predictor of disease progression in patients who are not transplanted.6 These investigators showed that the FCSS was correlated with the WPSS and transfusion dependence. However, a formal validation of the FCSS, particularly in the transplantation setting, has been lacking. Thus, we sought to examine the relevance of FCSS in a large cohort of patients (who were not included in the original dataset) to validate the initial findings.
As in our initial analysis, the present study showed a significant correlation of risk of posttransplantation relapse with pretransplantation FCSS scores, although the association between FCSS and overall survival did not reach significance. This difference may be related to the emergence of additional therapies for posttransplantation relapse, such as nonmyeloablative second HCT and hypomethylating therapy, which had not been available for the initially reported cohort. These emerging therapies may well have extended the survival of patients who relapsed after transplantation. In the initial study, the chief cause for decreased survival in patients with severe FCSS and moderate FCSS scores was the increased hazard of relapse. There was no significant difference in the hazard of relapse between the normal/mild and moderate FCSS categories. Indeed, the point estimate for hazard of relapse was insignificantly lower in the moderate FCSS compared the normal FCSS category. This finding may have represented a type II error; however, it is clear that the significant differences seen in the likelihood ratio test for relapse and RFS were driven largely by the contrast of normal/mild and moderate FCSS to severe FCSS rather than normal/mild FCSS to moderate FCSS. The data show that dyspoiesis that is detectable by flow cytometric analysis clearly results in increased risk of relapse after HCT. It is conceivable that the intrinsic changes within the myeloid cells that led to dyspoeitic changes identifiable by flow cytometric analysis also led to increased resistance to a graft-versus-leukemia effect and to increased resistance to chemotherapy. Those intrinsic changes may occur independently from peripheral cytopenias, transfusion burden, and cytogenetic alterations, and as such, would not be captured by the WPSS or the IPSS.
Similar to the previous study, the FCSS scores were associated with relapse even after adjusting for IPSS. Importantly, even among patients with less than 5% myeloid marrow blasts, myeloblast dyspoiesis was associated with an increased hazard of relapse. The agreement between IPSS and FCSS appeared to be weakest in patients with intermediate-1 or intermediate-2 risk MDS. Therefore, the application of the FCSS in patients with intermediate-1 or intermediate-2 risk disease by IPSS may be most useful in those patients who otherwise may not be offered therapy, specifically HCT at the initial time of diagnosis. In addition, it may be appropriate to offer patients with intermediate-1 risk disease with severe or moderate FCSS scores more aggressive therapy earlier in the course than would otherwise be considered. To our knowledge, no study has been conducted to evaluate the relationship between response to novel therapies and FCSS scores. Ultimately, the dyspoiesis measured by FCSS may be associated with response or lack of response to specific agents, and the FCSS may prove to be useful not only from the prognostic standpoint but may also be useful in guiding therapeutic decisions.
This validation study in a large cohort of patients with MDS confirmed our initial finding that FCSS scores are associated with posttransplantation outcomes, particularly relapse. The FCSS was associated with increased risk of relapse even after adjustment for IPSS. Furthermore, the presence of myeloid dyspoiesis was associated with relapse risk even among patients with less than 5% myeloid marrow blasts. Thus, the FCSS can be a useful prognostic tool, particularly in those patients who would not be characterized as having aggressive disease by currently applied standard criteria.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the HCT teams at the Fred Hutchinson Cancer Research Center and the Seattle VA Puget Sound Health Care System for their contributions, all patients for their participation in these trials, Joanne Greene and Franchesca Nguyen for maintaining the patient database, and Bonnie Larson and Helen Crawford for help with manuscript preparation.
This work was supported in part by the National Institutes of Health, Bethesda, MD (grants HL084054, HL36444, CA18029, CA15704, and HL82941).
National Institutes of Health
Authorship
Contribution: B.L.S. performed primary data review, provided input to the statistical analysis, and drafted the manuscript; D.A.W. performed all flow data analysis, assisted with writing the manuscript, and designed the analysis; M.R.L. verified flow data analysis and designed the analysis; D.M. reviewed all pathology specimens and performed WHO classification; W.M.L. performed all statistical analyses; and H.J.D. designed the analysis, provided input to the statistical analysis, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests. D.A.W. and M.R.L. are employees of HematoLogics, Inc.
Correspondence: Bart L. Scott, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: bscott@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal