Abstract
MT1-MMP plays a key role in endothelial function, as underscored by the angiogenic defects found in MT1-MMP deficient mice. We have studied the molecular interactions that underlie the functional regulation of MT1-MMP. At lateral endothelial cell junctions, MT1-MMP colocalizes with tetraspanin CD151 (Tspan 24) and its associated partner α3β1 integrin. Biochemical and FRET analyses show that MT1-MMP, through its hemopexin domain, associates tightly with CD151, thus forming α3β1 integrin/CD151/MT1-MMP ternary complexes. siRNA knockdown of HUVEC CD151 expression enhanced MT1-MMP-mediated activation of MMP2, and the same activation was seen in ex vivo lung endothelial cells isolated from CD151-deficient mice. However, analysis of collagen degradation in these experimental models revealed a diminished MT1-MMP enzymatic activity in confined areas around the cell periphery. CD151 knockdown affected both MT1-MMP subcellular localization and its inclusion into detergent-resistant membrane domains, and prevented biochemical association of the metalloproteinase with the integrin α3β1. These data provide evidence for a novel regulatory role of tetraspanin microdomains on the collagenolytic activity of MT1-MMP and indicate that CD151 is a key regulator of MT1-MMP in endothelial homeostasis.
Introduction
MT1-MMP is an important proteinase, with both direct actions on target substrates and indirect actions through its activation of a metalloproteinase cascade involving matrix metalloproteinase 2 (MMP2). Fibroblasts and tumor cells derived from MT1-MMP-deficient mice show impaired collagenolytic activity.1 MT1-MMP is essential for bone maturation and lung development, but MT1-MMP function in other processes seems to be compensated in knockout mice by other metalloproteinases.2 Supporting this, the phenotype of MMP2/MT1-MMP double-knockout mice is more severe, and these mice die in the first hours after birth.3
MT1-MMP gene expression is regulated by signaling via βcatenin/LEF4, Egr, NFAT, and Rac. Maturation by furin cleavage of the prodomain in MT1-MMP takes place intracellularly, so that the metalloproteinase is already mature when it reaches the plasma membrane. Hence, most regulation of MT1-MMP occurs at the plasma membrane.4,5 MT1-MMP is inhibited by tissue inhibitors of matrix proteinases 2-4 (TIMPs 2-4), although TIMP2 is also necessary for the appropriate activation of MMP2 by MT1-MMP and forms a ternary complex with the 2 proteases. Other secreted proteins such as testican have also been shown to inhibit MT1-MMP activity. The membrane glycoprotein reversion-inducing cysteine-rich protein with Kazal motifs (RECK) is an inhibitor of MT1-MMP, MMP2, and MMP9.6 In addition, MT1-MMP activity is self-regulated by its autocatalytic processing, which removes the catalytic domain and may act as a negative regulatory mechanism.7 Finally, MT1-MMP activity and turnover are regulated by hemopexin-dependent oligomerization.8
Intracellular trafficking of MT1-MMP is necessary for its participation in cell migration and is thought to replenish the plasma membrane with active new MT1-MMP molecules via a Rab8-dependent exocytic pathway.9 Thus, cells expressing MT1-MMP mutants, which fail to internalize, show enhanced MMP2 activation but a reduced invasive capability.6 Internalization of MT1-MMP occurs both via the clathrin and the caveolae pathways,10,11 and MT1-MMP internalization is regulated by its inclusion in lipid rafts.12
Cell membrane tetraspanin-based microdomains are generated by lateral association of tetraspanin proteins with several other transmembrane proteins, including integrins and immunoglobulin (Ig) superfamily members.13 Tetraspanins are key regulators of the functions and signaling activities of their associated partners in diverse cellular processes. Tetraspanin interactions have been reported for several proteases. For example, tetraspanin CD151 binds soluble pro-MMP7, facilitating its maturation.14 However, only MT1-MMP15 and some ADAM (A Disintegrin and Metalloproteinase) proteins16,17 have been shown to be included in tetraspanin microdomains. Human umbilical vein endothelial cells (HUVECs) express a repertoire of tetraspanins, including CD9, CD81, and CD151, which are localized mainly at cell-cell junctions in association with α3β1 integrin.18 The tight stoichiometry and affinity of the interaction of CD151 with α3 integrin suggest important roles for these complexes in key endothelial functions, such as polarity, motility, and angiogenesis.18 Inherited mutation of CD151 in humans causes severe renal and skin disorders.19 In contrast, CD151-deficient mice have a milder phenotype, with defects in platelet aggregation and keratinocyte migration20 ; however, renal defects21 and angiogenesis impairment22 have recently been reported in these mice. In this report, we address the functional relationship between tetraspanins and the metalloproteinase MT1-MMP in primary endothelial cells.
Methods
Cells and cell cultures
HUVECs were obtained and cultured as previously described.18 Cells were used at third passage in all assays. Before functional assays, cells were cultured overnight in human endothelial serum-free medium (Invitrogen, Carlsbad, CA) and seeded onto dishes coated with 10 μg/mL of collagen I. Mouse lung endothelial cells (MLECs) were isolated from wild-type (WT) and CD151-null mice as previously described.23 Institutional Review Board approval for these studies was obtained from the National Center of Cardiovascular Research.
Antibodies
The following monoclonal antibodies (mAbs) have been described previously18,24 : anti–human CD151 (LIA1/1), anti-CD9 (VJ1/20), anti-β1 integrin (TS2/16), anti-α3 integrin (VJ1/6), anti–MT1-MMP (LEM-2/15 and LEM-2/63), and anti–transferrin receptor (FG2/12), and anti-CD59 (VJ1/12). Polyclonal anti–mouse CD151 (140190) was as described.21 The anti–human-CD151 mAbs 8C3 and 11B1 were kindly provided by Dr K. Sekiguchi (Osaka University, Osaka, Japan) and Dr L. K. Ashman (University of Newcastle, Newcastle, Australia), respectively. Polyclonal anti–MT1-MMP Ab2 and Ab325 were donated by Dr J. Keski-Oja (University of Helsinki, Helsinki, Finland). Polyclonal anti-α3 integrin was provided by Dr R. O. Hynes (Howard Hughes Medical Institute, Chevy Chase, MD). Anti-FLAG M2 mAb, anti-vimentin mAb, and anti-caveolin polyclonal antibody were purchased from Sigma-Aldrich (St Louis, MO).
Flow cytometry, immunofluorescence, and confocal microscopy
For flow cytometry assays, HUVECs were detached with cell dissociation buffer (Invitrogen), stained, and analyzed as described.18 For immunofluorescence experiments, cells were fixed in 2% paraformaldehyde. Staining was performed as previously described18 using Alexa Fluor 488 goat antimouse IgG (H + L) conjugate and Rhodamine Red-X-Affinipure streptavidin as fluorescent reagents (Invitrogen). Samples (Figures 4B, 5A,B,D, and 6A) were viewed under an Axioscop-40 fluorescence microscope (Carl Zeiss, Jena, Germany) equipped with a 63×/1.4 NA oil immersion objective. Images were acquired with an Axiocam HRM digital camera and Axiovision software, release 4.2 (all Carl Zeiss). Alternatively, samples (Figures 1A and 2A) were analyzed on a Zeiss DMI6000 inverted epifluorescence microscope fitted with an HC × PLAPO 63/1.4-0′6 oil objective and a Leica TCS-SP5 confocal laser scanning unit equipped with Ar and He/Ne laser beams (Leica Microsystems, Wetzlar, Germany) and controlled by Leica confocal software (LASAF, version 1.8). Cytofluorograms were calculated using Leica Confocal Software and analyzed the colocalization spots on the image stack by depicting the fluorescence intensity of each pixel in both detection channels, so that colocalizing elements will appear in the diagonal of the diagram.26
To measure fluorescence resonance energy transfer (FRET) between YFP-tagged and CFP-tagged receptor pairs, the constructs were transiently expressed in HUVECs or HeLa cells, and FRET efficiency examined by the acceptor photobleaching method. CFP and YFP emission signals were collected before and after YFP photobleaching using the 458-nm or 514-nm laser lines, respectively. For each pixel, FRETeff was calculated, using Leica Confocal Software (Leica), from the increase in donor fluorescence. CFP- and YFP-MT1-MMP have been previously described,9 YFP-CD151 and CFP-α3 integrin were provided by Dr K. Sekiguchi.27
Coimmunoprecipitation assays
Coimmunoprecipitation experiments were performed as previously described.18,28 HUVEC lysates were obtained with Tris-buffered saline containing 1 mM Ca2+, 1 mM Mg2+, protease inhibitors, and 1 of 1% Brij96, 1% digitonin, or 1% Triton X-100. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was run under nonreducing conditions to avoid overlapping of the immunoprecipitating antibody with CD9 and MT1-MMP bands. For vascular endothelial (VE)–cadherin immunodetection, a parallel gel under reducing conditions was performed.
HeLa cells were transiently transfected by the calcium phosphate method with FLAG-tagged WT and mutant MT1-MMP constructs,29 kindly provided by Dr Seiki (University of Tokyo, Tokyo, Japan). Expression of the mutants was analyzed 48 hours after transfection by flow cytometry with anti-FLAG mAb; the efficiency of transfection ranged from 40% to 95% of FLAG-positive cells. HeLa cell monolayers were lysed with 1% Brij96 in Tris-buffered saline containing 1 mM Ca2+, 1 mM Mg2+, and protease inhibitors, and cell lysates were immunoprecipitated as described.18
Zymography assays
HUVEC or MLEC culture supernatants or total cell lysates were resolved under nonreducing conditions in 10% sodium dodecyl sulfate–polyacrylamide gels containing 0.1% gelatin or 1 mg/mL fibrinogen (Calbiochem, San Diego, CA), and processed as described.24
Small interference RNA assay
To knock down the expression of endothelial tetraspanins, RNA duplexes corresponding to the target sequences were used as described.30 The target sequences were as follows: CATGTGGCACCGTTTGCCT and CCTGCTGCGCCTGTACTTC for CD151, GAGCATCTTCGAGCAAGAA and ACCTTCACCGTGAAGTCCT for CD9. Target sequences and negative control oligonucleotide were from Eurogentec (Seraing, Belgium). Oligonucleotides were transfected into HUVECs with oligofectamine (Invitrogen). To enrich for cells with low tetraspanin expression, cells were trypsinized and negatively selected with anti-CD151 or anti-CD9 magnetic-coated beads (Dynabeads, M450 Goat anti–Mouse IgG; Dynal Biotech ASA, Oslo, Norway). Isolated cells were counted and seeded at confluence for experiments.
Sucrose density gradient fractionation
HUVECs grown to confluence in 100-mm dishes were rinsed with phosphate-buffered saline (PBS) and lysed for 20 minutes at 4°C in 250 μL of 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Triton X-100. Cell lysates were homogenized by repeated passing through a 22-gauge needle. The volume of extracts was made up to 1 mL (40% sucrose) and placed at the bottom of a 3-mL 5% to 30% linear sucrose gradient. Gradients were centrifuged for 18 hours at 39 000g at 4°C in a Beckman SW41 rotor. Sequential 300-μL fractions were harvested from the top of the tube.
Collagen degradation assays
Collagen I degradation was assayed with MLECs derived from WT, MT1-MMP-, or CD151-deficient mice. Cells were seeded onto 1 mg/mL rat-tail Collagen I (Roche Pharmaceuticals, Basel, Switzerland) for 24 hours. After fixing cells in 2% paraformaldehyde, collagen I was immunostained with anti-collagen I mAb (Sigma-Aldrich), and samples were viewed under an Axioscop-40 fluorescence microscope (Carl Zeiss, Jena, Germany). Dark areas around ECs, corresponding to collagen I degradation, were quantified by a thresholding-binarization method using ImageJ software.
For DQ-collagen degradation experiments, glass coverslips were coated at 10 μg/mL with DQ-collagen (Invitrogen). HUVECs or MLECs were plated in human endothelial serum-free medium and fixed 24 hours later. DQ collagen is heavily labeled with fluorescein so that fluorescence is quenched until proteolytically cleaved. The increase in fluorescence on digestion is proportional to the proteolytic activity and proteolysis of this matrix can be visualized by fluorescence microscopy as an increment in the fluorescent signal.
Results
MT1-MMP physically interacts with tetraspanin CD151 in HUVECs
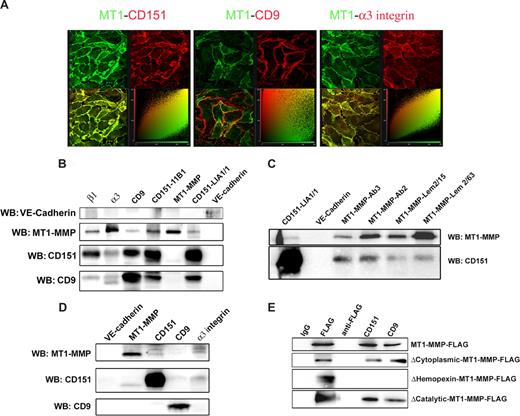
In confluent HUVECs grown on a β1-integrin–dependent substrate, MT1-MMP localizes to cell-cell junctions.31 We addressed the possible association of MT1-MMP with tetraspanin/α3 integrin complexes. Double staining for MT1-MMP and CD151 in HUVECs cultured on collagen I showed clear colocalization at lateral junctions (Figure 1A). MT1-MMP also colocalized with tetraspanin-associated integrin α3. Double staining for MT1-MMP and CD9, another endothelial tetraspanin that is highly expressed, showed only partial colocalization (Figure 1A, see cytofluorogram).
CD151 forms ternary complexes with MT1-MMP and α3β1 integrin at intercellular contacts between primary human endothelial cells. (A) HUVECs seeded at confluence on collagen I–coated coverslips were double-stained for MT1-MMP and 1 of tetraspanin CD151, tetraspanin CD9, or the tetraspanin-associated integrin α3. Samples were analyzed by confocal microscopy. The maximal projection is shown together with the cytofluorogram, in which colocalization is depicted as pixel accumulation along the central diagonal. (B) HUVEC monolayers were lysed in buffer containing 1% Brij96. Samples were immunoprecipitated with the following mAbs: anti–VE-cadherin (TEA1/31), anti–MT1-MMP (LEM-2/63), anti-CD151 (11B1 and LIA1/1), anti-CD9 (VJ1/20), anti-α3 integrin (VJ1/6), and anti-β1 integrin (TS2/16). Immunoprecipitates were analyzed by Western blot against VE-cadherin, MT1-MMP, CD151, and CD9. (C) HUVEC monolayers were lysed in 1% Brij96-containing lysis buffer. Samples were immunoprecipitated with mAbs anti–VE-cadherin (TEA1/31), anti–MT1-MMP (LEM-2/63 and LEM-2/15) and anti-CD151 (LIA1/1), and with anti–MT1-MMP pAbs (Ab2 and Ab3). Immunoprecipitates were analyzed by Western blot against MT1-MMP and CD151. (D) HUVEC monolayers were lysed in buffer containing 1% digitonin. Samples were immunoprecipitated with mAbs anti–VE-cadherin (TEA1/31), anti–MT1-MMP (LEM-2/63), anti-CD151 (11B1), anti-CD9 (VJ1/20), and anti-α3 integrin (VJ1/6). Immunoprecipitates were analyzed by Western blot against MT1-MMP, CD151, and CD9. (E) HeLa cells transiently transfected with different FLAG-tagged constructs for MT1-MMP (full-length and deletion mutants lacking the cytoplasmic, hemopexin, or catalytic domains) were lysed in 1% Brij96-containing buffer and immunoprecipitated with anti-FLAG mAb and antitetraspanin antibodies against CD151 and CD9 or with negative control X63 (IgG). Anti-FLAG mAb without cellular lysates (anti-FLAG) is shown to discriminate nonspecific bands. Membranes were probed with biotinylated anti-FLAG mAb.
CD151 forms ternary complexes with MT1-MMP and α3β1 integrin at intercellular contacts between primary human endothelial cells. (A) HUVECs seeded at confluence on collagen I–coated coverslips were double-stained for MT1-MMP and 1 of tetraspanin CD151, tetraspanin CD9, or the tetraspanin-associated integrin α3. Samples were analyzed by confocal microscopy. The maximal projection is shown together with the cytofluorogram, in which colocalization is depicted as pixel accumulation along the central diagonal. (B) HUVEC monolayers were lysed in buffer containing 1% Brij96. Samples were immunoprecipitated with the following mAbs: anti–VE-cadherin (TEA1/31), anti–MT1-MMP (LEM-2/63), anti-CD151 (11B1 and LIA1/1), anti-CD9 (VJ1/20), anti-α3 integrin (VJ1/6), and anti-β1 integrin (TS2/16). Immunoprecipitates were analyzed by Western blot against VE-cadherin, MT1-MMP, CD151, and CD9. (C) HUVEC monolayers were lysed in 1% Brij96-containing lysis buffer. Samples were immunoprecipitated with mAbs anti–VE-cadherin (TEA1/31), anti–MT1-MMP (LEM-2/63 and LEM-2/15) and anti-CD151 (LIA1/1), and with anti–MT1-MMP pAbs (Ab2 and Ab3). Immunoprecipitates were analyzed by Western blot against MT1-MMP and CD151. (D) HUVEC monolayers were lysed in buffer containing 1% digitonin. Samples were immunoprecipitated with mAbs anti–VE-cadherin (TEA1/31), anti–MT1-MMP (LEM-2/63), anti-CD151 (11B1), anti-CD9 (VJ1/20), and anti-α3 integrin (VJ1/6). Immunoprecipitates were analyzed by Western blot against MT1-MMP, CD151, and CD9. (E) HeLa cells transiently transfected with different FLAG-tagged constructs for MT1-MMP (full-length and deletion mutants lacking the cytoplasmic, hemopexin, or catalytic domains) were lysed in 1% Brij96-containing buffer and immunoprecipitated with anti-FLAG mAb and antitetraspanin antibodies against CD151 and CD9 or with negative control X63 (IgG). Anti-FLAG mAb without cellular lysates (anti-FLAG) is shown to discriminate nonspecific bands. Membranes were probed with biotinylated anti-FLAG mAb.
Tetraspanin interactions are routinely detected by protein solubilization with mild detergents, of which Brij96/97 provides the highest specificity.13 To assess the association of MT1-MMP and tetraspanins, HUVECs were lysed in 1% Brij96 and immunoprecipitated for tetraspanins CD9 and CD151. In accordance with colocalization data, MT1-MMP was readily detected in immunoprecipitates of α3 integrin and CD151. Similar results were obtained with 2 independent anti-CD151 mAbs (Figure 1B). The amount of MT1-MMP recovered in CD9 immunoprecipitates varied from experiment to experiment. In reciprocal experiments, a very weak signal of CD151 form could be observed in MT1-MMP immunoprecipitates. Because the yield of CD151 coimmunoprecipitated with anti-MT1-MMP mAb was rather low, we performed the coimmunoprecipitation experiment with a battery of anti–MT1-MMP Abs, all of which were able to coimmunoprecipitate tetraspanin CD151 (Figure 1C). The anti–MT1-MMP LEM-2/63, which was the most efficient at immunoprecipitating MT1-MMP from Brij96 lysates, usually pulls down the lower molecular weight band of CD151 (Figure 1B,D). This band, which also appeared in the anti-CD151 immunoprecipitates, might correspond either to a low-glycosylated form or to a post-lysis proteolytic product in the immunoprecipitates.
To further explore the nature of MT1-MMP complexes with tetraspanins and the integrin α3β1, we performed the immunoprecipitation analysis in HUVEC lysates obtained with 1% digitonin as the lysis detergent. This detergent has been reported to disrupt tetraspanin-tetraspanin associations while leaving intact associations between tetraspanins and their direct partners.28 As expected, under these conditions the amounts of CD9 coimmunoprecipitated with CD151 and vice versa were greatly reduced. However, MT1-MMP was still detected in immunoprecipitates of CD151 and α3β1 (Figure 1D) and at very low abundance in anti-CD9 immunoprecipitates. The anti–MT1-MMP mAb LEM-2/63 also coprecipitated CD151 from digitonin extracts. These data point to the existence of a ternary MT1-MMP:CD151:α3β1-integrin complex in endothelial cells.
To map the MT1-MMP domain involved in interaction with tetraspanins, we transfected HeLa cells with constructs encoding FLAG-tagged WT or deletion-mutant MT1-MMP. Tetraspanins coimmunoprecipitated with WT MT1-MMP and with mutants lacking the catalytic domain or the cytoplasmic tail. In contrast, no association was found with a MT1-MMP mutant lacking the hemopexin domain (Figure 1E).
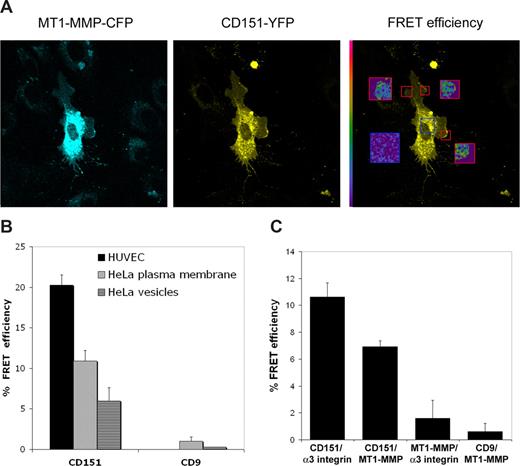
To examine tetraspanin–MT1-MMP interaction in situ, we transfected HeLa or HUVECs with fluorescent CD151-YFP and MT1-MMP-CFP constructs; these fluorescent protein tags form an appropriate pair for FRET, which will occur only if the fluorophores are within a distance of a few nanometers. This approach confirmed the association of MT1-MMP with CD151. FRET was detected both at plasma membrane contact sites with neighboring cells (Figure 2A) and in intracellular vesicles, probably of the endosomal compartment. Moreover, FRET efficiencies were clearly higher in primary HUVECs than in HeLa cells (Figure 2B). No FRET signal was obtained with CD9-YFP as acceptor molecule (Figure 2B,C).
FRET analysis of MT1-MMP/CD151 association. (A) Confocal fluorescence images showing the subcellular localization of MT1-MMP-CFP and CD151-YPF fusion proteins in primary HUVECs. Right panel shows FRET efficiency images by acceptor photobleaching of the areas depicted with a red square on the YFP image. The blue square corresponds to the background FRET efficiency in a nonbleached region. Pseudocolor scale is depicted at the left of the FRET image. (B) Chart depicts the quantification of FRET efficiency by acceptor photobleaching in HeLa and HUVECs for the pairs MT1-MMP-CFP/CD151-YFP and MT1-MMP-CFP/CD9-YFP. Data shown are mean plus or minus SE of n = 3 for HUVECs and n = 8 for HeLa. (C) Chart depicts the quantification of FRET efficiency by acceptor photobleaching in HeLa cells for the pairs α3 integrin-CFP/CD151-YFP; MT1-MMP-CFP/CD151-YFP; α3 integrin-CFP/MT1-MMP-YFP, and MT1-MMP-CFP/CD9-YFP. Data shown are mean plus or minus SE of n = 8.
FRET analysis of MT1-MMP/CD151 association. (A) Confocal fluorescence images showing the subcellular localization of MT1-MMP-CFP and CD151-YPF fusion proteins in primary HUVECs. Right panel shows FRET efficiency images by acceptor photobleaching of the areas depicted with a red square on the YFP image. The blue square corresponds to the background FRET efficiency in a nonbleached region. Pseudocolor scale is depicted at the left of the FRET image. (B) Chart depicts the quantification of FRET efficiency by acceptor photobleaching in HeLa and HUVECs for the pairs MT1-MMP-CFP/CD151-YFP and MT1-MMP-CFP/CD9-YFP. Data shown are mean plus or minus SE of n = 3 for HUVECs and n = 8 for HeLa. (C) Chart depicts the quantification of FRET efficiency by acceptor photobleaching in HeLa cells for the pairs α3 integrin-CFP/CD151-YFP; MT1-MMP-CFP/CD151-YFP; α3 integrin-CFP/MT1-MMP-YFP, and MT1-MMP-CFP/CD9-YFP. Data shown are mean plus or minus SE of n = 8.
In complementary experiments, HeLa cells were transfected with the following FRET pairs: CD151/α3β1 integrin; CD151/MT1-MMP; MT1-MMP/α3β1 integrin, and CD9/MT1-MMP. In these experiments, the highest FRET efficiency was measured with the CD151/α3β1 integrin, confirming the closeness of this association (Figure 2C). CD151/MT1-MMP showed a clearly positive but lower FRET signal, whereas CD9/MT1-MMP was indistinguishable from a negative FRET control. The MT1-MMP/α3β1 integrin pair showed an irregular behavior, with areas of positive signal interspersed with areas of background FRET levels (Figure 2C).
Reducing CD151 expression augments MT1-MMP–dependent MMP2 activation
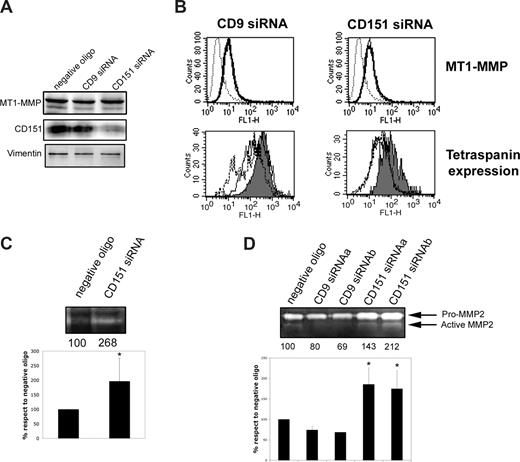
To explore the possible regulatory role of CD151 in MT1-MMP function at the plasma membrane, HUVECs were transfected with CD151-specific siRNA. The reduction in CD151 expression (55%-70%; 65% reduction in the experiment in Figure 3A) did not affect total expression of MT1-MMP or its levels at the cell surface, as measured by Western blot and flow cytometry, respectively (Figure 3A,B). Two approaches were used to explore the effect of CD151 interference on MT1-MMP activity. First, direct MT1-MMP fibrinolytic activity was assessed by fibrinogen zymography. CD151 interference increased fibrinogen degradation by confluent HUVECs (Figure 3C). Second, because MT1-MMP is the main activator of pro-MMP2 in endothelial cells, MMP2 activity was assessed by gelatin zymography. CD151 interference increased the relative and total amounts of active MMP2 (Figure 3D). This effect was confirmed with 2 independent CD151 siRNA target sequences (siRNAa and siRNAb). In contrast, CD9 knockdown with 2 independent siRNAs did not alter the prevalence of mature MMP2 (Figure 3D).
Reducing CD151 expression augments MT1-MMP–dependent MMP2 activation. (A) Western blot analysis of the expression of MT1-MMP and CD151 in total lysates of HUVECs transfected with negative oligonucleotide or with siRNA specific for CD9 or CD151. Vimentin is shown as a loading control. (B) Flow cytometric analysis of the membrane expression of MT1-MMP in HUVECs transfected with (left) CD9 or (right) CD151 siRNA (thick lines). (Toppanels) Thin lines show MT1-MMP expression in cells transfected with negative control oligonucleotide. Dotted lines correspond to the negative control X63. (Bottom panels) Flow cytometry charts of tetraspanin expression in siRNA transfected cells. Gray-filled profiles correspond to negative oligonucleotide-transfected cells; dotted lines correspond to oligo a and thin lines to oligo b, for both CD9 (left) and CD151 (right) interfered cells. (C) Fibrinogen zymography showing direct MT1-MMP protease activity in total lysates of HUVECs transfected with negative control or CD151-specific oligonucleotides. Numbers below the gel show the quantification of the fibrinolytic activity of MT1-MMP in the experiment shown. The chart shows the mean fibrinolytic activity of MT1-MMP normalized with respect to cells transfected with negative control oligonucleotide (± SD; n = 3). *P < .02 in Student t test. (D) Gelatin zymography of culture supernatants from HUVECs transfected with 2 different target siRNA sequences for CD9 or CD151. Numbers show the quantification of the gelatinolytic activity of mature MMP2 in the experiment shown. The chart shows the mean gelatinolytic activity of mature MMP2 normalized with respect to cells transfected with negative control oligonucleotide (± SD; n = 8 for CD151 siRNAa, n = 2 for CD151 siRNAb and CD9 siRNAs a and b). *P < .02 in a 1-way analysis of variance with respect to negative control oligonucleotide with Dunnett comparison test.
Reducing CD151 expression augments MT1-MMP–dependent MMP2 activation. (A) Western blot analysis of the expression of MT1-MMP and CD151 in total lysates of HUVECs transfected with negative oligonucleotide or with siRNA specific for CD9 or CD151. Vimentin is shown as a loading control. (B) Flow cytometric analysis of the membrane expression of MT1-MMP in HUVECs transfected with (left) CD9 or (right) CD151 siRNA (thick lines). (Toppanels) Thin lines show MT1-MMP expression in cells transfected with negative control oligonucleotide. Dotted lines correspond to the negative control X63. (Bottom panels) Flow cytometry charts of tetraspanin expression in siRNA transfected cells. Gray-filled profiles correspond to negative oligonucleotide-transfected cells; dotted lines correspond to oligo a and thin lines to oligo b, for both CD9 (left) and CD151 (right) interfered cells. (C) Fibrinogen zymography showing direct MT1-MMP protease activity in total lysates of HUVECs transfected with negative control or CD151-specific oligonucleotides. Numbers below the gel show the quantification of the fibrinolytic activity of MT1-MMP in the experiment shown. The chart shows the mean fibrinolytic activity of MT1-MMP normalized with respect to cells transfected with negative control oligonucleotide (± SD; n = 3). *P < .02 in Student t test. (D) Gelatin zymography of culture supernatants from HUVECs transfected with 2 different target siRNA sequences for CD9 or CD151. Numbers show the quantification of the gelatinolytic activity of mature MMP2 in the experiment shown. The chart shows the mean gelatinolytic activity of mature MMP2 normalized with respect to cells transfected with negative control oligonucleotide (± SD; n = 8 for CD151 siRNAa, n = 2 for CD151 siRNAb and CD9 siRNAs a and b). *P < .02 in a 1-way analysis of variance with respect to negative control oligonucleotide with Dunnett comparison test.
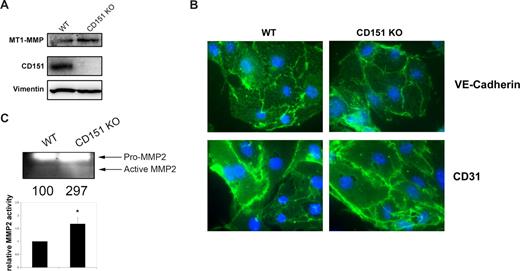
To examine MT1-MMP activity in the complete absence of CD151, we isolated MLECs from CD151-deficient mice.21 These cells expressed comparable amounts of MT1-MMP to WT MLECs (Figure 4A) and had normal subcellular localization of the endothelial markers CD31 and VE-cadherin (Figure 4B). Consistent with the siRNA experiments in HUVEC, gelatin zymography of culture supernatants from CD151−/− MLECs also revealed an increase in the active form of MMP2 (Figure 4C).
MLECs derived from CD151-deficient mice show higher MMP2 activity. (A) Western blot analysis of the expression of MT1-MMP and CD151 in total lysates of MLECs derived from WT or CD151-deficient animals (CD151 KO). Vimentin is shown as loading control. (B) Immunofluorescence staining of the endothelial specific markers VE-cadherin and CD31 in MLECs derived from WT and CD151-deficient mice. Nuclei were stained with Hoechst (blue). (C) Gelatin zymography of culture supernatants of MLECs derived from WT or CD151-deficient mice. Numbers depict the quantification of the gelatinolytic activity of mature MMP2 in the experiment shown. The chart below depicts the mean gelatinolytic activity of mature MMP2 normalized with respect to cells derived from WT mice plus or minus SE. *P < .05, Student t test.
MLECs derived from CD151-deficient mice show higher MMP2 activity. (A) Western blot analysis of the expression of MT1-MMP and CD151 in total lysates of MLECs derived from WT or CD151-deficient animals (CD151 KO). Vimentin is shown as loading control. (B) Immunofluorescence staining of the endothelial specific markers VE-cadherin and CD31 in MLECs derived from WT and CD151-deficient mice. Nuclei were stained with Hoechst (blue). (C) Gelatin zymography of culture supernatants of MLECs derived from WT or CD151-deficient mice. Numbers depict the quantification of the gelatinolytic activity of mature MMP2 in the experiment shown. The chart below depicts the mean gelatinolytic activity of mature MMP2 normalized with respect to cells derived from WT mice plus or minus SE. *P < .05, Student t test.
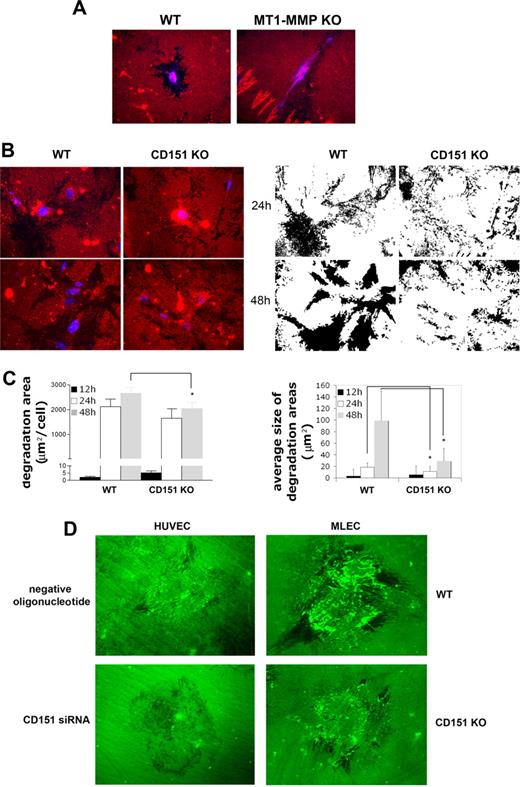
CD151 is required for a normal pattern of MT1-MMP– dependent collagenolysis
To assess MT1-MMP function in the absence of CD151, MLECs derived from WT- or CD151-deficient mice were seeded onto collagen I and the degradation of this matrix protein was monitored over time. Collagen I degradation in this assay is highly dependent on MT1-MMP activity, as demonstrated by the marked reduction observed in MLECs from MT1-MMP–deficient mice (Figure 5A). Remarkably, collagen degradation by CD151-deficient MLECs was already evident 12 hours after seeding, whereas it was very scarce in WT cells at this time, being the degraded area per cell larger in KO samples (Figure 5C). However, 24 and 48 hours after seeding, the degraded area per cell was bigger in WT samples, though the difference only reached statistical significance at 48 hours (Figure 5C). Interestingly, the pattern of degradation was very different between WT- and CD151-deficient MLEC: WT MLECs degraded the matrix homogeneously across their ventral surface, leaving a trail of degradation behind, whereas collagenolysis by CD151-deficient cells occurred in confined areas restricted to the cell periphery (Figure 5B). By 48 hours, culture plates of CD151−/− MLECs were speckled with small degradation areas that were sometimes difficult to link to an individual cell, a phenomenon that might be explained by the faster random migration observed for these cells.22 Therefore, the average size of the degraded areas was significantly smaller in CD151−/− samples at 24 and 48 hours (Figure 5C).
Abnormal collagen degradation by CD151-deficient cells. (A) Immunostaining of collagen I to show degradation by MLECs derived from WT or MT1-MMP deficient animals after 24-hour growth on 1 mg/mL rat-tail collagen I. Autofluorescence (blue) was acquired to reveal cell positions. (B) Immunostaining of collagen I to show degradation at 24 hours and 48 hours by WT or CD151 KO MLECs. Nuclei were stained with Hoechst to show the position of the cells. Monochrome images show the masks of the collagen-denuded areas (black) in the same fields. (C) Quantification of total collagen degradation area (left panel: total denuded area/number of cells; mean ± SE) or the average size of the degradation areas (right panel: mean ± SD) from WT and CD151 KO mice. A minimum of 50 cells in 20 or more 63× fields were counted in 2 independent MLEC preparations. *P < .05, Mann-Whitney t test. (D) HUVECs transfected with negative control oligonucleotide or CD151-specific siRNA (left) and MLECs derived from WT or CD151 KO mice (right) were seeded onto DQ-collagen–coated coverslips, fixed, and visualized by fluorescence microscopy.
Abnormal collagen degradation by CD151-deficient cells. (A) Immunostaining of collagen I to show degradation by MLECs derived from WT or MT1-MMP deficient animals after 24-hour growth on 1 mg/mL rat-tail collagen I. Autofluorescence (blue) was acquired to reveal cell positions. (B) Immunostaining of collagen I to show degradation at 24 hours and 48 hours by WT or CD151 KO MLECs. Nuclei were stained with Hoechst to show the position of the cells. Monochrome images show the masks of the collagen-denuded areas (black) in the same fields. (C) Quantification of total collagen degradation area (left panel: total denuded area/number of cells; mean ± SE) or the average size of the degradation areas (right panel: mean ± SD) from WT and CD151 KO mice. A minimum of 50 cells in 20 or more 63× fields were counted in 2 independent MLEC preparations. *P < .05, Mann-Whitney t test. (D) HUVECs transfected with negative control oligonucleotide or CD151-specific siRNA (left) and MLECs derived from WT or CD151 KO mice (right) were seeded onto DQ-collagen–coated coverslips, fixed, and visualized by fluorescence microscopy.
To directly visualize collagen degradation, HUVECs and MLECs were seeded onto DQ-collagen. This collagen is so heavily labeled with fluorescein that its fluorescence is quenched, and the increase in fluorescence upon digestion is proportional to proteolytic activity. Thus, the degradation of this matrix can be visualized by fluorescence microscopy as an increment in the fluorescent signal (Figure 5D). This experiment confirmed that, in the absence of CD151, cells scarcely degraded DQ-collagen. In many cases, black areas could be observed that lacked a rim of fluorescence, which might indicate that these collagen-denuded areas arise from collagen internalization or retraction without protease processing. Where DQ-positive signals were detected in cells lacking CD151, they were again restricted to the cell periphery, and no ventral collagen degradation was observed.
CD151 knockdown affects MT1-MMP subcellular localization and its association with integrin α3β1
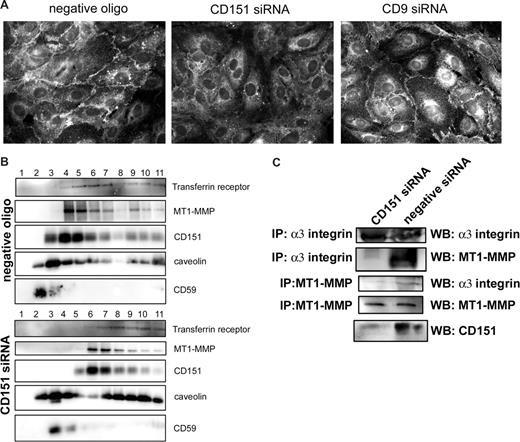
MT1-MMP activity at the plasma membrane needs to be finely regulated by a strict control of its membrane compartmentalization and subcellular localization. The pattern of collagen degradation by CD151-deficient cells suggested a dysregulation of MT1-MMP function. To examine this in more detail, we analyzed the subcellular localization of MT1-MMP in CD151-interfered HUVECs. Cells transfected with the negative control oligonucleotide showed the typical localization of MT1-MMP at lateral junctions, and this pattern was unaffected by siRNA knockdown of CD9; in contrast, the pattern of MT1-MMP staining in CD151-interfered cells was diffuse (Figure 6A).
Silencing CD151 expression in HUVECs alters MT1-MMP subcellular localization and its association to α3β1 integrin. (A) HUVECs transfected with negative control oligonucleotide or with CD9- or CD151-specific siRNA were plated at confluence onto collagen I–coated coverslips and stained with anti–MT1-MMP mAb. (B) HUVECs transfected with the negative control oligonucleotide or CD151-specific siRNA were lysed in 0.5% Triton X-100 and fractioned on a linear sucrose gradient (40%-30%-5%). Fractions were recovered sequentially from the top of the gradient and probed by Western blot with antibodies to transferrin receptor, MT1-MMP, CD151, caveolin, and CD59. (C) HUVEC monolayers, transfected with negative control oligonucleotide or with CD151-specific siRNA, were lysed in buffer containing 1% Triton X-100. Samples were immunoprecipitated with anti–MT1-MMP (LEM-2/63) and anti-α3 integrin (VJ1/6) mAbs. Immunoprecipitates were analyzed by Western blot against α3 integrin and MT1-MMP. A sample of total lysate was probed for CD151.
Silencing CD151 expression in HUVECs alters MT1-MMP subcellular localization and its association to α3β1 integrin. (A) HUVECs transfected with negative control oligonucleotide or with CD9- or CD151-specific siRNA were plated at confluence onto collagen I–coated coverslips and stained with anti–MT1-MMP mAb. (B) HUVECs transfected with the negative control oligonucleotide or CD151-specific siRNA were lysed in 0.5% Triton X-100 and fractioned on a linear sucrose gradient (40%-30%-5%). Fractions were recovered sequentially from the top of the gradient and probed by Western blot with antibodies to transferrin receptor, MT1-MMP, CD151, caveolin, and CD59. (C) HUVEC monolayers, transfected with negative control oligonucleotide or with CD151-specific siRNA, were lysed in buffer containing 1% Triton X-100. Samples were immunoprecipitated with anti–MT1-MMP (LEM-2/63) and anti-α3 integrin (VJ1/6) mAbs. Immunoprecipitates were analyzed by Western blot against α3 integrin and MT1-MMP. A sample of total lysate was probed for CD151.
The compartmentalization of MT1-MMP is largely dependent on its presence at caveolae, and tetraspanin microdomains are also partially recovered from the light (caveolae-enriched) fractions of a sucrose gradient.32 We analyzed the sucrose gradient fractionation of MT1-MMP in HUVECs transfected with CD151 siRNA. In control cells, a fraction of MT1-MMP was recovered from the light, caveolin-enriched fractions2-6 ; however, the partitioning of MT1-MMP did not follow the pattern of a typical raft marker, such as CD59, but instead overlapped with that of CD151 (Figure 6B). When CD151 expression was knocked down, the remaining CD151 and MT1-MMP were both shifted toward the bottom of the gradient (Figure 6B). Alterations in tetraspanin expression have been reported to affect the cell membrane repertoire of gangliosides,33 probably leading to a more general alteration of raft domains. Accordingly, the partitioning of caveolin was also affected, whereas CD59, a GPI-linked membrane protein, remained confined to the light fractions of the gradient (Figure 6B).
Because MT1-MMP compartmentalization at the plasma membrane is altered, and both biochemical and FRET analyses suggest that CD151 acts as a molecular linker between MT1-MMP and integrin α3β1, we analyzed the effect of silencing CD151 on the biochemical association of MT1-MMP with α3. As shown in Figure 6C, the amounts of MT1-MMP recovered in α3 immunoprecipitates, and vice versa, were severely reduced (> 50%) in HUVECs transfected with the siRNA directed against tetraspanin CD151.
Discussion
In this study, we provide evidence that inclusion in tetraspanin microdomains represents an important mechanism for regulating the enzymatic activity of MT1-MMP in endothelial cells. Our data support a model in which CD151 acts as a molecular linker between MT1-MMP and α3β1 integrin, directing MT1-MMP to endothelial lateral junctions under conditions of cell confluence and regulating its collagenolytic activity. These results thus point to CD151 as a key regulator of endothelial homeostasis.
Tetraspanin interactions have been shown to regulate cell invasion and protease expression.13,34 However, only MT1-MMP15 and some ADAM proteins16,17 have been shown to be included in tetraspanin microdomains. MT1-MMP might be regulated by different tetraspanins at different subcellular locations, such as its lysosomal degradation directed by CD6315 and its activity at the plasma membrane directed by CD151 (this report). On the other hand, CD151 has been detected in endothelial endosomes,35 a route that is also used in MT1-MMP recycling.36 FRET signal was observed between MT1-MMP and CD151 in intracellular vesicles, so it is feasible that association of MT1-MMP with CD151 is conserved through several subcellular compartments and that tetraspanin microdomains regulate the trafficking of the enzyme.
MT1-MMP activity at the plasma membrane needs to be finely regulated by a strict control of its membrane compartmentalization and subcellular localization. Our data suggest that tetraspanins are involved in both types of regulation. Knockdown of CD151 expression induces a shift of MT1-MMP partitioning on sucrose gradients toward the dense, soluble fractions. Because tetraspanins are recovered in light fractions of sucrose gradients under some conditions,32 it seems probable that MT1-MMP buoyancy will be related to its inclusion into tetraspanin-enriched microdomains rather than into classical lipid rafts. Moreover, tetraspanin microdomains are affected by palmitoylation,37 and several tetraspanin partners are palmitoylated proteins.13 Palmitoylation of MT1-MMP has been reported, and mutations of the palmitoylated residues affect the internalization of the enzyme.38
The biochemical and FRET data indicate the existence of ternary complexes in which MT1-MMP and α3β1 integrin are linked through their tight associations with CD151. These complexes are detected with different detergents, including digitonin and Triton X-100, which have been reported to disrupt tetraspanin-tetraspanin interactions. Moreover, the ability of the anti-CD151 mAbs to coimmunoprecipitate MT1-MMP correlates with their affinity for α3β1-associated CD151,27,39 FRET was readily detected with MT1-MMP/CD151 and α3β1/CD151 pairs, but was very variable when MT1-MMP was cotransfected with the α3 integrin. This heterogeneous profile would support an indirect association for this pair, in which insertion into the same tetraspanin-enriched microdomain might occasionally bring the 2 fluorescent tags close enough for FRET to occur. Finally, when CD151 was silenced, the biochemical association of MT1-MMP with the integrin was greatly reduced. This correlates with an earlier report showing that biochemical interactions of α3β1 integrin with a series of as yet undetermined molecules is lost in cells derived from CD151-deficient mice.22
It is feasible that association with tetraspanins plays an essential role in controlling the access of MT1-MMP to its substrates, both by regulating its subcellular localization and by including target proteins in the appropriate microdomains. Both CD44 and the α3 integrin chain are tetraspanin-associated proteins and are cleaved by MT1-MMP.6 MT1-MMP is also able to process laminins 5 and 10, which are ligands of CD151-associated α3β1 integrin. Furthermore, our immunoprecipitation data reveal the existence of ternary MT1-MMP:CD151:α3β1 complexes. Thus, in the absence of CD151, the functional association of MT1-MMP with α3β1 is perturbed, and collagenolysis is affected as a result. However, overall MT1-MMP proteolytic activity seems to be enhanced in cells with reduced CD151 expression, which would suggest that partition of MT1-MMP into tetraspanin microdomains might also have a negative regulatory function by impeding the shedding of other transmembrane receptors not included in these microdomains. Under quiescent conditions, inclusion of MT1-MMP into tetraspanin-enriched microdomains at lateral junctions might be important for maintaining the membrane-associated protease in a dormant state.
MT1-MMP can associate with both αvβ3 and β1 integrins, but at different cellular compartments and in different scenarios (extracellular matrix and migration-regulated).31 However, we have previously reported that CD151 does not associate with αv integrin in HUVECs.18 The data presented in the present study thus provide a molecular basis for the association with the β1 chain. Both types of complex may well coexist, with the association of MT1-MMP with α3β1 integrin being mediated by CD151 and related to processing of substrates such as collagen I, and a CD151-independent association of MT1-MMP with alternative partners such as αvβ3 integrin being preferentially involved in proMMP2 activation.
Several aspects of proteinase biology have been revealed by the phenotypes of proteinase-deficient mice. In parallel, some of the phenotypes presented by CD151-deficient mice might reflect dysregulated activity of MT1-MMP. CD151-deficient mice exhibit defects in keratinocyte migration and platelet aggregation,20 which has been shown to involve MT1-MMP–dependent MMP2 activation.40 More recent work with CD151-deficient mice has identified a defect in pathologic angiogenesis.41 Although the authors of that study claimed that metalloproteinase activity was not affected, a clear up-regulation of MMP2 could be observed, even though mature MMP2 was not properly resolved in the zymographs. We have reproduced the angiogenic defect in CD151-interfered HUVECs, and by blocking mAbs, demonstrated that this defect is related to nonfunctional MT1-MMP in these cells (M.Y.-M., unpublished results, 2006). An independent strain of CD151 KO mice displays renal defects.21 In both knockout strains, the expression of CD151-associated α3β1 integrin is not altered, although cells show defects in spreading and morphogenesis.22 All these phenotypes suggest a role for CD151 in matrix deposition or reorganization that might not be exclusively related to its association with α3β1 integrin. Accordingly, our data show that CD151 null endothelial cells were able to proteolyze collagen but only in confined areas at the cell periphery; there was no apparent matrix degradation at the ventral surface. Under physiologic conditions, the formation of ternary complexes with MT1-MMP and α3β1 integrin might allow CD151 to finely coordinate extracellular matrix-protein adhesion and degradation during migration and matrix assembly. This could explain the morphogenic defects observed in mice deficient in the expression of this tetraspanin.
In conclusion, localization to tetraspanin microdomains might represent a novel mechanism for regulating the enzymatic activity of MT1-MMP, whereby its subcellular localization and access to different substrates are controlled through the regulation of its compartmentalization at the plasma membrane.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. S. Apte and Dr K. Tryggvason for providing MT1-MMP–deficient mice, Simon Bartlett for editing the manuscript, and Dr Pilar Martin, Dr Manuel Gomez, and Dr Beatriz Gálvez for their invaluable contribution to this work.
This work was supported by the Ministerio de Educación y Ciencia (grants BFU2005-08435/BMC), Ayuda a la Investigación Básica 2002 from Juan March Foundation, and European Network (MAIN LSHG-CT-2003-502935; F.S.-M.), Contrato-Investigador FIS 0019 from Instituto de Salud Carlos III (M.Y.-M.), and the Ministerio de Educación y Ciencia (grant SAF2005-02228; A.G.A.).
Authorship
Contribution: M.Y.-M. designed and performed research, analyzed data, and wrote paper; O.B., N.S., and A.S. contributed vital new reagents; P.G., A.B., D.M., L.G., and M.S.-V. performed research; M.A.A., M.C.M., and A.G.A. analyzed data; and F.S.-M. designed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francisco Sánchez-Madrid, Servicio de Inmunología, Hospital de la Princesa, Universidad Autónoma de Madrid, C/ Diego de León 62, 28006, Madrid, Spain; e-mail: fsanchez.hlpr@salud.madrid.org.