Abstract
Infusion of epinephrine-activated human sickle erythrocytes (SS RBCs) into nude mice promotes both SS RBC and murine leukocyte adhesion to vascular endothelium in vivo. We hypothesized that interaction of epinephrine-stimulated SS RBCs with leukocytes leads to activation of leukocytes, which then adhere to endothelial cells (ECs). In exploring the underlying molecular mechanisms, we have found that coincubation in vitro of epinephrine-treated SS RBCs with human peripheral blood mononuclear cells (PBMCs) results in robust adhesion of PBMCs to ECs. Sham-treated SS RBCs had a similar but less pronounced effect, whereas neither sham- nor epinephrine-treated normal RBCs activated PBMC adhesion. PBMC activation was induced via at least 2 RBC adhesion receptors, LW and CD44. In response to SS RBCs, leukocyte CD44 and β2 integrins mediated PBMC adhesion to ECs, a process that involved endothelial E-selectin and fibronectin. SS RBCs activated adhesion of both PBMC populations, lymphocytes and monocytes. Thus, our findings reveal a novel mechanism that may contribute to the pathogenesis of vaso-occlusion in sickle cell disease, in which SS RBCs act via LW and CD44 to stimulate leukocyte adhesion to endothelium, and suggest that RBC LW and CD44 may serve as potential targets for antiadhesive therapy designed to prevent vaso-occlusion.
Introduction
In sickle cell disease (SCD), clinical and experimental observations have suggested a role for leukocytes, including polymorphonuclear neutrophils (PMNs) and monocytes, in the pathophysiology of sickle cell vaso-occlusion.1-4 Elevated leukocyte counts have been correlated with poor outcome in patients with SCD,5-7 and the clinical benefit of hydroxyurea treatment was observed to follow a reduction in the peripheral blood PMN counts, even without the anticipated increase in protective fetal hemoglobin.8
Like endothelial cells (ECs), circulating PMNs of patients with SCD display an activated phenotype, as shown by the expression of activated β2 integrins9 and cytokine-inducible CD64 (FcγRI),10 by increased release of leukocyte elastase, and by increased shedding of L-selectin and CD16 (FcγRIII).11 Leukocyte activation has also been suggested in transgenic sickle mice, in vivo, by an increased number of adherent leukocytes recruited to the endothelium compared with nonsickle control animals, after either hypoxia and reoxygenation or tumor necrosis factor–α (TNF-α) as an inflammatory stimulus.12,13 In addition, PMNs have also been shown to adhere to sickle red blood cells (SS RBCs) in static adhesion assays in vitro.14 Thus, activation of either leukocytes or endothelium, or both4,15,16 may lead to adherence of leukocytes,13,17,18 suggesting a direct participation of leukocytes in sickle cell vaso-occlusion.
We recently observed that, when the adhesive function of human SS RBCs was up-regulated with the stress hormone epinephrine,19,20 marked vaso-occlusion occurred and was associated with murine leukocyte adhesion to vascular endothelium in nude mice in vivo.20 It is well established that the SS RBC receptor LW binds not only to EC αvβ319 but also to leukocyte β1 (α4β1) and β2 integrins, including αLβ2 (LFA-1) and αMβ2 (Mac-1).21-23 We therefore hypothesized that interaction with SS RBCs could induce activation of leukocyte adhesion molecules, leading to leukocyte adherence to endothelium, and that interactions between SS RBCs and leukocytes might involve SS RBC LW. However, whereas PMN activation and adhesion have been relatively well studied in SCD, the activation of mononuclear leukocytes (lymphocytes and monocytes) has received less attention. We therefore focused on elucidating the molecular interactions between SS RBCs and mononuclear leukocytes that may lead to activation and adhesion of these cells to ECs. Our study has now identified 2 adhesion receptors expressed on SS RBCs, LW and CD44, which represent major contributors to activation of leukocyte CD44 and β2 integrins to mediate adherence of peripheral blood mononuclear cells (PBMCs) to ECs involving endothelial E-selectin and fibronectin. Adhesion of activated PBMCs to ECs included the 2 populations comprising PBMCs: lymphocytes and monocytes. Thus, whereas SS RBCs are known to adhere to endothelium in sickle cell vaso-occlusion,19,20,24 they also appear to play a major role as potent signaling cells capable of amplifying the vaso-occlusive process by activating PBMCs, which in turn adhere to endothelium.
Methods
Endothelial cells
Primary human umbilical vein endothelial cells (HUVECs) were grown as monolayers in EBM2 medium (Lonza Walkersville, Walkersville, MD) supplemented with EGM2 (Lonza Walkersville). EC passage was accomplished with trypsinization, and cells past the 5th passage were discarded.
Antibodies
Antibodies used included the following monoclonal antibodies (mAbs, as purified immunoglobulin (Ig) unless otherwise noted): 5E9 (anti–transferrin receptor as ascitic fluid diluted 1:100, generously provided by Dr Barton F. Haynes, Duke University, Durham, NC)25 ; BS46 (anti-LW)26 ; MP30-1 (anti-CD47)27 ; A3D8 (anti-CD44)28 ; anti-β2 integrins (CTB104; Santa Cruz Biotechnology, Santa Cruz, CA); anti–E-selectin (clone HAE-1f, Ancell, Bayport, MN); anti–P-selectin (CTB201, Santa Cruz Biotechnology); antifibronectin (IST-4, Sigma-Aldrich, St Louis, MO); and LM609 (specific for αvβ3 integrin, generously provided by Dr David Cheresh, Scripps Institute, La Jolla, CA).29 Rabbit polyclonal antibodies included antibody to purified human fibronectin (F3648, Sigma-Aldrich)30 ; protein-A affinity column purified anti-CD44 generated in our laboratory, and antithrombospondin (generously provided by Dr Leslie V. Parise, University of North Carolina, Chapel Hill, NC). The murine myeloma protein P3 × 63/Ag8 (P3 ascitic fluid diluted 1:500) was used as a nonreactive control murine Ig for mAbs.31 Normal rabbit immunoglobulin was used as a control for rabbit antisera. Antibodies were used at saturating dilutions unless otherwise indicated. Conjugated antibodies included fluorescein isothiocyanate (FITC)–conjugated mouse anti–human CD14 (AbD Serotec, Raleigh, NC); phycoerythrin-conjugated mouse anti–human CD15 and anti–human CD3 (BD Biosciences, San Jose, CA); FITC-conjugated goat anti–mouse Ig (Jackson ImmunoResearch Laboratories, West Grove, PA); and FITC-conjugated goat anti–rabbit Ig (Sigma-Aldrich).
PBMC separation
Blood from healthy donors diluted 1:1 in phosphate-buffered saline (PBS) was layered on top of Lymphoprep density gradient media at 1.077 plus or minus 0.001 g/mL (Greiner Bio-One, Frickenhausen, Germany) to separate PBMCs. Tubes were centrifuged for 30 minutes at 400g. PBMCs on top of the separation media were carefully collected and then washed 3 times with PBS. Blood collection from human subjects with and without sickle cell disease was approved by the Institutional Review Board of Duke University, and informed consent was obtained in accordance with the Declaration of Helsinki.
Collection, preparation, and treatment of human RBCs
Fresh blood samples from patients homozygous for hemoglobin S and from normal controls were collected into citrate tubes. RBCs were separated from the buffy coat and platelet-rich plasma by gravity at 4°C for at least 2 hours. Plasma and buffy coat were removed by aspiration, and RBCs were washed at least 5 times in sterile PBS with 1.26 mM Ca2+ and 0.9 mM Mg2+ (pH 7.4). Packed RBCs were analyzed for leukocyte and platelet contamination using an Automated Hematology Analyzer Sysmex K-1000 (Sysmex, Kobe, Japan).
Before coincubation with PBMCs, aliquots of RBCs were treated with 20 nM epinephrine for 1 minute at 37°C or sham-treated with the same buffer without the active agent. Treated RBCs were then washed 4 times with 4 mL PBS with Ca2+ and Mg2+.
Activation of PBMC adhesion by SS RBCs
PBMCs were labeled with PKH 26 red fluorescent cell linker kit (Sigma-Aldrich) following the manufacturer's instructions. To activate PBMC adhesion by SS RBCs, fluorescent PBMCs were coincubated with epinephrine- or sham-treated packed SS or normal RBCs at a RBC:PBMC ratio of 10:1 for 15 or 30 minutes at 37°C. In some experiments, RBCs were labeled with PKH 67 green fluorescent cell linker kit (Sigma-Aldrich) before coincubation with red fluorescence-labeled PBMCs.
In other experiments, RBC-PBMC mixtures were depleted of SS RBCs using RBC lysis buffer according to the manufacturer's instructions (eBioscience, San Diego, CA), followed by centrifugation and aspiration of supernatants. PBMCs were then washed 3 times before adhesion assays.
Flow chamber assays
Graduated height flow chambers were used to quantify adhesion of PBMCs or isolated lymphocytes or monocytes to HUVECs cultured on slides coated with 2% gelatin, as previously described in detail.19 Adhesion assays were visualized with a Nikon Eclipse TE300 inverted epi-fluorescence microscope (Nikon, Inc., Melville, NY), using a 20×/0.45 Plan Fluor ELWD objective lens (Nikon). Images were acquired using a RS Photometrics Coolsnap digital camera (Photometrics, Tucson, AZ) connected to both the inverted microscope and a Power Mac G4 computer (Apple, Cupertino, CA), and were processed with Scanalytics IPLab software version 3.5.2 (Scanalytics, Rockville, MD) and Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA).
Reticulocyte enrichment and depletion
To test the effect of SS reticulocytes and mature SS RBCs on normal PBMC adhesion, SS reticulocytes were separated from mature SS RBCs based on transferrin receptor expression, a marker of reticulocytes, using mAb 5E9 and goat anti–mouse IgG-coated micro-bead affinity columns (MACS; Miltenyi Biotec, Auburn, CA), following the manufacturer's instructions.19
Flow cytometric analysis
Preparation of soluble LW and soluble CD44
Preparation of the cDNA construct encoding recombinant soluble LW (sLW) was previously described in detail.20 The recombinant soluble extracellular portion of CD44 (sCD44) was prepared similarly, using the following primers: forward primer, 5′-ACC ATG GAC AAG TTT TGG TGG-3′, reverse primer, 5′-TTC TGG AAT TTG GGG TGT CC-3′.
Inhibition assays
To identify the RBC adhesion receptor reactive with PBMCs, SS RBCs were incubated for 30 minutes with 25 μg/mL anti-CD47, anti-CD44, anti-LW, or with both anti-CD44+ anti-LW antibodies, or P3 myeloma protein, washed, treated with epinephrine, washed again, and then coincubated with fluorescently labeled PBMCs. Fifteen minutes later, SS RBCs were lysed from PBMC-RBC mixtures before PBMC adhesion assays.
To identify whether the leukocyte receptor(s) interacting with SS RBCs included CD44, SS RBCs were incubated for 45 minutes with 50 μg/mL sCD44 or with sLW (as a control), washed, treated with epinephrine, washed again, and then coincubated with fluorescently labeled PBMCs. Fifteen minutes later, SS RBCs were lysed from PBMC-RBC mixtures before PBMC adhesion assays. To examine leukocyte β2 integrin interaction with SS RBCs, fluorescently labeled PBMCs were preincubated with 25 μg/mL anti-β2 integrin antibody, washed, and then coincubated with epinephrine-treated SS RBCs for 15 minutes before adhesion assays.
To identify the leukocyte receptor involved in PBMC adhesion to HUVECs, fluorescently labeled PBMCs were first activated by coincubation with epinephrine-treated SS RBCs for 15 minutes. SS RBCs were then lysed from PBMC-RBC mixtures, and PBMCs were incubated for 30 minutes with 25 μg/mL anti-CD47, anti-LW, anti-β2 integrin, or anti-CD44 antibody, or P3, and then washed before assays of leukocyte adhesion.
To confirm the receptor on PBMCs that bound to HUVECs, HUVECs were incubated for 1 hour at 37°C with 50 μg/mL sLW or sCD44, and then washed before leukocyte adhesion assays.
The EC ligands that bound to PBMCs were identified by incubating HUVECs with 50 μg/mL LM609 (anti-αvβ3), anti-CD44, anti-fibronectin, antithrombospondin, anti–P-selectin, or anti–E-selectin antibody for 1 hour at 37°C before leukocyte adhesion assays.
CD44 phosphorylation
To define whether SS RBCs induce leukocyte CD44 phosphorylation, PBMCs were depleted of endogenous adenosine triphosphate stores and 32P-labeled as previously described,19 before incubation with sham or epinephrine-treated SS RBCs for 15 or 30 minutes at 37°C. SS RBCs were then lysed from RBC-PBMC mixtures, and immunoprecipitation of CD44 from PBMCs using A3D8 antibody and the control P3 myeloma protein was performed as previously described in detail.19 Immunoprecipitated proteins were separated by 10% polyacrylamide–sodium dodecyl sulfate gel electrophoresis before transfer to nitrocellulose membrane. Total CD44 protein was detected by immunoblotting using A3D8 antibody. Phosphorylation of CD44 was detected by PhosphorImager (Storm 840, GE Healthcare, Piscataway, NJ) and quantified using ImageQuant Software, version 1.2 (GE Healthcare).
Adhesion of lymphocytes and monocytes
To determine the effect of SS RBCs on adhesion of lymphocytes and monocytes separately, lymphocytes and monocytes were isolated from PBMCs by cell sorting using a FACScan flow cytometer (BD Biosciences). Lymphocytes were separated from PBMCs by removing both FITC-conjugated CD14+ and phycoerythrin-conjugated CD15+ cells. Monocytes were isolated from PBMCs by removing cells stained by FITC-conjugated anti-CD3 mAb.
Statistical analysis
Data were compared using parametric analyses (GraphPad Prism 4 Software, San Diego, CA), including repeated and nonrepeated measures of analysis of variance. One-way analyses of variance were followed by Bonferroni corrections for multiple comparisons (multiplying the P value by the number of comparisons). A P value less than .05 was considered significant.
Results
We first examined our human SS RBC preparations for possible contamination by platelets and leukocytes. All normal RBC preparations showed immeasurable (0 cells/μL) leukocytes and platelets. Among all SS RBC preparations (0.13 ± 0.01 × 106/μL RBCs) tested, a few showed a low level of contamination by leukocytes (0.2 ± 0.06 × 103/μL). However, when similar numbers of isolated sickle leukocytes were coincubated with PBMCs, they did not induce adhesion of PBMCs to HUVECs (data not shown), making it unlikely that sickle leukocytes contaminating SS RBC preparations participated in the effects induced by SS RBCs on PBMCs.
SS RBCs induce PBMC adhesion to endothelium
We hypothesized that, to induce activation of PBMCs by SS RBCs to adhere to ECs, an initial contact step and adhesive interaction between SS RBCs and mononuclear leukocytes were required. Indeed, SS RBCs have been shown to adhere to normal PMNs, and this recognition results in activation of the PMN respiratory burst.14 To determine the effects of SS RBCs on PBMCs and subsequent PBMC adhesion to ECs, green fluorescence-labeled sham- or epinephrine-treated SS RBCs were coincubated with red fluorescence-labeled PBMCs before assays of adhesion of PBMC-RBC mixtures to HUVECs. SS RBCs, which adhered to some degree to nonactivated ECs, interacted with PBMCs and induced adhesion of PBMCs to HUVECs (Figure 1A). Interaction of PBMCs with epinephrine-treated SS RBCs, which adhered strongly to HUVECs, also resulted in PBMC adhesion to HUVECs (Figure 1B).
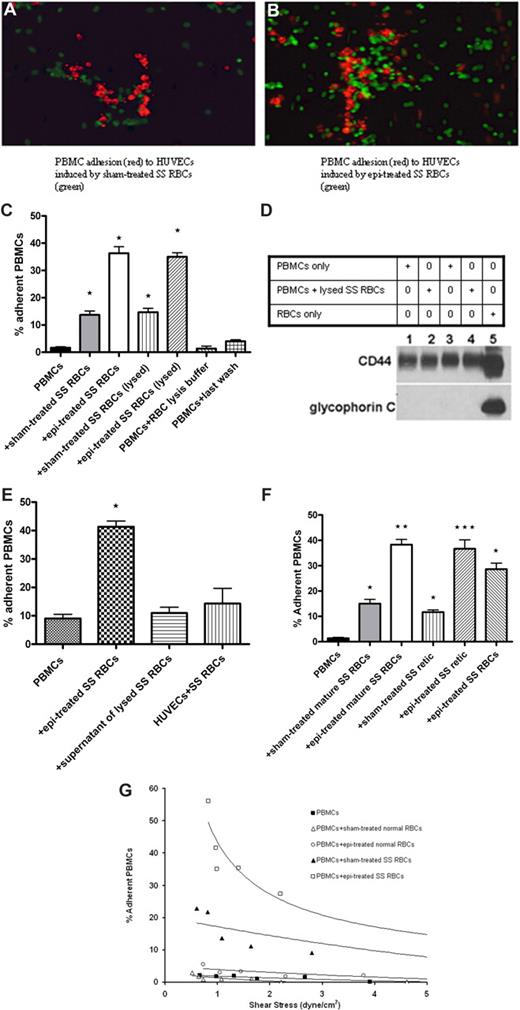
SS RBCs, but not normal RBCs, induced increased PBMC adhesion to HUVECs. (A,B) Sham-treated (A) or epinephrine (epi)–treated (B) SS RBCs (green) were coincubated with PBMCs (red). PBMC-RBC mixtures were then tested for adhesion to HUVECs. Photomicrographs using ×20 magnification show that sham-treated SS RBCs (A), which adhered to some degree to nontreated HUVECs, induced adhesion of PBMCs to HUVECs. Epinephrine-treated SS RBCs (B), which adhered strongly to nonactivated HUVECs, also induced PBMC adhesion to HUVECs. Photomicrograph of adhesion of PBMCs (not coincubated with SS RBCs) is not shown because such PBMCs did not visibly adhere to HUVECs. (C) PBMCs were tested for adhesion to HUVECs in the presence of sham- or epi-treated SS RBCs, or after lysis of sham- or epi-treated SS RBCs. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs. (D) PBMCs separated from blood obtained from 2 different donors (donor 1, lanes 1 and 2; donor 2, lanes 3 and 4) were analyzed alone (lanes 1 and 3) or after coincubation with ABO-matched SS RBCs (lanes 2 and 4). For PBMC-RBC mixtures, after 30 minutes of incubation, cells were treated with RBC lysis buffer, then washed free of lysed RBCs. Proteins (50 μg protein per lane) obtained from both types of PBMC preparations, as well as RBCs only (lane 5), were then analyzed for the presence of RBC proteins by Western blot using antiglycophorin C and anti-CD44 (a positive control for both RBCs and leukocytes) antibodies, and P3 myeloma protein as a negative control (data not shown). Western blot analysis showed that, after RBC lysis, leukocyte preparations were free of detectable RBC proteins. (E) PBMCs not coincubated with SS RBCs did not significantly adhere to HUVECs previously coincubated with SS RBCs for 15 minutes. Similarly, the supernatant potentially containing free heme and reactive oxygen species obtained from lysed SS RBCs did not induce PBMC adhesion to ECs. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs. (F) Separation of SS reticulocytes (retic) and mature SS RBCs was accomplished using anti–transferrin receptor mAb 5E9 and goat anti–mouse IgG-coated magnetic microbeads. PBMCs were coincubated with sham-treated mature SS RBCs, epi-treated mature SS RBCs, sham-treated SS retic, epi-treated SS retic, or epi-treated unseparated SS RBCs. Adhesion of PBMCs to HUVECs was then tested. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments. *P < .05 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with sham-treated mature SS RBCs; ***P < .001 compared with PBMCs coincubated with sham-treated SS retic. (G) Adhesion of PBMCs to HUVECs after coincubation with sham- or epi-treated normal RBCs versus sham- or epi-treated SS RBCs, respectively. One representative experiment is presented (n = 3).
SS RBCs, but not normal RBCs, induced increased PBMC adhesion to HUVECs. (A,B) Sham-treated (A) or epinephrine (epi)–treated (B) SS RBCs (green) were coincubated with PBMCs (red). PBMC-RBC mixtures were then tested for adhesion to HUVECs. Photomicrographs using ×20 magnification show that sham-treated SS RBCs (A), which adhered to some degree to nontreated HUVECs, induced adhesion of PBMCs to HUVECs. Epinephrine-treated SS RBCs (B), which adhered strongly to nonactivated HUVECs, also induced PBMC adhesion to HUVECs. Photomicrograph of adhesion of PBMCs (not coincubated with SS RBCs) is not shown because such PBMCs did not visibly adhere to HUVECs. (C) PBMCs were tested for adhesion to HUVECs in the presence of sham- or epi-treated SS RBCs, or after lysis of sham- or epi-treated SS RBCs. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs. (D) PBMCs separated from blood obtained from 2 different donors (donor 1, lanes 1 and 2; donor 2, lanes 3 and 4) were analyzed alone (lanes 1 and 3) or after coincubation with ABO-matched SS RBCs (lanes 2 and 4). For PBMC-RBC mixtures, after 30 minutes of incubation, cells were treated with RBC lysis buffer, then washed free of lysed RBCs. Proteins (50 μg protein per lane) obtained from both types of PBMC preparations, as well as RBCs only (lane 5), were then analyzed for the presence of RBC proteins by Western blot using antiglycophorin C and anti-CD44 (a positive control for both RBCs and leukocytes) antibodies, and P3 myeloma protein as a negative control (data not shown). Western blot analysis showed that, after RBC lysis, leukocyte preparations were free of detectable RBC proteins. (E) PBMCs not coincubated with SS RBCs did not significantly adhere to HUVECs previously coincubated with SS RBCs for 15 minutes. Similarly, the supernatant potentially containing free heme and reactive oxygen species obtained from lysed SS RBCs did not induce PBMC adhesion to ECs. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs. (F) Separation of SS reticulocytes (retic) and mature SS RBCs was accomplished using anti–transferrin receptor mAb 5E9 and goat anti–mouse IgG-coated magnetic microbeads. PBMCs were coincubated with sham-treated mature SS RBCs, epi-treated mature SS RBCs, sham-treated SS retic, epi-treated SS retic, or epi-treated unseparated SS RBCs. Adhesion of PBMCs to HUVECs was then tested. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments. *P < .05 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with sham-treated mature SS RBCs; ***P < .001 compared with PBMCs coincubated with sham-treated SS retic. (G) Adhesion of PBMCs to HUVECs after coincubation with sham- or epi-treated normal RBCs versus sham- or epi-treated SS RBCs, respectively. One representative experiment is presented (n = 3).
To further evaluate the levels of PBMC adherence to HUVECs, red fluorescence-labeled PBMCs were coincubated with nonlabeled sham- or epinephrine-treated SS RBCs before quantifying adhesion of PBMCs to nonactivated HUVECs. Because the only cell population visualized was red fluorescence-labeled normal mononuclear leukocytes, the quantitation of adherent PBMCs did not include any remaining nonlabeled leukocytes from SCD patients. Adhesion of PBMCs coincubated with SS RBCs was increased (14% ± 1.5% adherent cells) at a shear stress of 1 dyne/cm2, compared with adhesion of PBMCs not coincubated with SS RBCs (2% ± 0.3%; P < .001; Figure 1C). Epinephrine-activated SS RBCs, however, had a stronger effect on PBMCs than nonactivated SS RBCs and gave rise to 36% plus or minus 2.4% adherent mononuclear leukocytes (P < .001; Figure 1C). Thus, epinephrine-activated SS RBCs appeared to be potent activators of PBMC adhesion to endothelium.
Interaction of SS RBCs with endothelium may cause alteration of endothelial characteristics with up-regulation of adhesion molecule expression.34,35 To exclude the possibility that increased PBMC adhesion was the result of endothelial responses to SS RBCs, PBMC-RBC mixtures were treated with RBC lysis buffer to lyse SS RBCs before PBMC adhesion assays. Adhesion of PBMCs washed free of lysed sham-treated or epinephrine-treated SS RBCs was similar to adhesion of PBMCs in the presence of either sham-treated or epinephrine-treated SS RBCs, respectively (Figure 1C). The presence of RBC remnants within or attached to PBMCs was also ruled out. Western blot analysis using an antibody against glycophorin C, expressed only on RBCs, showed that leukocyte preparations were free of RBC membrane proteins after RBC lysis (Figure 1D). Furthermore, to confirm that SS RBCs did not have an effect on ECs, SS RBCs were coincubated with HUVECs for 15 minutes. After washing to eliminate nonadherent SS RBCs, leukocytes not previously coincubated with SS RBCs did not significantly adhere to HUVECs (P > .05, Figure 1E). These results suggest that increased PBMC adhesion to HUVECs was not the result of SS RBC–induced endothelial changes.
The possible effects of residual epinephrine, RBC lysis buffer, as well as reactive oxygen species or free heme that may derive from SS RBCs or be released by damaged SS RBCs were also considered. PBMCs were incubated with the supernatant from the last wash of SS RBC treatment with epinephrine, with RBC lysis buffer, or with the supernatant potentially containing free heme and reactive oxygen species from lysed SS RBCs, in conditions similar to those used for PBMC coincubation with RBCs before adhesion assays. Exposure of PBMCs to the supernatant obtained from the last wash of SS RBC treatment with epinephrine, RBC lysis buffer, or the supernatant obtained from lysed SS RBCs did not affect PBMC adhesion to ECs (Figure 1C,E), suggesting that activation of PBMC adhesion was specifically the result of the effect of interaction with intact SS RBCs.
We next asked which SS RBC population (mature SS RBCs or SS reticulocytes) activated PBMC adhesion. Reticulocyte-enriched and -depleted (mature) SS RBCs were first analyzed for expression of transferrin receptor, a reticulocyte marker, by flow cytometry. In each of 3 experiments, approximately 9% of unseparated SS RBCs were positive for transferrin receptor expression. After separation, more than 94% of the reticulocyte-enriched cells were transferrin receptor positive, whereas the reticulocyte-depleted population reacted with the anti–transferrin receptor antibody no more strongly than with the control murine myeloma protein P3. PBMC adhesion assays showed that all 3 SS RBC populations (reticulocyte-enriched, reticulocyte-depleted, and unseparated), pretreated or not with epinephrine, induced significant PBMC adhesion to HUVECs (Figure 1F).
In contrast to the PBMC adhesion induced by sham-treated and epinephrine-treated SS RBCs, neither sham-treated nor epinephrine-treated normal RBCs were capable of activating adhesion of PBMCs to HUVECs (Figure 1G).
SS RBC surface proteins LW and CD44 interact with PBMCs
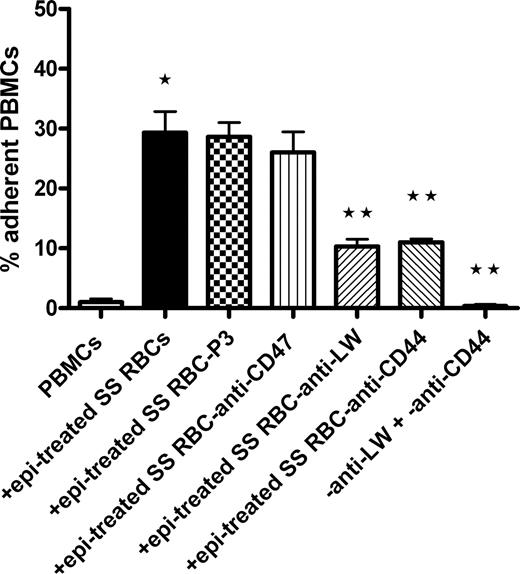
The glycoprotein LW on RBCs binds to leukocyte β1 and β2 integrins.21-23 We explored the possibility that LW on SS RBCs is involved in interaction and activation of PBMC adhesion using antibodies against different adhesion molecules present on SS RBCs. The negative control murine myeloma protein P3 and murine antibody against CD47, an RBC thrombospondin receptor, both failed to abrogate the ability of epinephrine-treated SS RBCs to induce adhesion of PBMCs to HUVECs (Figure 2). However, antibody against LW inhibited by 63% plus or minus 7% the ability of epinephrine-treated SS RBCs to subsequently induce PBMC adhesion to HUVECs (P < .001). Surprisingly, the effect of SS RBCs on PBMC adhesion to ECs was also significantly blocked (61% ± 2.7% inhibition) when epinephrine-treated SS RBCs were preincubated with anti-CD44 antibody (P < .001). Antibodies against LW and CD44 had an additive inhibitory effect; together, they completely prevented epinephrine-treated SS RBCs from activating PBMC adhesion (P < .001). These results strongly argue that LW and CD44 on SS RBCs are both involved in RBC interaction with PBMCs and thus in RBC-induced activation of PBMC adhesion to ECs.
Activation of PBMC adhesion to endothelium is induced by SS RBC LW and CD44. Inhibition of PBMC interaction with epi-treated SS RBCs was attempted by preincubation of SS RBCs with antibodies against the RBC receptors CD47, LW, and CD44; P3 was used as a nonreactive control antibody. Adhesion of PBMCs coincubated with such epi-treated SS RBCs was then assayed. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with SS RBCs preincubated with P3, then treated with epi.
Activation of PBMC adhesion to endothelium is induced by SS RBC LW and CD44. Inhibition of PBMC interaction with epi-treated SS RBCs was attempted by preincubation of SS RBCs with antibodies against the RBC receptors CD47, LW, and CD44; P3 was used as a nonreactive control antibody. Adhesion of PBMCs coincubated with such epi-treated SS RBCs was then assayed. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with SS RBCs preincubated with P3, then treated with epi.
Leukocyte CD44 and β2 integrin involvement in interactions with SS RBCs
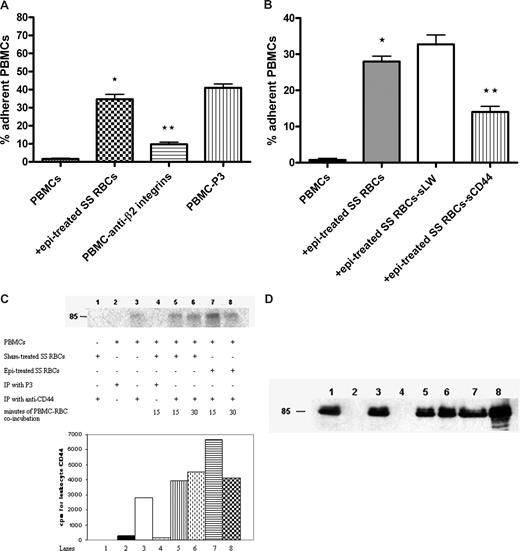
Leukocyte β1 (α4β1) and β2 integrins are known counterreceptors for RBC LW. Antibody against leukocyte β2 integrins blocked the effect of epinephrine-treated SS RBCs on PBMC adhesion by 76% plus or minus 2% (P < .001, Figure 3A). Furthermore, because leukocyte CD44 can mediate cell-cell adhesion,36 we were interested in determining whether leukocyte CD44 also interacts with RBCs. To block interaction of SS RBCs with leukocyte CD44, epinephrine-treated SS RBCs were preincubated with sCD44 or sLW (as a control) and then washed before coincubation with PBMCs. In contrast to sLW, sCD44 protein preincubation with SS RBCs significantly abrogated the subsequent effect of epinephrine-treated SS RBCs on PBMC adhesion to HUVECs by 57% plus or minus 2.3% (Figure 3B), suggesting that leukocyte CD44 bound to SS RBCs.
Leukocyte β2 integrins and CD44 are involved in interaction with SS RBCs. (A,B) Inhibition of PBMC interaction with epi-treated SS RBCs was performed as described in “Inhibition assays.” Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. (A) PBMC adhesion was measured after preincubation of PBMCs with anti-β2 integrin antibody followed by exposure to epi-treated SS RBCs. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs preincubated with P3. (B) PBMC adhesion was measured after exposure to epi-treated SS RBCs, or epi-treated SS RBCs preincubated with sCD44 or sLW. Error bars show SEM of 4 different experiments. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with SS RBCs preincubated with sLW, then treated with epi. (C) Phosphorylation of leukocyte CD44. Inorganic 32P radiolabeled intact PBMCs were coincubated or not with sham-treated SS RBCs for 15 minutes or 30 minutes, or with epi-treated SS RBCs for 15 minutes or 30 minutes. Leukocyte CD44 was immunoprecipitated (IP) with anti-CD44 antibody or P3 as a control, as indicated. RBC CD44 was IP from sham-treated SS RBCs with anti-CD44 antibody. The cpm shown are quantitative data of the radioactive band at 85 kDa. One representative experiment is shown (n = 3). (D) Total immunoprecipitates (lanes 1-8) obtained using the same conditions as described in panel C but immunostained with A3D8 mAb against CD44.
Leukocyte β2 integrins and CD44 are involved in interaction with SS RBCs. (A,B) Inhibition of PBMC interaction with epi-treated SS RBCs was performed as described in “Inhibition assays.” Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. (A) PBMC adhesion was measured after preincubation of PBMCs with anti-β2 integrin antibody followed by exposure to epi-treated SS RBCs. Error bars show SEM of 3 different experiments. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs preincubated with P3. (B) PBMC adhesion was measured after exposure to epi-treated SS RBCs, or epi-treated SS RBCs preincubated with sCD44 or sLW. Error bars show SEM of 4 different experiments. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with SS RBCs preincubated with sLW, then treated with epi. (C) Phosphorylation of leukocyte CD44. Inorganic 32P radiolabeled intact PBMCs were coincubated or not with sham-treated SS RBCs for 15 minutes or 30 minutes, or with epi-treated SS RBCs for 15 minutes or 30 minutes. Leukocyte CD44 was immunoprecipitated (IP) with anti-CD44 antibody or P3 as a control, as indicated. RBC CD44 was IP from sham-treated SS RBCs with anti-CD44 antibody. The cpm shown are quantitative data of the radioactive band at 85 kDa. One representative experiment is shown (n = 3). (D) Total immunoprecipitates (lanes 1-8) obtained using the same conditions as described in panel C but immunostained with A3D8 mAb against CD44.
Leukocyte CD44 can undergo phosphorylation as a result of activation. PhosphorImager analysis of immunoprecipitated CD44 and negative control immune complexes obtained from 32P-radiolabeled PBMCs indicated the presence of a modestly phosphorylated CD44 band at 85 kDa on nonstimulated PBMCs (Figure 3C lane 3). Stimulation of PBMCs with sham-treated SS RBCs for 15 minutes (Figure 3C lane 5) resulted in a 1.4-fold increased in CD44 phosphorylation. Leukocyte CD44 phosphorylation increased slightly more (by 1.6-fold) when PBMCs were exposed to sham-treated SS RBCs for 30 minutes (Figure 3C lane 6). Stimulation with epinephrine-treated SS RBCs for 15 minutes (Figure 3C lane 7) resulted in a 2.4-fold increase in leukocyte CD44 phosphorylation. However, 30-minute exposure to epinephrine-treated SS RBCs induced a lower increase in leukocyte CD44 phosphorylation (1.5-fold, Figure 3C lane 8). Phosphorylation was specific for leukocyte CD44 and did not include RBC CD44 because SS RBCs had not been radiolabeled, and a CD44 immunoprecipitate obtained from sham-treated RBCs was not phosphorylated (Figure 3C lane 1). Immunoprecipitates from PBMCs with anti-CD44 antibody contained similar amounts of CD44 immunoprecipitated in each sample (Figure 3D). These results suggest that SS RBCs were able to induce substantially increased phosphorylation of leukocyte CD44 and that phosphorylation occurred fairly quickly after exposure of PBMCs to SS RBCs.
Activated CD44 and β2 integrins mediate PBMC adhesion to endothelium
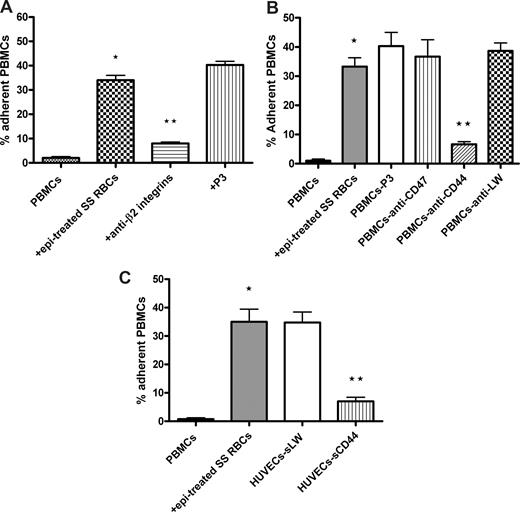
Leukocyte CD44 and β2 integrins can both mediate leukocyte interaction with endothelium.36,37 We therefore evaluated whether SS RBCs induced leukocyte β2 integrins and CD44 to mediate PBMC adhesion to ECs. Incubation of PBMCs with antibodies against β2 integrins and CD44 significantly inhibited (80% ± 2.3% and 83% ± 4.5% inhibition, respectively; P < .001) subsequent adhesion to ECs of activated PBMCs washed free of lysed epinephrine-treated SS RBCs (Figure 4A,B). In contrast, neither the control P3 protein nor antibodies to CD47 or LW were able to block activated PBMC adhesion to HUVECs. Similarly, sCD44 protein preincubated with HUVECs before adhesion assays also effectively inhibited activated PBMC adhesion by 81% plus or minus 1.8% (P < .001) (Figure 4C), whereas sLW failed to block PBMC adhesion. These results strongly suggest that PBMC adhesion to endothelium is mediated at least in part by leukocyte β2 integrins and CD44, after activation by epinephrine-stimulated SS RBCs.
Activated leukocyte β2 integrins and CD44 are involved in PBMC adhesion to ECs. Inhibition of PBMC adhesion with antibody (A,B) and recombinant protein (C) was performed as described in “Inhibition assays.” For all experiments, PBMC adhesion was activated by epi-treated SS RBCs as indicated. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments for panels A and B and SEM of 4 different experiments for panel C. (A) Inhibition of PBMC adhesion was performed by incubation of activated PBMCs washed free of lysed epi-treated SS RBCs with anti-β2 integrin antibody. P3 protein was used as a nonreactive control for both panels A and B. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with activated PBMCs preincubated with P3. (B) Inhibition of PBMC adhesion was performed by incubation of activated PBMCs washed free of lysed epi-treated SS RBCs with anti-CD47, anti-LW, or anti-CD44 antibody. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with activated PBMCs preincubated with P3. (C) Confluent cultures of HUVECs were incubated without recombinant protein or with sLW or sCD44 protein, washed, and then tested for their ability to support adhesion of activated PBMCs washed free of lysed epi-treated SS RBCs. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with epi-treated SS RBCs.
Activated leukocyte β2 integrins and CD44 are involved in PBMC adhesion to ECs. Inhibition of PBMC adhesion with antibody (A,B) and recombinant protein (C) was performed as described in “Inhibition assays.” For all experiments, PBMC adhesion was activated by epi-treated SS RBCs as indicated. Results are presented as percentage of adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments for panels A and B and SEM of 4 different experiments for panel C. (A) Inhibition of PBMC adhesion was performed by incubation of activated PBMCs washed free of lysed epi-treated SS RBCs with anti-β2 integrin antibody. P3 protein was used as a nonreactive control for both panels A and B. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with activated PBMCs preincubated with P3. (B) Inhibition of PBMC adhesion was performed by incubation of activated PBMCs washed free of lysed epi-treated SS RBCs with anti-CD47, anti-LW, or anti-CD44 antibody. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with activated PBMCs preincubated with P3. (C) Confluent cultures of HUVECs were incubated without recombinant protein or with sLW or sCD44 protein, washed, and then tested for their ability to support adhesion of activated PBMCs washed free of lysed epi-treated SS RBCs. *P < .001 compared with unstimulated PBMCs; **P < .001 compared with PBMCs coincubated with epi-treated SS RBCs.
Endothelial E-selectin and fibronectin are involved in PBMC adhesion
Expression of some adhesion molecules is up-regulated on HUVECs when plated on gelatin, fibronectin, collagen, or fibrinogen.33 Because HUVECs were cultured on glass slides precoated with gelatin before adhesion assays, we first examined adhesion molecule expression by HUVECs cultured on plastic versus on gelatin using flow cytometric analysis. Approximately half of the cells expressed αvβ3 integrin, and most were negative for P-selectin whether cultured on plastic or on gelatin. However, the levels of expression of αvβ3 and P-selectin were up-regulated on HUVECs cultured on gelatin (Table 1). When cultured on gelatin, the percentage of HUVECs expressing thrombospondin, fibronectin, and E-selectin and the levels of expression of these antigens were both highly up-regulated compared with the expression of these antigens by cells grown on plastic. The percentage of cells expressing CD44 increased only very modestly when HUVECs were grown on gelatin versus on plastic.
Target ligand expression on HUVECs by flow cytometry
| Antibody (target) . | % positive HUVECs cultured on plastic (MFI)* . | % positive HUVECs cultured on gelatin (MFI)* . |
|---|---|---|
| P3 (negative control) | 2.3 ± 0.6 (113 ± 6.1) | 2 ± 0.6 (129 ± 8.3) |
| Anti-αvβ3 | 49.7 ± 3.5 (127 ± 4.4) | 50 ± 2.5 (304 ± 19.8) |
| Anti-CD44 | 6 ± 1.5 (121 ± 3.6) | 9 ± 3.5 (131 ± 8.2) |
| Antithrombospondin† | 56.3 ± 3.5 (159 ± 8.1) | 87 ± 2.5 (800 ± 23) |
| Antifibronectin† | 8.3 ± 1.5 (170 ± 4.6) | 59 ± 4 (693 ± 21.8) |
| Anti–E-selectin† | 2.3 ± 0.6 (115 ± 7) | 37 ± 2.6 (252 ± 4.7) |
| Anti–P-selectin | 1.7 ± 0.6 (108 ± 2.5) | 3 ± 0.6 (174 ± 3.6) |
| Antibody (target) . | % positive HUVECs cultured on plastic (MFI)* . | % positive HUVECs cultured on gelatin (MFI)* . |
|---|---|---|
| P3 (negative control) | 2.3 ± 0.6 (113 ± 6.1) | 2 ± 0.6 (129 ± 8.3) |
| Anti-αvβ3 | 49.7 ± 3.5 (127 ± 4.4) | 50 ± 2.5 (304 ± 19.8) |
| Anti-CD44 | 6 ± 1.5 (121 ± 3.6) | 9 ± 3.5 (131 ± 8.2) |
| Antithrombospondin† | 56.3 ± 3.5 (159 ± 8.1) | 87 ± 2.5 (800 ± 23) |
| Antifibronectin† | 8.3 ± 1.5 (170 ± 4.6) | 59 ± 4 (693 ± 21.8) |
| Anti–E-selectin† | 2.3 ± 0.6 (115 ± 7) | 37 ± 2.6 (252 ± 4.7) |
| Anti–P-selectin | 1.7 ± 0.6 (108 ± 2.5) | 3 ± 0.6 (174 ± 3.6) |
Mean percentage and mean of mean fluorescence intensity (MFI) of positive cells are shown (n = 3 different experiments, ± SEM).
P < .001 for % HUVECs that were positive with indicated antibody when cells were cultured on gelatin, compared with % HUVECs positive when cells were cultured on plastic.
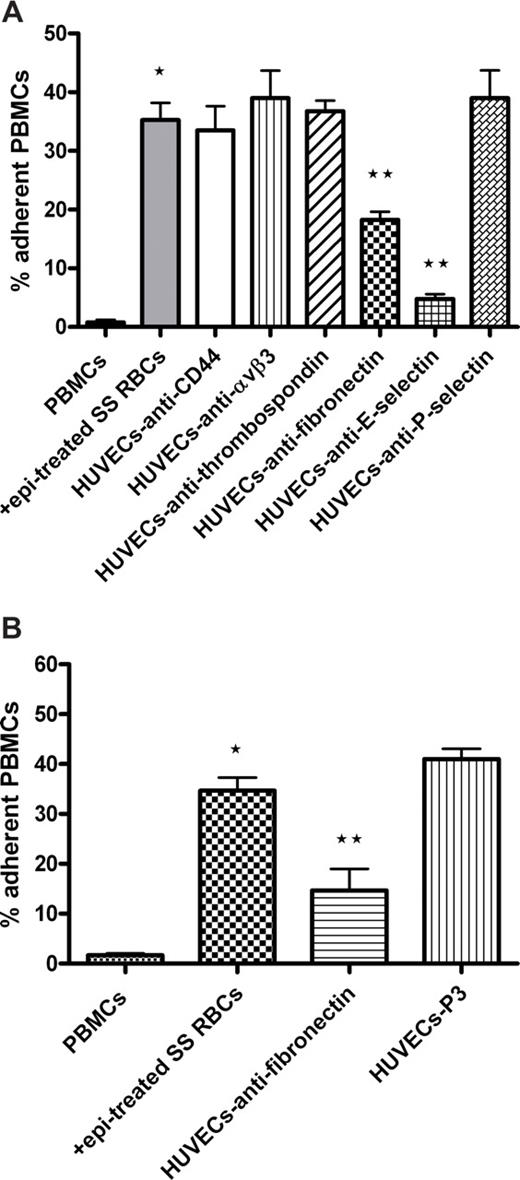
We next defined the molecule(s) on ECs involved in PBMC adhesion. Significant inhibition of activated PBMC adhesion to endothelium was observed when HUVECs were preincubated with monoclonal antibodies reactive with E-selectin (86% ± 3.3% inhibition, P < .01) and fibronectin (48% ± 2.9% inhibition, P < .01; Figure 5A). The involvement of fibronectin in leukocyte adhesion was confirmed using rabbit antibody to human fibronectin (65% ± 10.2% inhibition, P < .01; Figure 5B). In contrast, preincubation of HUVECs with antibodies to αvβ3 integrin, thrombospondin, CD44, and P-selectin failed to inhibit activated PBMC adhesion (Figure 5A). These data suggest that activated PBMCs bind to endothelial E-selectin and fibronectin.
Endothelial cell E-selectin and fibronectin are involved in adhesion of activated PBMCs. (A,B) HUVECs were preincubated with or without antibody against CD44, αvβ3 integrin, thrombospondin, fibronectin, E-selectin, or P-selectin. P3 protein was used as a nonreactive control antibody in panel B. HUVECs were washed and then tested for their ability to support adhesion of PBMCs activated with epi-treated SS RBCs. (A) Error bars show SEM of 4 different experiments measuring adhesion at a shear stress of 1 dyne/cm2. *P < .001 compared with unstimulated PBMCs; **P < .01 compared with PBMCs preincubated with epi-treated SS RBCs. (B) Error bars show SEM of 3 different experiments measuring adhesion at a shear stress of 1 dyne/cm2. *P < .001 compared with unstimulated PBMCs; **P < .01 compared with PBMCs preincubated with epi-treated SS RBCs.
Endothelial cell E-selectin and fibronectin are involved in adhesion of activated PBMCs. (A,B) HUVECs were preincubated with or without antibody against CD44, αvβ3 integrin, thrombospondin, fibronectin, E-selectin, or P-selectin. P3 protein was used as a nonreactive control antibody in panel B. HUVECs were washed and then tested for their ability to support adhesion of PBMCs activated with epi-treated SS RBCs. (A) Error bars show SEM of 4 different experiments measuring adhesion at a shear stress of 1 dyne/cm2. *P < .001 compared with unstimulated PBMCs; **P < .01 compared with PBMCs preincubated with epi-treated SS RBCs. (B) Error bars show SEM of 3 different experiments measuring adhesion at a shear stress of 1 dyne/cm2. *P < .001 compared with unstimulated PBMCs; **P < .01 compared with PBMCs preincubated with epi-treated SS RBCs.
SS RBCs can induce adhesion of both lymphocytes and monocytes to HUVECs
We next sought to determine whether PBMC adhesion induced by SS RBCs involved both cell populations, lymphocytes and monocytes. We used fluorescence cell sorting to isolate lymphocytes from both CD14+ and CD15+ cells. Monocytes were similarly isolated from CD3+ cells. Flow cytometric analysis showed that unseparated PBMCs consisted of 91% plus or minus 2% lymphocytes, 6% plus or minus 2% monocytes, and less than 1% double-positive CD14+ and CD15+ cells. After sorting, lymphocytes were enriched to 99.5% plus or minus 0.03% and were completely devoid of CD14+ and CD15+ cells. Monocytes were enriched to 99.6% plus or minus 0.05% and were devoid of CD3+ cells.
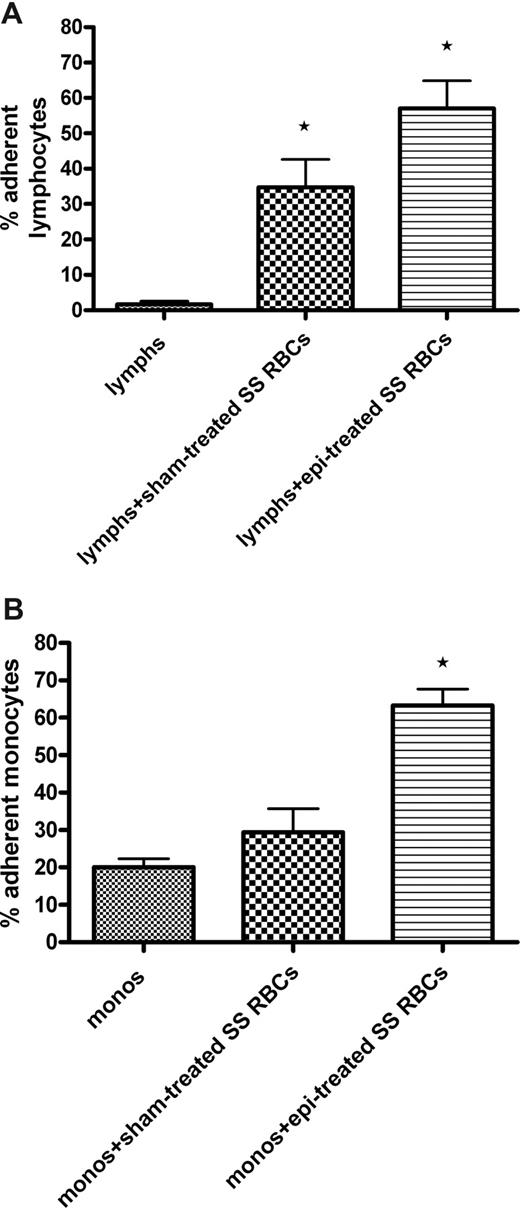
Adhesion assays demonstrated that sham-treated SS RBCs were capable of inducing significant up-regulation of adhesion of isolated lymphocytes (35% ± 8.0% adherent cells, P < .05) compared with adhesion of lymphocytes not exposed to SS RBCs (1.7% ± 0.9%; Figure 6A). Epinephrine-treated SS RBCs induced an even greater enhancement of lymphocyte adhesion to ECs (57% ± 7.8% adherent cells, P < .01 vs adhesion induced by sham-treated SS RBCs).
SS RBCs induce increased adhesion of both lymphocytes and monocytes to HUVECs. Lymphocytes (lymphs) and monocytes (monos) were isolated as described in “Adhesion of lymphocytes and monocytes.” Results are presented as percentage of adherent lymphocytes (A) or monocytes (B) at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments for both panels A and B. (A) Sham-treated SS RBCs or epi-treated SS RBCs were coincubated with isolated lymphocytes. Lymphocytes were then tested for adhesion to HUVECs. *P < .05 compared with unstimulated lymphs. (B) Sham-treated SS RBCs or epi-treated SS RBCs were coincubated with isolated monocytes. After incubation, monocytes were then tested for adhesion to HUVECs. *P < .01 compared with unstimulated monos.
SS RBCs induce increased adhesion of both lymphocytes and monocytes to HUVECs. Lymphocytes (lymphs) and monocytes (monos) were isolated as described in “Adhesion of lymphocytes and monocytes.” Results are presented as percentage of adherent lymphocytes (A) or monocytes (B) at a shear stress of 1 dyne/cm2. Error bars show SEM of 3 different experiments for both panels A and B. (A) Sham-treated SS RBCs or epi-treated SS RBCs were coincubated with isolated lymphocytes. Lymphocytes were then tested for adhesion to HUVECs. *P < .05 compared with unstimulated lymphs. (B) Sham-treated SS RBCs or epi-treated SS RBCs were coincubated with isolated monocytes. After incubation, monocytes were then tested for adhesion to HUVECs. *P < .01 compared with unstimulated monos.
However, adhesion of monocytes to HUVECs was markedly enhanced (63% ± 4.4% adherent cells, P < .01) only by epinephrine-activated SS RBCs (Figure 6B). Sham-treated SS RBCs were not able to significantly up-regulate adhesion of monocytes (29% ± 6.4%) compared with adhesion of monocytes not exposed to SS RBCs (20% ± 2.3%). These data suggest that epinephrine-activated SS RBCs can interact with and activate adhesion of both lymphocytes and monocytes.
Discussion
SS RBCs activate PBMC adhesion to ECs
The mechanisms leading to vaso-occlusion in patients with SCD are complex and involve interactions among SS RBCs, vascular endothelium, platelets, hemostatic plasma factors, and most probably leukocytes.1-4,20,24,38 Recently, we observed that murine leukocytes adhered to the vascular endothelium in vivo after infusion of epinephrine-activated human SS RBCs into nude mice.20 Our present in vitro data reveal an additional molecular mechanism that may contribute to the vaso-occlusive process, in which human SS RBCs act as a stimulus-promoting adhesion of human PBMCs to endothelium. Stimulation of SS RBCs by the stress hormone epinephrine led to a much more robust adhesion of PBMCs, including lymphocytes and monocytes. This PBMC adhesion is probably not the result of RBC-induced endothelial changes, which can occur in response to contact with SS RBCs, for the following reasons: PBMCs adhered even when SS RBCs were removed by hypotonic lysis before adhesion assays (Figure 1C), and incubation of endothelial cells with SS RBCs alone did not significantly increase subsequent PBMC adhesion to the endothelial cells (Figure 1E).
Epinephrine up-regulated the effect of both SS reticulocytes and mature SS RBCs on PBMC adhesion, although prior studies showed that dense (mostly mature) SS RBC adherence to polymorphonuclear leukocyte monolayers exceeded that of light (more immature) SS RBCs.14 The previously demonstrated ability of unseparated SS RBCs, SS reticulocytes, and mature SS RBCs to respond to epinephrine by increased activation of RBC adhesive function19 may partly explain the effect induced by these 3 SS populations on PBMC adhesion. However, the failure of epinephrine-stimulated normal RBCs, which have a much longer life span compared with SS RBCs, to activate PBMC adhesion may be a result of their previously demonstrated decreased ability to respond to epinephrine,19 probably the result of age-related loss of multiple protein kinase activities.39
SS RBC–PBMC interaction involves RBC LW and CD44
The initial step of leukocyte activation is recognition, and LW binds to leukocyte β1 and β2 integrins.21-23 We confirmed through inhibition studies that LW on epinephrine-stimulated SS RBCs interacted with PBMCs, resulting in subsequent PBMC adhesion to ECs. Our study also suggests that SS RBC CD44 contributes to RBC-leukocyte interactions that activate PBMC adhesion. Whereas our findings show that both LW and CD44 on SS RBCs had an additive effect on PBMCs, they also raise the possibility that, in addition to its effect on LW,19 epinephrine may also enhance the adhesive characteristics of RBC CD44 via a PKA-dependent pathway. CD44 has a predicted PKA site at serine 316.40 Thus, epinephrine-activation of SS RBCs, which leads to phosphorylation of LW via a PKA-dependent pathway,19 may also lead to phosphorylation of RBC CD44. Alternatively, similar to LW, which is activated to some degree on nonstimulated SS RBCs,19 CD44 on SS RBCs may already be sufficiently active to participate in RBC-leukocyte interactions.
Leukocyte β2 integrins and CD44 interact with SS RBCs
Our data, taken together with previously published work,22,23,36 suggest that leukocyte β2 integrins and CD44 interact with both RBCs and endothelial cells. Whereas incubation of PBMCs with antibody to β2 integrins before exposure to SS RBCs inhibited subsequent PBMC adhesion to ECs, antibody to β2 integrins also appeared to inhibit adhesion of PBMCs after they had been activated by SS RBCs. Therefore, our data neither support nor refute the possibility that SS RBC–PBMC interaction involves β2 integrins. Indeed, the best evidence for a role of β2 integrins in SS RBC–PBMC interaction is our data showing that antibody to LW blocks activation of PBMC adhesion (Figure 2), taken together with the data of Hermand et al clearly demonstrating the binding activity of LW to β2 integrins.22,23 Furthermore, the fact that sCD44 inhibits the ability of subsequently washed SS RBCs to activate leukocyte adhesion suggests that SS RBCs bind to leukocyte CD44. These results, along with results showing that anti-CD44 antibody incubated with SS RBCs had a similar inhibitory effect, raise the possibility but do not prove that CD44 on RBCs may interact with leukocyte CD44.
In resting nucleated cells, CD44 is found to be constitutively phosphorylated.40 However, conversion from the inactive to active form of leukocyte CD44 occurs in specific cell types in response to appropriate stimuli, including TNFα, IL-1, lipopolysaccharide, super-antigen, and T-cell mitogens.41-43 Our data also show that CD44 on nonactivated PBMCs is modestly phosphorylated. Activation of PBMCs by SS RBCs induced both increased CD44 phosphorylation as well as increased adhesion to endothelium mediated at least in part by CD44. Furthermore, the level of leukocyte CD44 phosphorylation increased in proportion to the degree of activation of adhesion, with epinephrine-treated SS RBCs causing more CD44 phosphorylation than nontreated SS RBCs. It is probable that β2 integrins also undergo activation as a result of stimulation of PBMCs by SS RBCs.
Leukocyte β2 integrins and CD44 mediate PBMC adhesion to ECs, a process involving endothelial E-selectin and fibronectin
Activation of both leukocyte β2 integrins and CD44 by SS RBCs resulted in PBMC adhesion to ECs. β2 integrins and CD44 on PMNs have previously been shown to interact with endothelium.44 Leukocyte CD44 is also involved in rolling and adhesion of activated CD4+ T helper (Th) lymphocytes to TNF-α–activated vascular endothelium in mice.36 Studies to date also suggest that a threshold level of CD44 expression as well as conversion to the active phosphorylated form is required to engage hyaluronan.45 Our data suggest that the ability of PBMC CD44 to mediate adhesion to endothelium requires cell activation, a process possibly associated with increased leukocyte CD44 phosphorylation (Figures 3,4).
Activated PBMCs adhered to endothelial E-selectin and fibronectin, both highly expressed on HUVECs cultured on gelatin. Previous studies have demonstrated that leukocyte CD44, although originally described as a hyaluronan receptor,46 can also bind to E-selectin44 and fibronectin,47 whereas β2 integrins bind to fibronectin.48 In contrast, P-selectin was not well expressed on HUVECs cultured on gelatin and did not appear to be involved in activated PBMC adhesion to ECs.
SS RBCs induce increased adhesion of both lymphocytes and monocytes to HUVECs
There is a growing body of data showing that activated monocytes4 and PMNs10 adhere to endothelium in SCD. We have now observed that both lymphocytes and monocytes can be activated to adhere to endothelium by a novel potent stimulus, epinephrine-activated SS RBCs. We therefore suggest that, in addition to neutrophils and monocytes, lymphocytes, which represent the second largest population of circulating leukocytes, may also contribute to the pathophysiology of SCD. Activation and adherence of both lymphocytes and monocytes could induce production of multiple cytokines and may cause stimulation of the vascular endothelium to increase its expression of ligands for blood cell adhesion molecules. These processes would result in tissue damage and inflammation, which could then predispose to vaso-occlusion involving both leukocytes and SS RBCs, as well as further tissue damage. However, it is probable that several other mechanisms also lead to inflammatory responses in SCD. For example, the potential hypoxic environment in the microcirculation can also trigger an increase in hemoglobin autoxidation, which augments superoxide pro-duction in RBCs. Consequently, RBCs release H2O2, which diffuses to the endothelium, thereby leading to P-selectin–dependent leukocyte recruitment.49
In conclusion, our data suggest a central role for SS RBCs and, in particular, for epinephrine-activated SS RBCs. SS RBCs probably not only participate in sickle cell vaso-occlusion through adhesion to vascular endothelium but also act as critical signaling cells that activate mononuclear leukocyte adhesion to endothelium as well.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jean-Pierre Cartron (Paris, France) for providing the LW cDNA from which we derived our sLW construct.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK065040) (R.Z.), and National Heart, Lung, and Blood Institute, National Institutes of Health (R01 HL079915) (R.Z., M.J.T.).
National Institutes of Health
Authorship
Contribution: R.Z. designed and supervised all of the research study, performed most of the experiments, analyzed and interpreted all data, and wrote the manuscript; A.C. performed some leukocyte adhesion assays; K.X. helped with some lymphocyte adhesion assays and CD44 phosphorylation; M.B. prepared soluble LW and CD44; and M.J.T. contributed to the design of the research study and helped edit the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marilyn J. Telen or Rahima Zennadi, Duke University Medical Center, Box 2615, Durham, NC 27710; e-mail: telen002@mc.duke.edu or zenna001@mc.duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal