Abstract
The liver has been generally considered an organ prone to tolerance induction and maintenance. However, whether and how the unique liver microenvironment contributes to tolerance maintenance is largely unknown. Here, we used liver fibroblastic stromal cells to mimic the liver microenvironment and found that liver stroma could induce Lin−CD117+ progenitors to differentiate into dendritic cells (DCs) with low CD11c, MHC II but high CD11b expression, high IL-10, but low IL-12 secretion. Such regulatory DCs could inhibit T-cell proliferation in vitro and in vivo, induce apoptosis of the activated T cells, and alleviate the damage of autoimmune hepatitis. Furthermore, liver stroma–derived macrophage colony-stimulating factor (M-CSF) was found to contribute to the generation of such regulatory DCs. Regulatory DC–derived PGE2 and T cell–derived IFN-gamma were responsible for the regulatory function. The natural counterpart of regulatory DCs was phenotypically and functionally identified in the liver. Importantly, Lin−CD117+ progenitors could be differentiated into regulatory DCs in the liver once transferred into the liver. Infusion with liver regulatory DCs alleviated experimental autoimmune hepatitis. Therefore, we demonstrate that the liver microenvironment is highly important to program progenitors to differentiate into regulatory DCs in situ, which contributes to the maintenance of liver tolerance.
Introduction
The liver is a unique organ in which induction of tolerance may be favored over induction of immunity. There is a great deal of experimental and clinical evidence that support such an observation. For example, administration of antigens via the portal vein was found to induce immune tolerance,1 and allogeneic liver transplantation could be established and maintained even without immunosuppression.2 In addition, pathogens, such as hepatitis B virus, can cause chronic infection in the liver even after the initiation of immune response.3,4 The phenomenon of “liver tolerance” has drawn much attention; however, the underlying mechanisms are not fully understood
Up to now, most studies on the mechanisms of liver tolerance mainly focused on the behavior of lymphocytes and antigen-presenting cells (APCs) in the liver. Natural killer (NK) and NKT cells rich in the liver can secrete chemokines to trap the activated T cells to undergo cell death in liver,5-7 which was proposed as one reason for liver tolerance. Another explanation was that some DCs in the liver secrete IL-10, which in turn induces tolerance.7-9 However, considering that such cells exist all over the body, we wonder why and how they can preferentially induce tolerance in the liver.
As a heterogeneous population of APCs, DCs play pivotal roles in the initiation of immunity and induction of immunologic tolerance depending on their maturation state and subsets.10-12 Recently, DCs with regulatory functions have attracted much attention because they can inhibit T-cell response and inflammation. On the basis of the long-term culture system of splenic stromal cells,13 we and others have demonstrated that the splenic microenvironment is important for the differentiation of regulatory DCs because hematopoietic progenitors and mature DCs can differentiate into regulatory DCs induced by splenic stroma.14-16 It would be interesting to know whether a similar mechanism exists in other organs such as liver. Specifically, we are very curious about the role of the liver microenvironment in controlling immunity and maintaining tolerance.
Liver stroma had been shown to support hematopoiesis in the embryonic development,17 and in certain disease states in adult life to sustain focal intrahepatic extramedullary hae-matopoiesis.18 The transferred bone marrow cells could localize in the liver and differentiate into mature hepatocytes,19 Kupffer cells, and hepatic satellite cells.20 On the basis of our previous studies14,16 and the observations that cells with progenitor properties are present in the liver21 and that liver stroma function to drive differentiation of many kinds of cells, we wonder whether liver stroma can drive the differentiation of progenitors to the cells, which in turn contributes to liver tolerance.
In this study, we used liver stromal cells (LSCs) to mimic the liver microenvironment and found that the liver stroma could induce Lin−CD117+ progenitors to differentiate into regulatory DCs, which could alleviate the damage of autoimmune hepatitis. Our results demonstrate that the liver microenvironment plays an important role in maintaining liver tolerance by programming generation of regulatory cells in the liver.
Methods
Mice
C57BL/6J and BALB/c mice were obtained from Joint Ventures Sipper BK Experimental Animal (Shanghai, China). DO11.10 (H-2d) mice, IFN-γ knock-out mice, and enhanced green fluorescent protein (EGFP)–transgenic mice were from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained in the specific pathogen–free facility and used at 6 to 10 weeks of age. All animal experiments were undertaken in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Secondary Military Medical University, Shanghai.
Antibodies and flow cytometry
Fluorescence-conjugated monoclonal antibodies (mAbs) and respective isotype controls for flow cytometry analysis were purchased from eBioscience (San Diego, CA), purified neutralizing antibodies (anti–macrophage colony-stimulating factor [M-CSF], anti–IL-6, anti–TGF-β1, anti–TGF-β2, anti–TGF-β3, anti-SCF, anti-VEGF, anti-HGFR, anti–B7H-1, and anti–IFN-γ) were from R&D Systems (Minneapolis, MN). Annexin V–FITC and fixation/permeabilization kits were from BD PharMingen, (San Diego, CA). For cell-surface marker or apoptosis staining, cells were incubated with fluorescence-conjugated mAbs or annexin V in labeling solution in the presence of 2.4G2 or rat sera. Fluorescence-conjugated isotype-matched irrelevant mAbs were set to establish background fluorescence. 7-AAD was used to exclude dead cells. For intracellular cytokine staining, cells were fixed, permeabilized, and incubated for 30 minutes at 4°C with fluorescence-conjugated mAbs. Flow cytometric analyses were conducted on a FACSCalibur or LSR II (BD Biosciences, San Jose, CA) and the data were analyzed with CELLQuest or FACSDiva software (BD Biosciences, San Jose, CA).
Liver stromal cell culture
The liver was resected from newborn C57BL/6 mice, cut into 1-mm squares, and cultured in RPMI 1640 medium supplemented with 20% fetal calf serum (FCS; PAA Laboratories, Pasching, Austria). The medium was half-changed every week. After several weeks, relatively fast-growing cells were harvested, and contaminating macrophages were eliminated by CD11b− selection using anti-CD11b microbeads (Miltenyi Biotec, Auburn, CA). Such cells, designated as LSCs, were expanded for following experiments. Morphology was examined under a phase-contrast microscope (Leica-DMIRB; Leica, Wetzlar, Germany), and the phenotype was examined by immunofluorescence and flow cytometry.
Differentiation of Lin−CD117+ progenitors on the monolayer of LSCs
Once LSC monolayer reached 50% confluence, culture medium was replaced with RPMI 1640 medium supplemented with 5% FCS. Bone marrow cells were enriched for Lin− with the Lineage Cell Depletion Kit (Miltenyi Biotec), which depleted cells by magnetically labeling with a cocktail of biotinylated antibodies against a panel of so-called “lineage” antigens (CD5, B220, CD11b, Gr-1, 7-4, and Ter-119) and antibiotin microbeads. Lin− bone marrow cells were subsequently used to enrich Lin−CD117+ progenitors by positive selection using CD117 microbeads (Miltenyi Biotec). The enriched Lin−CD117+ cells were seeded directly onto LSC monolayer at a density of 5 × 104 per well in 6-well plates. Except for the fact that half of the medium was removed and replaced with fresh medium every 3 days, the cells continued to be cultured for at least 7 days without passage. Finally, the differentiated cells were digested with 0.1% trypsin and 2 mM EDTA, purified with CD11c magnetic microbeads, and either analyzed or used in further experiments. In some experiments, transwell, neutralizing antibodies, stromal cell supernatant, and stromalcell–derived membrane proteins were used. Cell membrane proteins were prepared according to a method described by Varez-Silva.22
Generation of conventional DCs
Assay for phagocytic ability
The protocols were performed as described previously.14 Cells incubated with bovine serum albumin (BSA)–FITC at 4°C were used as a negative control.
Isolation and sorting of CD11clowCD11bhighIalow liver DCs
Nonparenchymal hepatic cells (NPCs) were isolated as described previously with modifications.23 The liver NPCs were recovered and further purified by density centrifugation using 30% and 70% Percoll at 1200g for 20 minutes at 4°C. The buffy layer over the 70% Percoll was aspirated, washed, and then labeled with fluorescence-conjugated anti-CD11c, anti–I-Ab, and anti-CD11b. Populations which expressed CD11clow-I-Ab-lowCD11bhi and with high forward and side scatter profiles were sorted by the Vantage SE (BD Biosciences).
Assay for antigen-specific T-cell proliferation
The in vitro and in vivo antigen-specific T-cell proliferation was measured as described previously.14,15 For in vitro assay, 105 CD4 T cells or 5 μM CFSE (Molecular Probes, Eugene, OR)–labeled CD4 T cells were cultured with 104 different magnetic positive selected or fluorescence-activated cell sorter (FACS)–sorted APCs (conventional DCs [cDCs], liver regulatory DCs [LRDCs], or liver CD11clow DCs) in the presence of 100 nM OVA323-339 peptide or cultured with antigen-loaded APCs. Antigen-loaded APCs were prepared by pulsing cDCs or LRDCs with 2 μM OVA323-339 peptide or 5 μM OVA protein at 37°C for 6 hours, then washing the APCs with cold phosphate-buffered saline (PBS) 3 times. After 5 days of culture, supernatants were collected for cytokine assay by enzyme-linked immunosorbent assay (ELISA), and cells were stained with anti-CD4–PE, 7-AAD, and annexin V–FITC. The number of CD4+ 7-AAD− cells or CD4+ annexin V+ 7-AAD− cells (apoptosis cells) in each well were counted for 56 seconds by FACS.16 In some experiments, transwell, blocking reagents, such as the indoleamine dioxygenase inhibitor 1-methyltryptophan, indomethacin (Sigma-Aldrich, St Louis, MO), and neutralizating antibodies (anti–B7-H1; anti–IL-10; anti–TGF-β1, anti–TGF-β2, anti–TGF-β3, and anti–IFN-γ) were used. For in vivo assay, 107 CD4 T cells from DO11.10 × C57BL/6 FI hybrid mice were labeled with 5 μM CFSE and then injected intraperitoneally into BALB/c × C57BL/6 FI hybrid mice. At 24 hours later, 5 × 106 APCs (cDCs or LRDCs) were pulsed with 2 μM OVA323-339 peptide or LCMV-NP309-328 peptide at 37°C for 6 hours, and then injected intraperitoneally into the mice transferred with DO11.10 × C57BL/6 FI CD4 T cells. After 4 days, single-cell suspensions prepared from hepatic draining lymph node, spleen, and liver NPCs were double-stained with APC-conjugated anti-CD4 mAb and analyzed for CFSE dilution by flow cytometry.
Transfer of Lin−CD117+ progenitors into the liver
A total of 5 × 105 Lin−CD117+ progenitors from bone marrow cells of EGFP-transgenic mice were injected through the superior mesenteric vein using a 30-gauge needle. After transfer of the progenitors, the mice peripheral blood, spleen, lymph node, bone marrow, thymus, and liver were harvested to prepare mononuclear cells, and the percentages of EGFP+ cells were assayed. Liver NPCs were gated for EGFP+ cells and further analyzed their CD11b, CD11c, and I-Ab expression in details. In some experiments, the liver was fixed by perfusing with 4% paraformaldehyde through a portal vein; frozen 20-μm thickness liver frozen sections were prepared and then observed and imaged by fluorescence microscope and phase-contrast microscope (Leica-DMIRB; Leica).
Alleviation of EAH by infusion of LRDCs
The experimental autoimmune hepatitis (EAH) model was established by immunizations with freshly prepared syngeneic liver S100 antigen in CFA.24 A total of 5 × 106 LRDCs or cDCs were injected intraperitoneally on days 0, 7, and 14. Disease severity was assessed by histology and serology taken on day 21, which accorded with the peak of disease activity. Alanine aminotransferase (ALT) in the sera and liver tissue histologic analysis were detected. Liver draining lymph node cells (2 × 106 cells/mL) were restimulated with S100 antigen or OVA (20 μg/mL) for 4 days, then IFN-γ in supernatants was assayed by ELISA. For analysis of T-cell turnover in liver, BrdU was administered to animals in drinking water (1 mg/mL) for 9 days combined with intraperitoneal injection (1 mg/mL) twice before the animals were killed; liver NPCs were prepared on day 21, then cells were stained with anti-BrdU and were CD4- or CD8-analyzed by FACS.
Statistical analysis
Data are shown as a mean plus or minus SD of 3 or more independent experiments. Statistical analysis for comparison of different groups was performed using the Student t test or analysis of variance (ANOVA) where appropriate. A P value less than .05 was considered significant.
Results
Liver stroma programs progenitor differentiation into DC-like cells
To study the effect of liver microenvironment on the differentiation of progenitor cells, we prepared LSCs with fibroblast characteristics through organotypic slice culture of neonatal liver (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Since there are varieties of NPCs in the liver, we first identified their cell type. Dil-Ac-LDL uptake experiment showed these cells were not liver endothelial cells. The characteristics of positive vimentin and desmin expression and negative cytokeratin-7 and albumin indicated that LSCs were fibroblasts (Figure S1A). The phenotype analysis declared that these cells expressed CD106 on their surface, but did not express CD105, CD54, CD80, CD86, CD40, I-Ab, or CD11b (Figure S1B), indicating that LSCs we prepared were fibroblasts, but not the liver endothelial cells, biliary epithelial cells, hepatocytes, macrophages, or DCs. These stromal cells expressed mRNA of α-smooth muscle actin (α-SMA), (α1) procollagen, MMP-2, and TlMP-1, but not that of glial fibrillary acidic protein (GFAP), further confirming that LSCs were fibroblasts (Figure S1C). Moreover, reverse transcriptase–polymerase chain reaction (RT-PCR) results showed that these cells expressed a variety of cytokines and chemokines, including MCP-1, SDF-1α, M-CSF, HGF, and SCF (Figure S1D,E), indicating these liver fibroblastic stromal cells may have effects on the progenitor differentiation.
The bone marrow–derived Lin−CD117+ progenitors were seeded onto the liver stroma. After being cocultured for 7 days, the progenitors adhered to the stromal cells, formed clusters, and proliferated and differentiated into loosely adherent cells with dendritics (Figure 1A). Transmission electron micrographs and Wright staining showed such differentiated DC-like cells had eccentric nuclei, long dendrites, and rich mitochondria in cytoplasm, similar with bone marrow–derived cDCs. However, they had rare lysosome, which is well known to be abundant in macrophages, which distinguished them from macrophages (Figure 1B). In addition, the following experiments showed these differentiated DC-like cells could inhibit T-cell proliferation and could be found in normal liver and even generated in situ from progenitors in the liver, so we called this kind of differentiated DC-like cell liver regulatory dendritic cells (LRDCs). However, different from the phenotype of cDCs, LRDCs expressed low CD11c and MHC II and high CD11b and B7-H1. Furthermore, they were negative for CD4, CD8, B220, Gr-1, and CD14 (Figure 1C), with a similar phenotype to that of immature DCs. In addition, the phagocytosis assay showed that LRDCs had more potent phagocytosis ability than cDCs (Figure 1E). All together, the results showed that LRDCs had characteristics of immature DCs. LPS is known to drive maturation of immature DCs, so we detected the phenotypic and functional changes of LRDCs stimulated with LPS. The physiological dose (1 ng/mL) and high dose (100 ng/mL) of LPS could not affect low expression of MHC class II and costimulatory molecules on LRDCs, indicating that LRDCs had a relatively stable phenotype (Figure 1D). LRDCs produced low levels of IL-12p70, but high IL-10 after LPS stimulation, which is different from LPS-induced high IL-12p70 and low IL-10 secretion in cDCs (Figure 1F). In addition, the intracellular cytokine staining confirmed this observation (Figure 1G). Accordingly, in contrast to the potent antigen-presenting ability of cDCs, LRDCs either stimulated with LPS or not showed weak ability to prime antigen-specific T-cell proliferation (Figure 1H). Interestingly, LRDCs alone did not secrete IL-2 (data not shown), but LRDCs could still induce T cells to secrete a certain level of IL-2 (Figure 1H). Collectively, these data demonstrated that the LSCs programmed progenitor cells to differentiate into a distinct subset of DCs with a unique cytokine profile and phenotype.
LSCs program Lin−CD117+ progenitor differentiation into DC-like cells. (A) Differentiation of bone marrow–derived Lin−CD117+ progenitor on the monolayer of LSCs at different times. CoolSNAP cf camera (Roper Scientific Photometrics, Tucson, AZ) mounted on a microscope (Leica-DMIRB) and Meta Imaging Series 5.0 (Molecular Devices, Downingtown, PA) were used to acquire images. Original magnification, 40× objective. (B) Morphology of DC-like cells differentiated from progenitors under liver stroma (we refer to them as LRDCs) and cDCs under phase-contrast microscope, transmission electron microscope (Philips CM120; Philips, Eindhoven, The Netherlands), and Wright staining after stimulation with or without LPS. Original magnification, 40× objective (phase-contrast microscope). Scale bars equal 2 μm (transmission electron microscope); 100× oil objective (Wright staining). (C) Phenotype of LRDCs and cDCs. Open histograms represent isotype control; numbers in histograms indicate the geometric mean fluorescence. (D) Phenotype of LRDCs matured with 0 ng/mL LPS (black line), 1 ng/mL LPS (dashed line) or 100 ng/mL LPS (long dotted line). Short dotted line represents isotype control. (E) The phagocytosis ability of LRDCs was assessed by incubating with 100 mg/mL FITC-conjugated BSA at 37°C or 4°C for 4 hours. Numbers in histogram indicate geometric mean fluorescence. (F,G) Expression of IL-10 and IL-12p70 by LRDCs stimulated with LPS was measured by ELISA (F) and intracellular cytokine staining (G). (H) Antigen-presenting ability of LRDCs. CD4 T cells from DO11.10 × C57BL/6 F1 hybrid mice cocultured with LRDCs, LPS-stimulated LRDCs (LRDC-LPS), and cDCs in the presence of OVA323-339 peptide or cocultured with OVA protein–loaded LRDCs (OVAp-LRDC), OVA323-339 peptide–loaded LRDCs (OVA-LRDC)/cDC(OVA-cDC). cDCs or LRDCs were pulsed with 2 μM OVA323-339 peptide or 5 μM OVA protein at 37°C for 6 hours, then washed with cold PBS 3 times. After 5 days, the total number of live CD4 T cells was counted by FACS, and IL-2 was analyzed by ELISA. Data represent mean plus or minus SD of triplicate wells and are representative of at least 3 independent experiments with similar results. *P < .001.
LSCs program Lin−CD117+ progenitor differentiation into DC-like cells. (A) Differentiation of bone marrow–derived Lin−CD117+ progenitor on the monolayer of LSCs at different times. CoolSNAP cf camera (Roper Scientific Photometrics, Tucson, AZ) mounted on a microscope (Leica-DMIRB) and Meta Imaging Series 5.0 (Molecular Devices, Downingtown, PA) were used to acquire images. Original magnification, 40× objective. (B) Morphology of DC-like cells differentiated from progenitors under liver stroma (we refer to them as LRDCs) and cDCs under phase-contrast microscope, transmission electron microscope (Philips CM120; Philips, Eindhoven, The Netherlands), and Wright staining after stimulation with or without LPS. Original magnification, 40× objective (phase-contrast microscope). Scale bars equal 2 μm (transmission electron microscope); 100× oil objective (Wright staining). (C) Phenotype of LRDCs and cDCs. Open histograms represent isotype control; numbers in histograms indicate the geometric mean fluorescence. (D) Phenotype of LRDCs matured with 0 ng/mL LPS (black line), 1 ng/mL LPS (dashed line) or 100 ng/mL LPS (long dotted line). Short dotted line represents isotype control. (E) The phagocytosis ability of LRDCs was assessed by incubating with 100 mg/mL FITC-conjugated BSA at 37°C or 4°C for 4 hours. Numbers in histogram indicate geometric mean fluorescence. (F,G) Expression of IL-10 and IL-12p70 by LRDCs stimulated with LPS was measured by ELISA (F) and intracellular cytokine staining (G). (H) Antigen-presenting ability of LRDCs. CD4 T cells from DO11.10 × C57BL/6 F1 hybrid mice cocultured with LRDCs, LPS-stimulated LRDCs (LRDC-LPS), and cDCs in the presence of OVA323-339 peptide or cocultured with OVA protein–loaded LRDCs (OVAp-LRDC), OVA323-339 peptide–loaded LRDCs (OVA-LRDC)/cDC(OVA-cDC). cDCs or LRDCs were pulsed with 2 μM OVA323-339 peptide or 5 μM OVA protein at 37°C for 6 hours, then washed with cold PBS 3 times. After 5 days, the total number of live CD4 T cells was counted by FACS, and IL-2 was analyzed by ELISA. Data represent mean plus or minus SD of triplicate wells and are representative of at least 3 independent experiments with similar results. *P < .001.
LRDCs inhibit conventional DC-primed CD4 T-cell proliferation
The LRDCs secreted high levels of IL-10, a key immunosuppressive cytokine secreted by many kinds of cells with regulatory functions. Thus, we wondered if LRDCs had immune regulatory function. As shown in Figure 2A, cDCs could effectively prime proliferation of OVA323-339 peptide–specific CD4+ T cells, whereas addition of LRDCs to the cDC/CD4 T coculture system resulted in the suppression of CD4 T-cell proliferation to 30%. Interestingly, just like the OVA323-339 peptide–loaded LRDCs (OVA-LRDCs), the LCMV-NP309-328 peptide–loaded LRDCs (LCMV-LRDC) also inhibited OVA323-339 peptide–loaded cDC (OVA-cDC)–induced proliferation of antigen-specific CD4 T cells. The data indicated that the inhibitory function of LRDCs was antigen nonspecific. CFSE-labeled OVA323-339 peptide–specific CD4 T cells were used as responders, and the results also demonstrated LRDCs suppressed CD4 T-cell divisions (Figure 2B). Although the proliferation of CD4 T cells was inhibited, high levels of IFN-γ and IL-2 were detected in supernatants of the LRDC/cDC/CD4 T coculture system (Figure 2C), indicating that LRDCs did not inhibit the Th1 cytokine secretion of CD4 T cells activated by cDCs. It has been shown that IFN-γ and IL-2 all can promote induction of regulatory T cells. What the biological significance of high IFN-γ and IL-2 production in the coculture system is for the immune regulation needs to be investigated in the future.
LRDCs inhibit antigen-specific CD4 T-cell proliferation both in vitro and in vivo. (A,C) CD4 T cells from DO11.10 × C57BL/6 F1 hybrid mice were cocultured with cDCs and/or LRDCs in the presence of OVA323-339 peptide, or CD4 T cells were cocultured with OVA323-339 peptide–loaded cDCs (OVA-cDC) in the presence or absence of OVA323-339 peptide–loaded LRDCs (OVA-LRDC) or control peptide LCMV-NP309-328–loaded LRDCs (LCMV-LRDC). After 5 days, the total number of live CD4 T cells was counted by FACS (A), and the cytokine release was detected by ELISA (C). *P < .001; ***P > .05. (B) CD4 T cells labeled with 5 μM CFSE were cocultured with cDCs and/or LRDCs in the presence of OVA323–339 peptide; the dilution of CFSE was then assayed by FACS. (D) CD4 T cells from DO11.10 × C57BL/6 F1 hybrid mice were labeled with 5 μM CFSE and then transferred intraperitoneally into BALB/c × C57BL/6 F1 hybrid mice; 24 hours later, antigen-loaded cDCs and/or LRDCs were also transferred into abdominal cavity. After 4 days, mononuclear suspensions from spleen, lymph nodes, and the liver were assayed for CFSE dilutions by flow cytometry. Numbers in bottom right indicate the percentages of proliferated CD4 T cells. Data are presented as mean plus or minus SD of triplicate samples. Data in panels A and C were pooled from 4 experiments. Data in panels B and D are representative of 3 independent experiments.
LRDCs inhibit antigen-specific CD4 T-cell proliferation both in vitro and in vivo. (A,C) CD4 T cells from DO11.10 × C57BL/6 F1 hybrid mice were cocultured with cDCs and/or LRDCs in the presence of OVA323-339 peptide, or CD4 T cells were cocultured with OVA323-339 peptide–loaded cDCs (OVA-cDC) in the presence or absence of OVA323-339 peptide–loaded LRDCs (OVA-LRDC) or control peptide LCMV-NP309-328–loaded LRDCs (LCMV-LRDC). After 5 days, the total number of live CD4 T cells was counted by FACS (A), and the cytokine release was detected by ELISA (C). *P < .001; ***P > .05. (B) CD4 T cells labeled with 5 μM CFSE were cocultured with cDCs and/or LRDCs in the presence of OVA323–339 peptide; the dilution of CFSE was then assayed by FACS. (D) CD4 T cells from DO11.10 × C57BL/6 F1 hybrid mice were labeled with 5 μM CFSE and then transferred intraperitoneally into BALB/c × C57BL/6 F1 hybrid mice; 24 hours later, antigen-loaded cDCs and/or LRDCs were also transferred into abdominal cavity. After 4 days, mononuclear suspensions from spleen, lymph nodes, and the liver were assayed for CFSE dilutions by flow cytometry. Numbers in bottom right indicate the percentages of proliferated CD4 T cells. Data are presented as mean plus or minus SD of triplicate samples. Data in panels A and C were pooled from 4 experiments. Data in panels B and D are representative of 3 independent experiments.
To further investigate whether LRDCs had regulatory capacity in vivo, we transferred CFSE-labeled DO11.10 × C57BL/6 F1 CD4 T cells into BALB/c × C57BL/6 F1 mice, and then intraperitoneally transferred OVA323-339 peptide–loaded cDCs together with or without peptide-loaded LRDCs. The dilution of CFSE in CD4 T cells from spleen, liver draining lymph nodes, and NPCs indicated that adoptive transfer of LRDCs also inhibited CD4 T-cell proliferation in vivo. Consistent with the results in Figure 2A, the irrelevant antigen LCMV-NP309-328 peptide–loaded LRDCscould also inhibit cDC-induced CD4 T-cell proliferation in vivo (Figure 2D), further indicating that LRDCs inhibit CD4 T-cell proliferation in an antigen-nonspecific manner. All together, these results indicated that LRDCs, generated from the progenitors after cocultured with liver stroma, could inhibit T-cell proliferation, thus exhibiting regulatory function.
Liver stroma–derived M-CSF is responsible for the generation of liver regulatory DCs
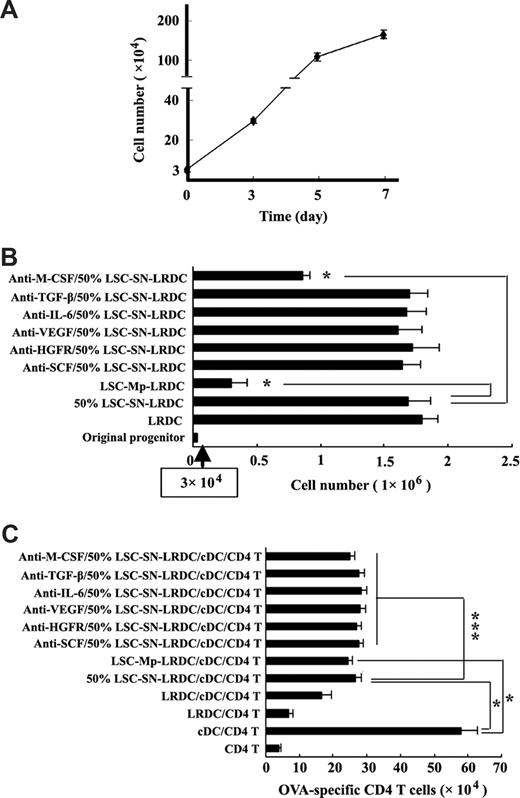
As shown in Figure 3A, liver stroma efficiently induced Lin−CD117+ progenitor proliferation and differentiation into LRDCs in vitro. To investigate the mechanisms for LRDCs differentiation under the liver stroma, we prepared the supernatants and membrane proteins of LSCs, and then investigated the induction of Lin−CD117+ progenitor differentiation by liver stroma was cell membrane protein or soluble factor dependent. As shown in Figure 3B,C, when cultured with 50% LSC supernatant, the progenitors proliferated and differentiated into regulatory cells, with a similar potency of coculture with LSCs. While cultured with LSC-derived membrane proteins, the progenitors could be differentiated into cells with regulatory functions, but the progenitors proliferated with less efficiency. These data indicated that LSCs could induce progenitor differentiation into LRDCs through both membrane molecules and soluble factors, whereas LSC-induced progenitor proliferation was mainly due to the soluble factors secreted by LSCs. To further identify which factor(s) from liver stroma involved in the proliferation and differentiation of progenitors to LRDCs, we added neutralizing antibodies to SCF, HGFR, VEGF, IL-6, TGF-β, or M-CSF, respectively, to the coculture system, and found that anti–M-CSF–neutralizing antibody could reduce progenitor proliferation efficiency to about 50%, whereas others had no effects (Figure 3B). In addition, the blockade of these cytokines, including M-CSF, had no effects on the regulatory function of LRDCs. All together, LSCs could induce differentiation of progenitors to LRDCs though both cell membrane proteins and soluble factors, and liver stroma–derived M-CSF was required for the proliferation of progenitors along differentiation of LRDCs.
Mechanisms for the differentiation of LRDCs from hematopoietic progenitors driven by liver stroma. (A) Dynamic observation of Lin−CD117+ progenitor proliferation under liver stroma. (B,C) Differentiation of progenitors to LRDCs in the presence of the precoated LSC-derived membrane proteins (LSC-Mp-LRDC) or 50% supernatant of LSCs that were harvested after culture for 24 hours (50%-LSC-SN-LRDC). In some groups, a variety of neutralizing antibodies were added into the culture system. After 7 days, the differentiated cells under different conditions were harvested and positively selected by using CD11c magnetic microbeads, cell numbers were counted (B), and the suppression of cDC-induced T-cell proliferation was assayed (C). The original number of Lin−CD117+ progenitors was 3 × 104. Data are shown as the mean plus or minus SD of triplicate samples and are representative of 4 independent experiments.*P < .001; ***P > .05 compared with 50% LSC-SN-LRDC (B), cDC/CD4 T, or 50% LSC-SN-LRDC/cDC/CD4T (C). LSC-Mp indicates LSC-derived membrane proteins; 50% LSC-SN, 50% culture supernatant of LSCs.
Mechanisms for the differentiation of LRDCs from hematopoietic progenitors driven by liver stroma. (A) Dynamic observation of Lin−CD117+ progenitor proliferation under liver stroma. (B,C) Differentiation of progenitors to LRDCs in the presence of the precoated LSC-derived membrane proteins (LSC-Mp-LRDC) or 50% supernatant of LSCs that were harvested after culture for 24 hours (50%-LSC-SN-LRDC). In some groups, a variety of neutralizing antibodies were added into the culture system. After 7 days, the differentiated cells under different conditions were harvested and positively selected by using CD11c magnetic microbeads, cell numbers were counted (B), and the suppression of cDC-induced T-cell proliferation was assayed (C). The original number of Lin−CD117+ progenitors was 3 × 104. Data are shown as the mean plus or minus SD of triplicate samples and are representative of 4 independent experiments.*P < .001; ***P > .05 compared with 50% LSC-SN-LRDC (B), cDC/CD4 T, or 50% LSC-SN-LRDC/cDC/CD4T (C). LSC-Mp indicates LSC-derived membrane proteins; 50% LSC-SN, 50% culture supernatant of LSCs.
Regulatory DC-derived PGE2 and T cell–derived IFN-γ are responsible for the regulatory function of liver regulatory DCs
To explore the mechanisms for the regulatory function of LRDCs, first we used a transwell coculture system to investigate whether the cell-cell contact was required for the suppression of T cells by LRDCs. When LRDCs were separated from CD4 T cells and cDCs by transwell inserts and cultured for 5 days, LRDCs failed to inhibit cDC-induced T-cell proliferation completely (Figure 4A,D), suggesting that cell-cell contact was required for the inhibitory functions of LRDCs. Then, we looked for the membrane molecule(s) involved in the effect. As shown in Figure 1C, LRDCs expressed high levels of B7-H1, which is reported to function as a negative regulator of T cells.25-27 However, anti–B7-H1–neutralizing antibody could not reverse the suppressive function of LRDCs (Figure 4D). The membrane molecule(s) of LSCs involved in the regulatory function of LRDCs need to be identified in the future.
Mechanisms for the inhibitory effects of LRDCs on the antigen-specific CD4 T-cell proliferation. (A-C) CD4 T cells of DO11.10 × C57BL/6 F1 hybrid mice were labeled with 5 μM CFSE and cocultured with cDCs or/and LRDCs in the presence of OVA323-339 peptide for 5 days; the divisions of CFSE (A) and intracellular staining for IFN-γ secretion (C) of CD4 T cells were then analyzed by FACS. PGE2 and IFN-γ released in the supernatant were assayed by ELISA (B). In the experiments using the transwell system, LRDCs were seeded into the upper chamber of the transwell with CD4 T cells and cDCs in the bottom of a 24-well plate. (D) A variety of neutralizing antibodies or blocking reagents were added into the coculture system, and the inhibitory functions of LRDCs were assayed. (E) Detection of T-cell apoptosis in the coculture system. (F,G) LRDCs or/and cDCs derived from IFN-γ knockout mice were used in the coculture system, CD4 T-cell proliferation was detected by FACS (F), and the level of IFN-γ in supernatant was assayed by ELISA (G). Data represent the mean plus or minus SD of triplicate wells and are representative at least from 3 independent experiments. *P < .001; **P < .01; ***P > .05.
Mechanisms for the inhibitory effects of LRDCs on the antigen-specific CD4 T-cell proliferation. (A-C) CD4 T cells of DO11.10 × C57BL/6 F1 hybrid mice were labeled with 5 μM CFSE and cocultured with cDCs or/and LRDCs in the presence of OVA323-339 peptide for 5 days; the divisions of CFSE (A) and intracellular staining for IFN-γ secretion (C) of CD4 T cells were then analyzed by FACS. PGE2 and IFN-γ released in the supernatant were assayed by ELISA (B). In the experiments using the transwell system, LRDCs were seeded into the upper chamber of the transwell with CD4 T cells and cDCs in the bottom of a 24-well plate. (D) A variety of neutralizing antibodies or blocking reagents were added into the coculture system, and the inhibitory functions of LRDCs were assayed. (E) Detection of T-cell apoptosis in the coculture system. (F,G) LRDCs or/and cDCs derived from IFN-γ knockout mice were used in the coculture system, CD4 T-cell proliferation was detected by FACS (F), and the level of IFN-γ in supernatant was assayed by ELISA (G). Data represent the mean plus or minus SD of triplicate wells and are representative at least from 3 independent experiments. *P < .001; **P < .01; ***P > .05.
Next, we wondered if cell contact–induced cytokine secretion contributed to the regulatory function of LRDCs. So, we screened the factors in the coculture system of LRDC/cDC/CD4 T cells, and found that the concentrations of PGE2 (Figure 4B) and IFN-γ (Figure 4B,C) were much higher than controls. Since PGE2 and IFN-γ are reported to be involved in the immunosuppression and tolerance induction,28-30 we blocked PGE2 and IFN-γ in the coculture system, and found that suppression of T-cell proliferation by LRDCs could be partially reversed, but blockade of IDO, IL-10, and TGF-β had no effects (Figure 4D). Similar results were also observed in the experiments using CFSE-labeled CD4 T cells (data not shown). These data demonstrated that PGE2 and IFN-γ are responsible for the regulatory function of LRDCs.
Recent studies provided supporting evidence for the role of IFN-γ in apoptosis of CD4 T cells during infectious diseases and tolerance induction.31-33 We then wondered if LRDCs could also induce apoptosis of CD4 T cells in addition to their inhibition of CD4 T-cell proliferation. As shown in Figure 4E, LRDCs could induce more CD4 T cells to apoptosis in the LRDC/cDC/CD4 T coculture system. Furthermore, anti–IFN-γ–neutralizing antibody could decrease the apoptosis induction, indicating that IFN-γ acted as the inducer of T-cell apoptosis and inhibitor of T-cell proliferation in the coculture system. As IFN-γ can be produced by DCs and T cells, we wonder which kind of cells secrete the high level of IFN-γ in the coculture system. We prepared cDCs and LRDCs from IFN-γ knockout mice, and checked whether IFN-γ secreted by cDCs or/and LRDCs was responsible for the suppressive effect on the proliferation of CD4 T cells. We found that the inhibition of T-cell proliferation remained almost unchanged in the coculture system when cDCs or/and LRDCs derived from IFN-γ knockout mice were used, thus indicating that the high level of IFN-γ in the LRDC/cDC/CD4 T coculture system was from the activated CD4 T cells, and CD4 T cell–derived IFN-γ in turn contributed to the induction of T-cell apoptosis and inhibition of T proliferation in an autocrine or paracrine manner (Figure 4F,G).
Identification of the natural counterpart of LRDCs in the liver
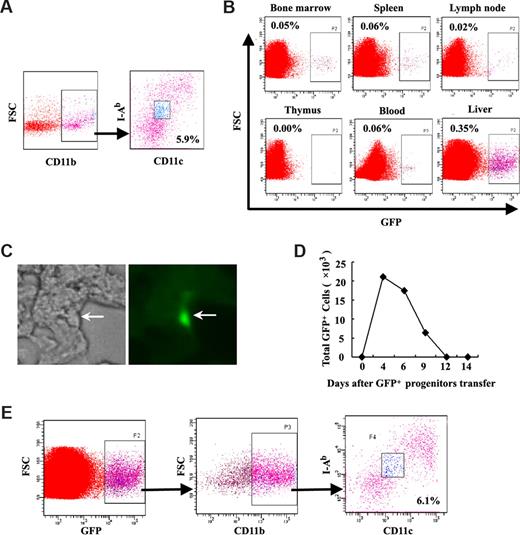
To investigate whether the natural counterpart of LRDCs exists in vivo, we analyzed liver NPCs of C57BL/6 mice on the basis of the phenotype of LRDCs. About 5.9% of CD11bhi liver NPCs were CD11clowI-Ab-low cells (Figure 5A). Further study showed that the freshly sorted CD11clowI-Ab-lowCD11bhi cells (marked as CD11clowDCs in Figure S2) had similar functional characteristics with LRDCs, indicating that the sorted CD11clowI-Ab-lowCD11bhi cells from liver NPCs were the natural counterpart of LRDCs in the liver. To find whether they have similar functions as LRDCs, we sorted these CD11bhiCD11clowI-Ab-low cells from liver NPCs, and found that these cells secreted high IL-10 and low IL-12 stimulated with or without LPS, with similar cytokine profiles of LRDCs (Figure S2A). Furthermore, we found that the sorted CD11bhiCD11clowI-Ab-low cells (CD11clowDCs) could inhibit CD4 T-cell proliferation (Figure S2B,C). Moreover, the sorted CD11bhiCD11clowI-Ab-low cells could induce apoptosis of the activated T cells (Figure S2D). In addition, the populations of CD11bhi CD11chi I-Ab-hi cells and CD11bhi CD11cnegI-Ab-neg cells were sorted; however, these populations could not inhibit mature DC–primed CD4 T-cell proliferation (data not shown). Altogether, the sorted CD11bhiCD11clowI-Ab-low cells from liver NPCs in vivo had similar functional characteristics as the LRDCs we identified in vitro, and could be the in vivo natural counterpart of LRDCs.
Characteristics of the natural counterpart to LRDCs in the liver. (A) Liver NPCs were labeled with fluorescence-conjugated CD11c, I-Ab, and CD11b, and the CD11clowI-Ab-lowCD11bhi cells were sorted by FACSDiva. (B) Bone marrow–derived EGFP+Lin−CD117+ progenitors were transferred into the liver through mesentery vein injection. After 6 days, single-cell suspensions from different organs of recipient mice were prepared and analyzed for the percentage of EGFP+ cells. (C) EGFP+ cells with dendrites in the liver after EGFP+ progenitors transfer were observed under microscope (Leica-DMIRB). Original magnification, 40× objective. (D) Kinetics of EGFP+ cell proliferation in the liver after EGFP+Lin−CD117+ progenitors were transferred into the liver. After EGFP+ progenitors were transferred, the liver NPC suspensions were prepared on different days and analyzed for the sum of EGFP+ cells. (E) At 6 days after progenitors were transferred, the liver NPC suspensions were prepared and gated EGFP+ cells were analyzed for the percentage of CD11bhiCD11clowI-Alow cells by flow cytometry. Data are representative of at least 3 independent experiments.
Characteristics of the natural counterpart to LRDCs in the liver. (A) Liver NPCs were labeled with fluorescence-conjugated CD11c, I-Ab, and CD11b, and the CD11clowI-Ab-lowCD11bhi cells were sorted by FACSDiva. (B) Bone marrow–derived EGFP+Lin−CD117+ progenitors were transferred into the liver through mesentery vein injection. After 6 days, single-cell suspensions from different organs of recipient mice were prepared and analyzed for the percentage of EGFP+ cells. (C) EGFP+ cells with dendrites in the liver after EGFP+ progenitors transfer were observed under microscope (Leica-DMIRB). Original magnification, 40× objective. (D) Kinetics of EGFP+ cell proliferation in the liver after EGFP+Lin−CD117+ progenitors were transferred into the liver. After EGFP+ progenitors were transferred, the liver NPC suspensions were prepared on different days and analyzed for the sum of EGFP+ cells. (E) At 6 days after progenitors were transferred, the liver NPC suspensions were prepared and gated EGFP+ cells were analyzed for the percentage of CD11bhiCD11clowI-Alow cells by flow cytometry. Data are representative of at least 3 independent experiments.
In situ generation of LRDCs from the progenitors transferred into liver
To find whether the natural counterpart of LRDCs in the liver are differentiated from the hematopoietic progenitors in liver, we prepared Lin−CD117+ hematopoietic progenitors from EGFP+ mice and then transferred the progenitors into the liver of C57BL/6 mice. Then, the liver NPCs were assayed at various time intervals. At 6 days after transfer, the percentage of EGFP+ cells in the liver NPCs was 0.35%, much higher than that of single-cell suspensions from other organs, indicating that the transferred EGFP+Lin−CD117+ cells were mainly detained and might differentiate in the liver (Figure 5B). We also observed EGFP+ cells with dendrites in the liver of recipient mice by fluorescent microscopy, suggesting that progenitors might differentiate into DC-like cells in situ (Figure 5C). We dynamically analyzed the differentiation of EGFP+Lin−CD117+ cells in the liver, and found that the transferred EGFP+ cells reached its peak on day 4, and persisted for about 2 weeks in the liver. Moreover, we found that transferred progenitors could differentiate into various cells, among which 6.1% of the liver NPCs were CD11clowI-Ab-lowCD11bhi (Figure 5E). These results demonstrated that the hepatic microenvironment could program progenitor differentiation into LRDCs in the liver in situ.
Suppression of experimental autoimmune hepatitis by infusion with LRDCs
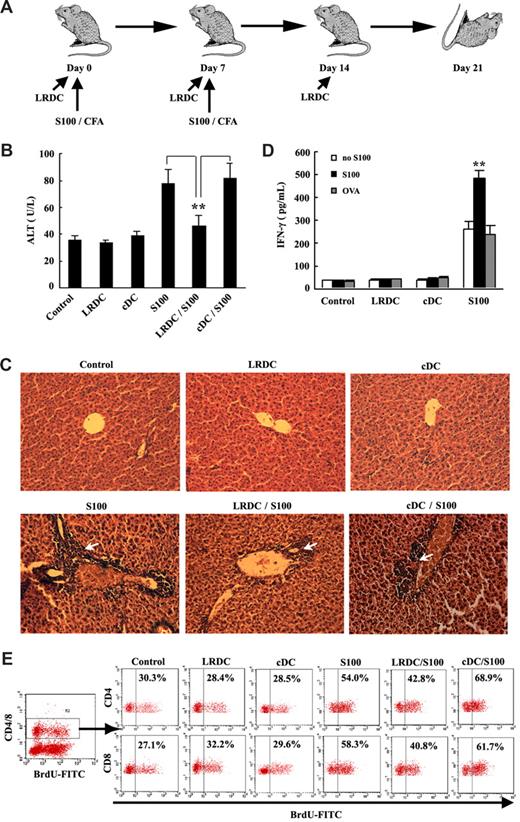
The liver tolerance might be resulted from the unique immune constitution in the liver. Loss of tolerance or overactivation of immune response may lead to autoimmune diseases.34 To prove the contribution of LRDCs to liver tolerance in vivo, we intraperitoneally transferred LRDCs into the mice to observe whether they had the preventive effect on EAH (Figure 6A). After the repeated immunizations with S100 antigen, symptoms of EAH appeared, such as bloody ascetic fluid, increase of serum ALT (Figure 6B), and chronic portal inflammation (Figure 6C). Compared with controls, the mice infused with LRDCs showed the lower level of ALT and the marked attenuation of portal inflammatory infiltrations. To find out the possible mechanisms for the attenuation of EAH by the transfer of LRDCs, we first detected whether there were S100-specific T cells primed in the S100-immunized mice. We harvested the cells of the liver draining lymph nodes in the S100-immunized mice and restimulated them with S100 antigen or irrelevant antigen OVA protein in vitro for 4 days. We found that stimulation with S100 antigen up-regulated IFN-γ production, while stimulation of OVA antigen failed, showing that S100-specific T cells were primed in the liver draining lymph nodes (Figure 6D). To find out if the protective effect of LRDCs was due to the inhibition of S100-specific T-cell proliferation, we measured the S100-specific T-cell responses in the liver of a hepatitis model with BrdU incorporation methods. The results showed that S100 immunization increased T-cell turnover in the liver, with the percentage of BrdU+ cells increasing from 30% to 54% for CD4 T cells and from 27% to 58% for CD8 T cells. LRDCs transfer could significantly inhibit the T-cell proliferation in the liver, thus confirming our speculation (Figure 6E). Collectively, these results indicated that LRDCs could contribute to maintain the liver tolerance in vivo.
Attenuation of experimental autoimmune hepatitis by infusion with LRDCs. (A) Schematic diagram of the induction and intervention of EAH. In the control, EAH was induced by double immunizations with liver S100 antigen in CFA. LRDC infusion was accomplished intraperitoneally 3 times in weekly intervals. Livers and blood were harvested for analysis 21 days after EAH induction. (B) Mean level of serum ALT in all groups (n = 6) was assayed. (C) Representative hematoxylin-eosin (HE) histologic observations of EAH lesions in animal liver after standard EAH induction. CoolSNAP cf camera (Roper Scientific Photometrics) mounted on a microscope (Leica-DMIRB) and Meta Imaging Series 5.0 (Molecular Devices) were used to acquire images. Arrow shows the characteristic periportal infiltrates in comparison with the minimal histologic lesions observed in EAH animals protected by LRDCs. Original magnification, 20×. (D) Liver draining lymph node cells isolated from the S100-immunized mice or LRDC/cDC-transferred mice were restimulated with S100 antigen or OVA antigen in vitro; the IFN-γ levels in the supernatant were then assayed by ELISA. (E) Turnover of T cells in the liver was measured by BrdU labeling. BrdU was administrated to mice via drinking water and intraperitoneal injection, and the liver NPCs were then prepared and assayed using FITC-labeled anti-BrdU antibody by FACS. Data represent the mean plus or minus SD of triplicate samples and are representative of 2 independent experiments. **P < .01.
Attenuation of experimental autoimmune hepatitis by infusion with LRDCs. (A) Schematic diagram of the induction and intervention of EAH. In the control, EAH was induced by double immunizations with liver S100 antigen in CFA. LRDC infusion was accomplished intraperitoneally 3 times in weekly intervals. Livers and blood were harvested for analysis 21 days after EAH induction. (B) Mean level of serum ALT in all groups (n = 6) was assayed. (C) Representative hematoxylin-eosin (HE) histologic observations of EAH lesions in animal liver after standard EAH induction. CoolSNAP cf camera (Roper Scientific Photometrics) mounted on a microscope (Leica-DMIRB) and Meta Imaging Series 5.0 (Molecular Devices) were used to acquire images. Arrow shows the characteristic periportal infiltrates in comparison with the minimal histologic lesions observed in EAH animals protected by LRDCs. Original magnification, 20×. (D) Liver draining lymph node cells isolated from the S100-immunized mice or LRDC/cDC-transferred mice were restimulated with S100 antigen or OVA antigen in vitro; the IFN-γ levels in the supernatant were then assayed by ELISA. (E) Turnover of T cells in the liver was measured by BrdU labeling. BrdU was administrated to mice via drinking water and intraperitoneal injection, and the liver NPCs were then prepared and assayed using FITC-labeled anti-BrdU antibody by FACS. Data represent the mean plus or minus SD of triplicate samples and are representative of 2 independent experiments. **P < .01.
Discussion
The liver is an organ that encounters varieties of incoming foreign antigens from the intestine, so it is essential to control the immune response in the liver at the appropriate time and extend and maintain tolerance to innocent antigens. However, the underlying mechanisms for maintenance of tolerance or control of immunity in the liver are still largely unknown. In this study, we demonstrated that liver stroma could program differentiation of bone marrow–derived progenitors into regulatory DCs. Most importantly, these LRDCs were capable of inducing apoptosis of the activated T cells, which gave a new explanation for liver tolerance maintenance. Furthermore, these LRDCs could also be generated in situ from the progenitors directly transferred into the liver, and the natural counterpart had been identified in the liver. Infusion with LRDCs also suppressed experimental autoimmune hepatitis. Together, these data suggest that the liver microenvironment is important to regulate the immune responses in the liver, providing a new way to understand the mechanisms for maintenance of liver tolerance.
As the major stromal cells in the liver, fibroblasts are located around the central vein and in the portal area. In some conditions, such as liver inflammation or liver injury, fibroblasts in the portal area proliferate, and hepatic satellite cells, primarily located in the parenchyma in normal mice, also can be activated and transformed into myofibroblast-like cells,35 suggesting that fibroblasts, as mesenchyma in the liver, contribute greatly to the functions of liver microenvironment. The LSCs we prepared had fibroblastic characteristics, so we used them to mimic the liver microenvironment.
LSCs secreted many kinds of cytokines and chemokines, including GM-CSF, M-CSF, and HGF. It has been shown that M-CSF and HGF can drive progenitor differentiation into DCs or regulatory DCs in vitro,36,37 which gives us clues to investigate whether LSCs can program progenitor differentiation into regulatory DCs. M-CSF is generally considered an important growth factor for development of monocyte/macrophage lineage.36,38 However, our results showed that blockade of M-CSF could decrease the number of the differentiated LRDCs, but did not change their inhibitory functions, indicating that M-CSF, although not responsible for the progenitor differentiation, was very important for the proliferation of progenitors and generation of LRDCs. Consistently, generation of DCs was found to be reduced by more than 85% in M-CSF–deficient osteopetrotic mice.39
Up to now, several kinds of regulatory DCs had been reported, such as CD11clowI-AlowCD11bhi splenic regulatory DCs and14,16 CD11clowCD45RB+DCs.15,40 Compared with these previously reported regulatory DCs, LRDCs shared common characteristics with them, including immature DC phenotype, high IL-10 and low IL-12p70 secretion, and inhibition of T-cell proliferation. But the mechanisms of LRDCs to inhibit T-cell proliferation were quite different. CD11clowCD45RB+DCs down-regulated T-cell response mainly by inducing regulatory T cells, and CD11clowI-AlowCD11bhi splenic regulatory DCs inhibited T-cell proliferation via nitric oxide; other regulatory DCs also exerted their regulatory function through IL-10. However, in addition to the involvement of PGE2 derived from LRDCs in the inhibition of T-cell proliferation, which outlined a new way for the mechanistic explanation for regulatory DCs, LRDCs could also induce the apoptosis of the activated T cells. This distinct characteristic of LRDCs might reflect the unique microenvironments in liver, which acts as the grave of the activated T cells.41,42 Thus, the fact that LRDCs induce apoptosis of the activated T cells might be an inherent characteristic for one of mechanisms for immune negative regulation in the liver.
Increasing evidences indicate that IFN-γ is required for the induction of tolerance for liver allografts in vivo by inhibiting activation and proliferation of T cells30,43 and also inducing caspase-8–dependent apoptosis of T cells.44 It is also reported that only naive CD4 T cells and Th2 cells express both types of IFN-γ receptor chains (IFN-γR1 and IFN-γR2), while expression of the IFN-γR2 is lost in Th1 cells.45,46 So, in our experiments, CD4 T cell–derived IFN-γ was likely to mediate the death of naive CD4 T cells and Th2 cells via paracrine manner, or induce the death of Th1 cells indirectly by inducing factors from LRDCs or cDCs such as nitric oxide, just as in a recent report.47 Moreover, PGE2 has been shown by us to be involved in the inhibitory functions of LRDCs. As an anti-inflammatory molecule, PGE2 can up-regulate indoleamine 2,3-dioxygenase expression in DCs or enhance the production of endogenous IL-10, thus inhibiting T-cell proliferation.28,29 Thus, LRDCs might exert their regulatory functions through several pathways.
The results that identification of the LRDC's natural counterpart in the liver and the in situ differentiation of progenitors to LRDCs in the liver confirm that the liver microenvironment could program bone marrow–derived progenitor differentiation into regulatory DCs and negatively regulate immune response in the liver. In vivo infusion with LRDCs can attenuate the pathogenesis of experimental autoimmune hepatitis, suggesting that a deep understanding of the roles of the liver microenvironment in the tolerance and immune homeostasis will be helpful to design strategies to eradicate chronic liver infections, treat liver cancer, or prolong liver allograft survival.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr M. Zhang, Dr H. Tang, Ms Q. Li, Mr S. Yue, Dr Y. Yao, and Dr X. Liu for helpful discussion and Ms R. Zhang for her excellent technical assistance.
This work was supported by grants from the National Key Basic Research Program of China (2007CB512403), the National Natural Science Foundation of China (30721091, 30490240), and the Shanghai Science and Technology Foundation.
Authorship
Contribution: S.X., Z.G., X.X., H.Y., and Q.W. performed experiments; S.X. and Z.G. analyzed data and wrote the paper; and X.C. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xuetao Cao, Institute of Immunology and National Key Laboratory of Medical Immunology, Second Military Medical University, 800 Xiangyin Road, Shanghai 200433, China; e-mail: caoxt@public3.sta.net.cn.
References
Author notes
*S.X. and Z.G. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal