Abstract
Following allogeneic blood and marrow transplantation (BMT), mature donor T cells can enhance engraftment, counteract opportunistic infections, and mount graft-versus-tumor (GVT) responses, but at the risk of developing graft-versus-host disease (GVHD). With the aim of separating the beneficial effects of donor T cells from GVHD, one approach would be to selectively deplete subsets of alloreactive T cells in the hematopoietic cell inoculum. In this regard, TCR Vβ repertoire analysis by CDR3-size spectratyping can be a powerful tool for the characterization of alloreactive T-cell responses. We investigated the potential of this spectratype approach by comparing the donor T-cell alloresponses generated in vitro against patient peripheral blood lymphocytes (PBLs) with those detected in vivo posttransplantation. The results indicated that for most Vβ families that exhibited alloreactive CDR3-size skewing, there was a robust overlap between the in vitro antipatient and in vivo spectratype histograms. Thus, in vitro spectratype analysis may be useful for determining the alloreactive T-cell response involved in GVHD development and, thereby, could serve to guide select Vβ family depletion for designer transplants to improve outcomes.
Introduction
Immunotherapeutic strategies have gained recognition as viable alternatives to more conventional modalities for the treatment of cancer because of their potential for curative effects. In this regard, adoptive T-cell therapy via allogeneic blood and marrow transplantation (BMT) has presented the first evidence to prove that antitumor effects could be achieved against hematologic malignancies.1-3 Theoretically, by extension, allogeneic BMT also represents one of the few potentially curative treatments for advanced solid tumors. However, donor T cell–mediated graft-versus-host disease (GVHD) continues to be the principal complication of allogeneic BMT, along with disease relapse and opportunistic infections.
The thrust of much effort in the field of BMT has been to develop tactics that could facilitate the separation of the beneficial graft-versus-tumor (GVT) effects from the deleterious effects of GVHD. A number of rational approaches for the separation of these 2 opposing processes have been developed by investigators based on differentials in cytotoxic mechanisms4-6 and antigen expression,7 as well as in cytokine and growth factor responses.8-11 Other modalities have included the introduction of a suicide gene into infused donor T cells12,13 and the delayed transplantation of regulatory T cells (Treg),14,15 both of which are theoretically designed to permit some level of alloreactivity, beneficial for GVT, before controlling effector T cells in an effort to minimize the development of GVHD. Alternatively, donor T cells have been administered as a delayed donor lymphocyte infusion (DLI) 1 to 3 months after transplantation, with the intent of providing the lymphocytes in an environment with diminished host inflammatory responses associated with the pretransplantation conditioning regimen.16,17
Several investigators have successfully separated donor responder T cells capable of mediating GVHD as opposed to GVT responses, through in vitro coincubation with host stimulators in a mixed lymphocyte culture (MLC), identifying the alloreactive cells by either their surface phenotype, proliferative potential, or retention of photoactive dyes. Subsequently, the alloreactive cells were depleted by magnetic cell separation,18-20 fluorescence-activated cell sorting,21-23 immunotoxins,24-26 or photodynamic cell purging.27,28 These manipulations reduced donor antihost alloreactive responses in vitro while preserving anti–third-party major histocompatibility complex (MHC), antitumor, and antiviral T-cell responses.25 Furthermore, these allodepletion approaches resulted in a reduction in GVHD development in murine models21,27,28 and in some cases have been adapted for the clinical situation, with some success.29
The underlying hypothesis driving all these transplantation strategies is that although T cells involved in GVHD and GVT responses are highly overlapping in their recognition of antigens between host tissues and tumor cells, the overlap is not complete and should therefore ultimately allow them to be separated. GVT responses may involve T-cell recognition of unique tumor-specific antigens presented by appropriate available MHC class I or II molecules, and there are many examples of these antigens, with some being used for the development of vaccines to boost posttransplantation responses.30-32 Alternatively, some of the shared host alloantigens may be tissue-specific and, if expressed only in the hematopoietic/lymphoid compartment, as in the case of minor histocompatibility antigen (miHA) HA-1,33 may not cause severe GVHD-related target organ damage. Thus, it may be possible to identify and select donor T cells that can provide beneficial GVT responses with minimal GVHD risk.
In this regard, TCR CDR3-size Vβ spectratype analysis can be used to characterize alloreactive and GVT-specific T-cell repertoire responses by their clonal and oligoclonal expansion of particular Vβ families. This approach could then lead to either negative selection of GVHD-reactive Vβ families or positive selection of GVT-specific Vβ families from the donor inoculum, which could then be provided as a DLI to BMT recipients weeks to months after transplantation.
Previously, we used the Vβ spectratype approach in murine models to define the nature of CD4+ and CD8+ T-cell involvement in GVHD to multiple miHA differences and in GVT responses to a syngeneic myeloid leukemia.34-36 Theoretically, given the high sensitivity of the PCR-based spectratyping technique, molecular analysis of alloreactive T-cell responses in vitro should identify the most potentially critical Vβ families involved in the later development of GVHD and GVT effects in patients. To this end, we tested the hypothesis that TCR-Vβ spectratype analysis of the donor T-cell repertoire of HLA-matched sibling (SIB) or matched unrelated donor (URD) pairs from in vitro, host-stimulated MLC would be predictive of the TCR-Vβ spectratype analysis of the T-cell repertoire in the patient after BMT. From this initial study, of 18 patient pairs, we observed a 68% overlap in the spectratype patterns between the donor antipatient in vitro and the patient after transplantation in vivo T-cell repertoires. Furthermore, this overlap was stable at several time points after transplantation.
Methods
Patients and donors
This study was conducted in accordance with Institutional Review Board–approved protocols at Hackensack University Medical Center (HUMC) and Thomas Jefferson University (TJU) and in accordance with the Declaration of Helsinki. Eighteen patients with hematologic malignancies were enrolled on study at HUMC and TJU. Sixteen patients received transplants from an HLA-matched SIB donor, and 2 patients received transplants from an HLA-matched URD. All patients received unmanipulated transplants; 15 were peripheral blood mononuclear cells (PBMCs) and 3 were bone marrow cells (BMs). The pretransplantation conditioning regimens consisted of busulfan (Bu; 12.8 mg/kg)/cytoxan (Cy; 60 mg/kg × 2; n = 9); total body irradiation (TBI; 12 Gy)/Cy (n = 3); fludarabine (Flu; 20-30 mg/m2 per day × 5)/TBI (n = 2); Flu/melfalan (Mel; 140 mg/m2; n = 1); and Flu/cytosine arabinoside (AraC; 2 g/m2 × 3)/Cy (n = 3). The posttransplantation GVHD prophylaxis consisted of either tacrolimus (Tac)/methotrexate (MTX; n = 12); cyclosporine A (CSA), mycophenolate mofotil (MMF; 1 g × 2 per day; n = 4); CSA/MTX (n = 1); or CSA plus an experimental CD4 inhibitory peptide, 802-237 (2.5 mg/kg per day × 4; n = 1).
Blood samples
PBLs were enriched from donor and patient peripheral blood samples as previously described38 by centrifugation over Ficoll-Paque-Plus (GE Healthcare, Piscataway, NJ). Vβ spectratype analysis of separated PBLs from the donor samples was used as the standard of comparison for the T-cell repertoires of the MLCs as well as for the posttransplantation samples.
MLCs
MLCs were set up according to standard techniques.39 Briefly, enriched PBLs from the transplant patients or a third party were irradiated (30 Gy) with a 137Ce source and used to stimulate responding T cells from their corresponding donors in 1-way MLC. Cells were incubated (37°C, 5% CO2) in T25 flasks in 10 mL RPMI 1640 complete medium (10% human serum, 5% Pen-Strep, 5% l-glutamine) at an R:S ratio of 1:2 (10 × 106 responders and 20 × 106 stimulators). After 9 days, responders were harvested and restimulated 8 days as before with the addition of 20 U/mL of rIL-2 (National Institute of Allergy and Infectious Diseases Reagent Program).
CDR3-size spectratype analysis
Total RNA was isolated from PBLs solubilized in UltraSpec (Biotecx Laboratories, Houston, TX) by chloroform extraction, isopropanol precipitation, and cDNA was prepared as previously described.38 Seminested PCR was performed using a panel of human Vβ sense oligoprimers and 2 Cβ antisense oligoprimers; the second Cβ being fluorescently labeled. The PCR products were run on either an ABI 3130 capillary gel system (Perkin Elmer-Applied Biosystems, Foster City, CA) at the Molecular Resource Facility of The University of Medicine and Dentistry of New Jersey or on a sequencing gel at the Nucleic Acid Facility of TJU. Spectratype analysis was performed using either the GeneMapper (version 3.7) or Genotyper Genescan software programs (Perkin Elmer-Applied Biosystems), which allow direct comparison of the Vβ T-cell repertoire from the MLCs and the patient posttransplantation. In brief, the software program identified each histogram peak by its PCR-size length and determined the area under each peak. The percentage area under the corresponding peaks between the donor and the MLC or patient posttransplantation samples were determined by direct comparison as previously described.40,41 A peak within the Vβ family spectratype histograms of the MLC and the patient posttransplantation samples was considered to be skewed if the area under the peak was at least 2-fold the area of the corresponding peak in the Vβ family spectratype histogram of the donor sample. This value was derived empirically from our preclinical mouse studies, whereby skewing of the non-GVHD relevant CD4+Vβ5 family35,42 could be generated by lowering the threshold area to below 2-fold.
Several technical issues contribute to making it routinely difficult to get data for all the Vβ families in the spectratype analysis. The presentation of the data for each Vβ family for each patient in the tables is dependent upon a resolvable spectratype for the donor sample, the MLC, and/or the patient posttransplantation samples. Each Vβ family constitutes a different percentage of the total T-cell repertoire. Some Vβ families comprise less than 1%, whereas others can be as much as 18%, and these numbers vary from person to person. The actual amount of peripheral blood obtained from each person can vary, and there is also variability among patients as to the total number of circulating T cells/cc of blood, especially early posttransplantation. All of these technical issues may result in an inadequate amount of mRNA for spectratype analysis, indicated as not determined (ND) in the summary tables.
Statistical considerations
In this descriptive analysis all values are expressed as percentage means plus or minus SEM. To establish that an MLC Vβ family spectratype was predictive of the patient posttransplantation sample Vβ family spectratype, the following criteria were used: (1) the observation of biased CDR3-size skewing in 1 or more identical PCR lengths between the MLC and most (≥ 50%) of the patient's posttransplantation samples, or (2) no skewing of any PCR lengths in the MLC nor in most (≥ 50%) of the patient posttransplantation samples. In cases where there was not a clear indication of majority outcome for the posttransplantation samples, the determination of predictive value was based on the last obtained patient sample.
Flow cytometric analysis
All fluorescently labeled monoclonal antibodies (mAbs) were purchased from Beckman Coulter (Miami, FL). PBLs were stained according to manufacture's instructions. Samples were analyzed using an FC500 flow cytometer (Beckman Coulter).
Results
Patient characteristics and clinical status
The 18 patients enrolled in this study ranged in age from 30 to 69 years (mean, 48.8 years; median, 49 years). The 14 females and 4 males presented with various underlying hematologic malignancies consisting of acute myelogenous leukemia (AML; n = 5), acute lymphoblastic leukemia (ALL; n = 3), myelodysplastic syndrome (MDS; n = 3), chronic myelogenous leukemia (CML; n = 2), non-Hodgkin lymphoma (NHL; n = 2), chronic lymphocytic leukemia (CLL; n = 1), large granulocytic leukemia (LGL; n = 1), and myelofibrosis (Myel; n = 1). Data are summarized in Table 1. The pretransplantation conditioning regimens consisted of Bu/Cy (n = 10), TBI/Cy (n = 3), Flu/TBI (n = 2), Flu/Mel (n = 1), or Flu/AraC/Cy (n = 2). The posttransplantation GVHD prophylaxis consisted of tacrolimus (Tac)/methotrexate (MTX; n = 12); cyclosporine (CSA)/mycophenolate mofotil (MMF; n = 2); CSA/MTX (n = 1) plus CD4 peptide (n = 1); or CSA/MMF/Tac (n = 1). Eight patients developed acute GVHD (stage II-IV), 3 of whom also developed chronic GVHD. An additional 7 patients developed only chronic GVHD, and 3 patients had no clinical manifestations of GVHD (Table 1). Furthermore, 1 patient with relapsing disease received a DLI at 193 days after transplantation. The majority of the patients who developed GVHD were treated with Tac and/or prednisone, and the times of PBL sampling after transplantation are as indicated (Table 1). Most of the patients (13 of 18) had at least 1 PBL collection obtained during the posttransplantation period of less than 100 days.
Patient characteristics
| Patient no./sex/age . | Disease . | GVHD prophylaxis . | GVHD staging (onset day) . | GVHD treatment . | Days after transplantation of sample collection(s) . |
|---|---|---|---|---|---|
| 1/M/46 | ALL | T/M | a/cGVHD (44/151) | P/MSC/T | 53, 98, 190 |
| 2/F/42 | ALL | C/M | aGVHD(116) | C/T/P/MF/AT | 116 |
| 3/F/48 | AML | T/M | aGVHD (35) | T/P | 35, 72 |
| 4/F/46 | AML | T/M | a/cGVHD(18/231) | P | 125, 231, 348, 528 |
| 5/M/44 | CML | T/M | cGVHD(227) | T/P | 64, 227, 362, 721 |
| 6/F/56 | AML | T/M | No GVHD | NA | 90 |
| 7/F/53 | LGL | T/M | No GVHD | NA | 28, 92, 185, 368 |
| 8/F/49 | Myel | T/M | cGVHD (120) | P | 338, 437 |
| 9/F/69 | MDS | C/MF | cGVHD (187) | P | 94, 200, 340 |
| 10/F/53 | NHL | C/MF | cGVHD (158) | P | 157, 271 |
| 11/F/55 | AML | T/M | cGVHD (120) | P/T | 34, 84, 153 |
| 12/F/47 | AML | T/M | aGVHD (64) | T/P | 99, 175 |
| 13/F/50 | CML | T/M | No GVHD; relapse | NA | 36, 180, 193, 344, 513 |
| 14/F/41 | MDS | T/M | cGVHD(230) | P/T/PH | 46, 79, 178, 228 |
| 15/F/57 | CLL | C/MF | aGVHD (18) | P/C/MF | 37 |
| 16/F/30 | T-ALL | C/M/4 | aGVHD (11) | C/M/MF/AT/OK/R/Z | 58 |
| 17/M/53 | MDS | T/M | a/cGVHD (79/153) | P/T | 25, 79, 166, 362 |
| 18/M/40 | NHL | C/MF | cGVHD (171) | C/MF/P/T | 105 |
| Patient no./sex/age . | Disease . | GVHD prophylaxis . | GVHD staging (onset day) . | GVHD treatment . | Days after transplantation of sample collection(s) . |
|---|---|---|---|---|---|
| 1/M/46 | ALL | T/M | a/cGVHD (44/151) | P/MSC/T | 53, 98, 190 |
| 2/F/42 | ALL | C/M | aGVHD(116) | C/T/P/MF/AT | 116 |
| 3/F/48 | AML | T/M | aGVHD (35) | T/P | 35, 72 |
| 4/F/46 | AML | T/M | a/cGVHD(18/231) | P | 125, 231, 348, 528 |
| 5/M/44 | CML | T/M | cGVHD(227) | T/P | 64, 227, 362, 721 |
| 6/F/56 | AML | T/M | No GVHD | NA | 90 |
| 7/F/53 | LGL | T/M | No GVHD | NA | 28, 92, 185, 368 |
| 8/F/49 | Myel | T/M | cGVHD (120) | P | 338, 437 |
| 9/F/69 | MDS | C/MF | cGVHD (187) | P | 94, 200, 340 |
| 10/F/53 | NHL | C/MF | cGVHD (158) | P | 157, 271 |
| 11/F/55 | AML | T/M | cGVHD (120) | P/T | 34, 84, 153 |
| 12/F/47 | AML | T/M | aGVHD (64) | T/P | 99, 175 |
| 13/F/50 | CML | T/M | No GVHD; relapse | NA | 36, 180, 193, 344, 513 |
| 14/F/41 | MDS | T/M | cGVHD(230) | P/T/PH | 46, 79, 178, 228 |
| 15/F/57 | CLL | C/MF | aGVHD (18) | P/C/MF | 37 |
| 16/F/30 | T-ALL | C/M/4 | aGVHD (11) | C/M/MF/AT/OK/R/Z | 58 |
| 17/M/53 | MDS | T/M | a/cGVHD (79/153) | P/T | 25, 79, 166, 362 |
| 18/M/40 | NHL | C/MF | cGVHD (171) | C/MF/P/T | 105 |
C indicates cyclosporine; M, methotrexate; T, tacrolimus; 4, CD4 peptide; MF, mycophenolate mofotil; AT, antithymocyte globulin; OK, OKT3 anti-CD3 antibody; R, rapamycin; Z, zenapax; P, prednisone; MSC, mesenchymal stem cells; aGVHD, acute GVHD; cGVHD, chronic GVHD; and PH, photopheresis.
Flow cytometry
Flow cytometric analysis was conducted to examine lymphocyte reconstitution using 2-color (FITC and PE) immunophenotyping. The proportions CD3+CD4+ helper T cells, CD4+CD45RA+ naive and CD4+CD45RO+ memory helper T cells, CD3+CD8+ cytotoxic T cells, and CD8+CD45RO+ naive and CD8+CD45RO+ memory cytotoxic T cells, as well as the CD19+ B-cell subsets were analyzed (Table 2). Reported percentages were derived from lymphocyte gating based on forward and side scattering. The data correspond to the spectratype time point indicated. Among the studied patients, cytotoxic T cells ranged between 10.4% and 48.9%. Memory and naive T cells within the cytotoxic T-cell population ranged between 36.2% and 85.9% and 17.3% and 76.2%, respectively. Helper T cells ranged between 4.6% and 35.7%, memory compartment ranging between 57.1% and 77.9% and naive T cells ranging between 23.8% and 46.2%. As expected, 75% of the patients presented an inverted CD4/CD8 ratio (0.47).
Phenotypic analysis of reconstituted lymphocyte populations
| Patient no. (days after transplantation) . | 1 (190) . | 5 (721) . | 6 (90) . | 7 (92) . | 8 (338) . | 9 (200) . | 10 (271) . | 12 (175) . | 13 (180) . | 17 (79) . |
|---|---|---|---|---|---|---|---|---|---|---|
| %CD3+CD4+ | 10.3 | 35.7 | 32.1 | 18.6 | 12.3 | 4.6 | 7.0 | 15.0 | 14.1 | 16.8 |
| %CD3+CD8+ | 24.4 | 10.4 | 17.1 | 48.9 | 63.4 | 16.0 | 27.5 | 43.6 | 27.5 | 46.5 |
| %CD19+ | 34.5 | 27.8 | 6.6 | 2.8 | 11.2 | 6.7 | 6.6 | 21.1 | 4.0 | 3.7 |
| %CD4+CD45RO+ | 66.8 | 74.8 | 63.9 | 76.2 | ND | 57.1 | ND | 76.6 | 77.9 | ND |
| %CD8+CD45RO+ | 85.9 | 48.6 | 36.2 | 44.8 | ND | 50.0 | ND | 38.4 | 64.2 | ND |
| %CD4+CD45RA+ | 33.2 | 22.9 | 39.9 | 23.8 | ND | 46.2 | ND | 30.5 | 37.6 | ND |
| %CD8+CD45RA+ | 17.3 | 50.8 | 74.1 | 52.9 | ND | 45.7 | ND | 76.2 | 35.8 | ND |
| Patient no. (days after transplantation) . | 1 (190) . | 5 (721) . | 6 (90) . | 7 (92) . | 8 (338) . | 9 (200) . | 10 (271) . | 12 (175) . | 13 (180) . | 17 (79) . |
|---|---|---|---|---|---|---|---|---|---|---|
| %CD3+CD4+ | 10.3 | 35.7 | 32.1 | 18.6 | 12.3 | 4.6 | 7.0 | 15.0 | 14.1 | 16.8 |
| %CD3+CD8+ | 24.4 | 10.4 | 17.1 | 48.9 | 63.4 | 16.0 | 27.5 | 43.6 | 27.5 | 46.5 |
| %CD19+ | 34.5 | 27.8 | 6.6 | 2.8 | 11.2 | 6.7 | 6.6 | 21.1 | 4.0 | 3.7 |
| %CD4+CD45RO+ | 66.8 | 74.8 | 63.9 | 76.2 | ND | 57.1 | ND | 76.6 | 77.9 | ND |
| %CD8+CD45RO+ | 85.9 | 48.6 | 36.2 | 44.8 | ND | 50.0 | ND | 38.4 | 64.2 | ND |
| %CD4+CD45RA+ | 33.2 | 22.9 | 39.9 | 23.8 | ND | 46.2 | ND | 30.5 | 37.6 | ND |
| %CD8+CD45RA+ | 17.3 | 50.8 | 74.1 | 52.9 | ND | 45.7 | ND | 76.2 | 35.8 | ND |
The data refer to percentages of total gated lymphocytes.
ND indicates not determined.
Comparative analysis of the responding T-cell repertoires in the MLC and transplant recipients
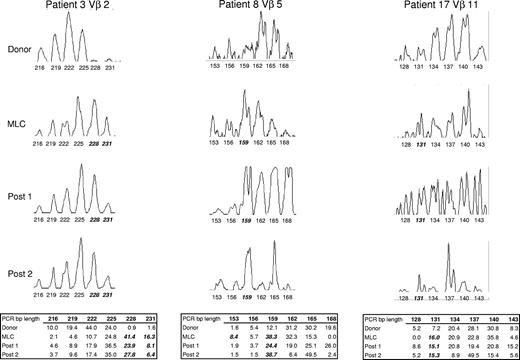
To determine the predictive value of Vβ use in the MLC, CDR3-size spectratype analysis was used to compare these alloreactive T-cell responses to those of the reconstituting repertoire of the patients receiving transplants. To this end, posttransplantation peripheral blood samples were collected at various time points (Table 1), PBLs were enriched by centrifugation over Ficoll-Paque-Plus, and RNA was isolated for the spectratype analysis. An example of the results obtained from a representative Vβ family from 3 donor/patient pairs can be seen in Figure 1. Spectratype histograms from the donors of patients 2, 8, and 17 for Vβ 2, 5, and 11, respectively, demonstrated a Gaussian-like distribution for the typical number of 6 to 8 peaks, indicative of the various CDR3-size lengths that one would expect in a broad complex repertoire that is not reacting to antigenic stimulation. In contrast, Vβ family skewing (bolded, italicized PCR lengths under peaks in Figure 1), as seen in the histograms of the MLC and posttransplantation samples, indicates clonal or oligoclonal expansion of specific T cells to antigen, that is, alloantigen, at least in the case of the MLC analysis and most likely in the patient samples, as well.
Representative CDR3-size spectratype analysis of a Vβ family from 3 donor/patient pairs. Spectratype histograms were generated from donor PBLs, the MLC, and 2 posttransplantation samples for each Vβ shown. The area under each peak matching the PCR basepair (bp) product length is determined by the software program as described in “CDR3-size spectratype analysis” and is indicated in the table as a percentage of the total histogram. A peak within a spectratype is compared with its corresponding peak in the donor sample and is considered to be skewed (indicated in bold italics) if the percentage area is at least 2-fold that of the donor.
Representative CDR3-size spectratype analysis of a Vβ family from 3 donor/patient pairs. Spectratype histograms were generated from donor PBLs, the MLC, and 2 posttransplantation samples for each Vβ shown. The area under each peak matching the PCR basepair (bp) product length is determined by the software program as described in “CDR3-size spectratype analysis” and is indicated in the table as a percentage of the total histogram. A peak within a spectratype is compared with its corresponding peak in the donor sample and is considered to be skewed (indicated in bold italics) if the percentage area is at least 2-fold that of the donor.
In a similar manner, the heterogeneity of 21 T-cell Vβ families was examined for the 18 donor/patient pairs and their respective MLCs. The spectratype histograms of the donor sample were used as the baseline for all comparisons of the individual peaks within a Vβ family. A spectratype histogram for a Vβ family was considered to be predictive if the same peak was skewed in both the MLC and in most (≥ 50%) of the patient's posttransplantation samples or if there was no skewing in either histogram. The results for the resolvable Vβ families, summarized in Table 3, indicated that, overall, 67.5% (± 2.1%; range, 40%-77%; median 69%) of the in vitro antihost T-cell responses were predictive of those in the patient posttransplantation. Of the 32.5% nonpredictive Vβ families, 7.8% (± 1.6%; range, 0%-18%; median 8%) exhibited skewing in the MLC but no skewing in the patient posttransplantation repertoire, 12.2% (± 1.6%; range, 0%-25.0%; median 12.5%) exhibited skewing in different peaks within the same Vβ family, and 12.9% (± 2.5%; range, 0%-40%; median 12%) showed skewing in the patient posttransplantation and none in the MLCs. Taken together, these results suggested that in vitro MLC is highly predictive of the patient posttransplantation T-cell responses.
Spectratype analysis of the responding T-cell repertoires in the MLC and transplant recipients
| Vβ . | Patient no. . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | |
| 1 | − | + | NO | NO | + | + | + | NO | + | NO | + | ND | − | + | ND | ND | NO | + |
| 2 | ND | + | + | + | + | + | + | − | + | NO | − | + | + | NP | NO | + | + | + |
| 3 | ND | ND | + | ND | NO | + | − | − | + | + | + | + | − | + | + | NO | + | + |
| 4 | + | + | + | + | NP | + | ND | + | + | + | NO | NP | + | NO | + | ND | NO | NP |
| 5 | − | + | − | + | − | ND | NO | + | + | ND | + | + | + | NP | NP | ND | − | + |
| 6 | + | ND | NP | + | + | + | NP | NO | − | + | + | NO | + | + | ND | ND | − | + |
| 7 | ND | + | − | NO | + | + | + | ND | − | ND | + | NO | + | + | ND | + | − | ND |
| 8 | NO | NO | + | + | + | − | ND | − | + | ND | + | + | − | ND | + | + | + | + |
| 9 | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | NP | − | + |
| 11 | ND | + | NP | + | − | + | + | ND | − | + | + | + | + | + | + | ND | + | ND |
| 12 | + | ND | + | ND | + | NP | ND | − | ND | + | ND | NP | + | + | ND | ND | ND | NO |
| 13 | + | − | − | + | + | ND | + | + | + | + | + | + | − | + | + | + | ND | + |
| 14 | + | + | ND | ND | + | − | + | + | + | ND | ND | ND | + | + | ND | NO | + | + |
| 15 | NO | + | + | + | + | + | + | NO | + | + | − | + | + | + | − | ND | + | NO |
| 16 | NP | + | NP | − | + | + | NO | ND | + | + | ND | + | NO | NO | NP | + | + | + |
| 17 | + | + | + | NO | + | + | NP | + | − | ND | + | + | + | + | ND | + | + | NP |
| 18 | + | + | ND | NO | + | + | ND | ND | + | ND | NP | ND | ND | NO | ND | ND | NO | ND |
| 19 | + | NP | + | ND | + | + | + | − | + | ND | + | + | + | + | + | ND | + | NO |
| 21 | + | + | ND | + | + | + | ND | ND | + | ND | + | ND | ND | ND | ND | + | + | ND |
| 22 | ND | ND | ND | ND | + | NP | − | ND | + | ND | ND | ND | ND | + | ND | ND | + | NP |
| 23 | + | − | + | + | NP | − | + | + | NO | − | NO | + | + | NP | − | + | + | + |
| % + | 68.8 | 76.5 | 58.8 | 68.8 | 76.2 | 73.7 | 62.5 | 40.0 | 75.0 | 75.0 | 70.6 | 75.0 | 66.7 | 68.4 | 58.3 | 72.7 | 63.2 | 64.7 |
| % − | 12.5 | 11.8 | 17.6 | 6.3 | 9.5 | 15.8 | 12.5 | 40.0 | 20.0 | 8.3 | 11.8 | 0.0 | 27.8 | 0.0 | 16.7 | 0.0 | 21.1 | 0.0 |
| % NO | 18.8 | 5.9 | 5.9 | 25.0 | 4.8 | 0.0 | 12.5 | 20.0 | 5.0 | 16.7 | 11.8 | 12.5 | 5.6 | 15.8 | 8.3 | 18.2 | 15.8 | 17.6 |
| % NP | 6.3 | 5.9 | 17.6 | 0.0 | 9.5 | 10.5 | 12.5 | 0.0 | 0.0 | 0.0 | 5.9 | 12.5 | 0.0 | 15.8 | 16.7 | 9.1 | 0.0 | 17.6 |
| Vβ . | Patient no. . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | |
| 1 | − | + | NO | NO | + | + | + | NO | + | NO | + | ND | − | + | ND | ND | NO | + |
| 2 | ND | + | + | + | + | + | + | − | + | NO | − | + | + | NP | NO | + | + | + |
| 3 | ND | ND | + | ND | NO | + | − | − | + | + | + | + | − | + | + | NO | + | + |
| 4 | + | + | + | + | NP | + | ND | + | + | + | NO | NP | + | NO | + | ND | NO | NP |
| 5 | − | + | − | + | − | ND | NO | + | + | ND | + | + | + | NP | NP | ND | − | + |
| 6 | + | ND | NP | + | + | + | NP | NO | − | + | + | NO | + | + | ND | ND | − | + |
| 7 | ND | + | − | NO | + | + | + | ND | − | ND | + | NO | + | + | ND | + | − | ND |
| 8 | NO | NO | + | + | + | − | ND | − | + | ND | + | + | − | ND | + | + | + | + |
| 9 | + | + | + | + | + | + | + | − | + | + | + | + | − | + | + | NP | − | + |
| 11 | ND | + | NP | + | − | + | + | ND | − | + | + | + | + | + | + | ND | + | ND |
| 12 | + | ND | + | ND | + | NP | ND | − | ND | + | ND | NP | + | + | ND | ND | ND | NO |
| 13 | + | − | − | + | + | ND | + | + | + | + | + | + | − | + | + | + | ND | + |
| 14 | + | + | ND | ND | + | − | + | + | + | ND | ND | ND | + | + | ND | NO | + | + |
| 15 | NO | + | + | + | + | + | + | NO | + | + | − | + | + | + | − | ND | + | NO |
| 16 | NP | + | NP | − | + | + | NO | ND | + | + | ND | + | NO | NO | NP | + | + | + |
| 17 | + | + | + | NO | + | + | NP | + | − | ND | + | + | + | + | ND | + | + | NP |
| 18 | + | + | ND | NO | + | + | ND | ND | + | ND | NP | ND | ND | NO | ND | ND | NO | ND |
| 19 | + | NP | + | ND | + | + | + | − | + | ND | + | + | + | + | + | ND | + | NO |
| 21 | + | + | ND | + | + | + | ND | ND | + | ND | + | ND | ND | ND | ND | + | + | ND |
| 22 | ND | ND | ND | ND | + | NP | − | ND | + | ND | ND | ND | ND | + | ND | ND | + | NP |
| 23 | + | − | + | + | NP | − | + | + | NO | − | NO | + | + | NP | − | + | + | + |
| % + | 68.8 | 76.5 | 58.8 | 68.8 | 76.2 | 73.7 | 62.5 | 40.0 | 75.0 | 75.0 | 70.6 | 75.0 | 66.7 | 68.4 | 58.3 | 72.7 | 63.2 | 64.7 |
| % − | 12.5 | 11.8 | 17.6 | 6.3 | 9.5 | 15.8 | 12.5 | 40.0 | 20.0 | 8.3 | 11.8 | 0.0 | 27.8 | 0.0 | 16.7 | 0.0 | 21.1 | 0.0 |
| % NO | 18.8 | 5.9 | 5.9 | 25.0 | 4.8 | 0.0 | 12.5 | 20.0 | 5.0 | 16.7 | 11.8 | 12.5 | 5.6 | 15.8 | 8.3 | 18.2 | 15.8 | 17.6 |
| % NP | 6.3 | 5.9 | 17.6 | 0.0 | 9.5 | 10.5 | 12.5 | 0.0 | 0.0 | 0.0 | 5.9 | 12.5 | 0.0 | 15.8 | 16.7 | 9.1 | 0.0 | 17.6 |
+ indicates MLC and post both skewed in same peak, or neither skewed; −, MLC not skewed and post sample skewed; NO, no overlapping skewing (MLC and post sample skewed in different peak); NP, not predictive (MLC skewed and post sample not skewed); and ND, not determined.
Given that skewing within a Vβ family represents expansion of reactive T cells and, in the case of the MLCs, would be indicative of allospecific responses, we next determined the percentage of overlapping skewing for each peak in the MLC Vβ family and its respective peak in the patient posttransplantation repertoire. Overall, a Vβ family was considered to be skewed in the patient's posttransplantation samples if biased CDR3-size skewing was observed in most (≥ 50%) samples. In cases where there was not a clear indication of majority outcome for the posttransplantation samples, the determination of skewing was based on the last obtained patient sample. The results, summarized in Table 4, indicated that, overall, there was 65.2% (± 2.8%; range, 50%-80%; median 68%) overlapping skewing for the resolvable Vβ families between the T-cell responses in the MLC and the patients' posttransplantation.
Overlapping skewing between the T-cell repertoires of the MLC and the posttransplantation patient samples
| Vβ . | Patient no. . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | |
| 1 | −/+ | +/+ | NO | NO | +/+ | −/− | −/− | NO | +/+ | NO | −/− | ND | −/+ | −/− | ND | ND | NO | +/+ |
| 2 | ND | −/− | +/+ | +/+ | +/+ | −/− | −/− | −/+ | −/− | NO | −/+ | +/+ | −/− | +/− | NO | +/+ | −/− | +/+ |
| 3 | ND | ND | +/+ | ND | NO | −/− | −/+ | −/+ | −/− | +/+ | −/− | +/+ | −/+ | −/− | +/+ | NO | −/− | +/+ |
| 4 | −/− | −/− | −/− | +/+ | +/+ | −/− | ND | −/− | −/− | +/+ | NO | +/− | −/− | NO | −/− | ND | NO | +/− |
| 5 | −/+ | +/+ | −/+ | +/+ | −/+ | ND | NO | +/+ | −/− | ND | +/+ | +/+ | +/+ | +/− | +/− | ND | −/+ | −/− |
| 6 | +/+ | ND | +/− | +/+ | −/− | −/− | +/− | NO | −/+ | +/+ | +/+ | NO | +/+ | +/+ | ND | ND | −/+ | +/+ |
| 7 | ND | −/− | −/+ | NO | −/− | +/+ | −/− | ND | −/+ | ND | +/+ | NO | −/− | +/+ | ND | −/− | −/+ | ND |
| 8 | NO | NO | +/+ | +/+ | −/− | −/+ | ND | −/+ | −/− | ND | +/+ | +/+ | −/+ | ND | −/− | −/− | +/+ | −/− |
| 9 | +/+ | −/− | +/+ | +/+ | −/− | −/− | −/− | −/+ | −/− | +/+ | +/+ | −/− | −/+ | −/− | +/+ | +/− | −/+ | +/+ |
| 11 | ND | +/+ | +/− | +/+ | −/+ | −/− | +/+ | ND | −/+ | +/+ | −/− | +/+ | +/+ | −/− | +/+ | ND | +/+ | ND |
| 12 | +/+ | ND | +/+ | ND | +/+ | +/− | ND | −/+ | ND | +/+ | ND | +/− | −/− | −/− | ND | ND | ND | ND |
| 13 | −/− | −/+ | −/+ | −/− | −/− | ND | +/+ | −/− | +/+ | −/− | −/− | −/− | −/+ | −/− | −/− | −/− | ND | −/− |
| 14 | −/− | +/+ | ND | ND | −/− | −/+ | −/− | −/− | +/+ | ND | ND | ND | +/+ | −/− | ND | NO | +/+ | +/+ |
| 15 | NO | +/+ | +/+ | +/+ | −/− | −/− | +/+ | NO | −/− | +/+ | −/+ | +/+ | −/− | +/+ | −/+ | ND | −/− | NO |
| 16 | +/− | −/− | +/− | −/− | −/− | −/− | NO | ND | −/− | +/+ | ND | +/+ | NO | NO | +/− | +/+ | −/− | +/+ |
| 17 | −/− | +/+ | +/+ | NO | −/− | −/− | +/− | +/+ | −/− | ND | +/+ | −/− | −/− | −/− | ND | +/+ | −/− | +/− |
| 18 | +/+ | +/+ | ND | NO | −/− | −/− | ND | ND | −/− | ND | +/− | ND | ND | NO | ND | ND | NO | ND |
| 19 | +/+ | +/− | +/+ | ND | −/− | −/− | +/+ | −/+ | −/− | ND | −/− | +/+ | +/+ | +/+ | +/+ | ND | +/+ | NO |
| 21 | +/+ | +/+ | ND | +/+ | +/+ | +/+ | ND | ND | +/+ | ND | −/− | ND | ND | ND | ND | −/− | −/− | ND |
| 22 | ND | ND | ND | ND | −/− | +/− | −/+ | ND | −/− | ND | ND | ND | ND | −/− | ND | ND | −/− | +/− |
| 23 | +/+ | −/+ | −/− | +/+ | +/− | −/+ | −/− | +/+ | NO | −/+ | NO | +/+ | −/− | +/+ | −/+ | −/− | +/+ | +/+ |
| % | 70 | 80 | 67 | 71 | 71 | 50 | 50 | 50 | 80 | 80 | 67 | 69 | 83 | 50 | 57 | 50 | 71 | 57 |
| Vβ . | Patient no. . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | |
| 1 | −/+ | +/+ | NO | NO | +/+ | −/− | −/− | NO | +/+ | NO | −/− | ND | −/+ | −/− | ND | ND | NO | +/+ |
| 2 | ND | −/− | +/+ | +/+ | +/+ | −/− | −/− | −/+ | −/− | NO | −/+ | +/+ | −/− | +/− | NO | +/+ | −/− | +/+ |
| 3 | ND | ND | +/+ | ND | NO | −/− | −/+ | −/+ | −/− | +/+ | −/− | +/+ | −/+ | −/− | +/+ | NO | −/− | +/+ |
| 4 | −/− | −/− | −/− | +/+ | +/+ | −/− | ND | −/− | −/− | +/+ | NO | +/− | −/− | NO | −/− | ND | NO | +/− |
| 5 | −/+ | +/+ | −/+ | +/+ | −/+ | ND | NO | +/+ | −/− | ND | +/+ | +/+ | +/+ | +/− | +/− | ND | −/+ | −/− |
| 6 | +/+ | ND | +/− | +/+ | −/− | −/− | +/− | NO | −/+ | +/+ | +/+ | NO | +/+ | +/+ | ND | ND | −/+ | +/+ |
| 7 | ND | −/− | −/+ | NO | −/− | +/+ | −/− | ND | −/+ | ND | +/+ | NO | −/− | +/+ | ND | −/− | −/+ | ND |
| 8 | NO | NO | +/+ | +/+ | −/− | −/+ | ND | −/+ | −/− | ND | +/+ | +/+ | −/+ | ND | −/− | −/− | +/+ | −/− |
| 9 | +/+ | −/− | +/+ | +/+ | −/− | −/− | −/− | −/+ | −/− | +/+ | +/+ | −/− | −/+ | −/− | +/+ | +/− | −/+ | +/+ |
| 11 | ND | +/+ | +/− | +/+ | −/+ | −/− | +/+ | ND | −/+ | +/+ | −/− | +/+ | +/+ | −/− | +/+ | ND | +/+ | ND |
| 12 | +/+ | ND | +/+ | ND | +/+ | +/− | ND | −/+ | ND | +/+ | ND | +/− | −/− | −/− | ND | ND | ND | ND |
| 13 | −/− | −/+ | −/+ | −/− | −/− | ND | +/+ | −/− | +/+ | −/− | −/− | −/− | −/+ | −/− | −/− | −/− | ND | −/− |
| 14 | −/− | +/+ | ND | ND | −/− | −/+ | −/− | −/− | +/+ | ND | ND | ND | +/+ | −/− | ND | NO | +/+ | +/+ |
| 15 | NO | +/+ | +/+ | +/+ | −/− | −/− | +/+ | NO | −/− | +/+ | −/+ | +/+ | −/− | +/+ | −/+ | ND | −/− | NO |
| 16 | +/− | −/− | +/− | −/− | −/− | −/− | NO | ND | −/− | +/+ | ND | +/+ | NO | NO | +/− | +/+ | −/− | +/+ |
| 17 | −/− | +/+ | +/+ | NO | −/− | −/− | +/− | +/+ | −/− | ND | +/+ | −/− | −/− | −/− | ND | +/+ | −/− | +/− |
| 18 | +/+ | +/+ | ND | NO | −/− | −/− | ND | ND | −/− | ND | +/− | ND | ND | NO | ND | ND | NO | ND |
| 19 | +/+ | +/− | +/+ | ND | −/− | −/− | +/+ | −/+ | −/− | ND | −/− | +/+ | +/+ | +/+ | +/+ | ND | +/+ | NO |
| 21 | +/+ | +/+ | ND | +/+ | +/+ | +/+ | ND | ND | +/+ | ND | −/− | ND | ND | ND | ND | −/− | −/− | ND |
| 22 | ND | ND | ND | ND | −/− | +/− | −/+ | ND | −/− | ND | ND | ND | ND | −/− | ND | ND | −/− | +/− |
| 23 | +/+ | −/+ | −/− | +/+ | +/− | −/+ | −/− | +/+ | NO | −/+ | NO | +/+ | −/− | +/+ | −/+ | −/− | +/+ | +/+ |
| % | 70 | 80 | 67 | 71 | 71 | 50 | 50 | 50 | 80 | 80 | 67 | 69 | 83 | 50 | 57 | 50 | 71 | 57 |
% indicates number of overlapping skewed Vβ families in the MLC and posttransplantation samples divided by total skewed MLC; +, overlapping skewed spectratype; −, no skewing; NO, no overlapping skewing (MLC and post sample skewed in different peak); and ND, not determined.
To further examine the antihost specificity of the responses elicited in vitro, additional MLCs were also generated from 2 donors against stimulator PBLs from a third-party donor. The results exhibited much less overlapping skewing (29% and 20% for donor/patient pairs 15 and 18, respectively) between the donor anti–third-party MLC responses and those seen in the patient posttransplantation (Table 5). When comparing the spectratype skewing of the donor antihost to donor anti–third-party MLC responses, overlaps of 28% and 7% were observed for donor/patient pairs 15 and 18, respectively. Taken together, these findings are consistent with the likelihood that the observed overlapping skewings in the patient posttransplantation are also reflective of alloreactive responses that could potentially be associated with the development of GVHD. Again, it should be noted that of the 18 patients after trans-plantation, 15 (83%) of them developed either acute or chronic GVHD.
Overlapping skewing between the T-cell repertoires of the donor anti–third-party MLC and the posttransplantation patient samples
| Vβ . | Patient no. . | |
|---|---|---|
| 15 . | 18 . | |
| 1 | ND | NO |
| 2 | −/+ | −/+ |
| 3 | NO | NO |
| 4 | +/− | −/− |
| 5 | NO | +/− |
| 6 | ND | NO |
| 7 | ND | ND |
| 8 | −/− | +/− |
| 9 | −/+ | +/+ |
| 11 | +/+ | ND |
| 12 | ND | ND |
| 13 | −/− | −/− |
| 14 | ND | −/+ |
| 15 | +/+ | +/+ |
| 16 | −/− | NO |
| 17 | ND | NO |
| 18 | ND | ND |
| 19 | NO | −/+ |
| 21 | ND | ND |
| 22 | ND | −/− |
| 23 | NO | NO |
| % | 29 | 20 |
| Vβ . | Patient no. . | |
|---|---|---|
| 15 . | 18 . | |
| 1 | ND | NO |
| 2 | −/+ | −/+ |
| 3 | NO | NO |
| 4 | +/− | −/− |
| 5 | NO | +/− |
| 6 | ND | NO |
| 7 | ND | ND |
| 8 | −/− | +/− |
| 9 | −/+ | +/+ |
| 11 | +/+ | ND |
| 12 | ND | ND |
| 13 | −/− | −/− |
| 14 | ND | −/+ |
| 15 | +/+ | +/+ |
| 16 | −/− | NO |
| 17 | ND | NO |
| 18 | ND | ND |
| 19 | NO | −/+ |
| 21 | ND | ND |
| 22 | ND | −/− |
| 23 | NO | NO |
| % | 29 | 20 |
% indicates number of overlapping skewed Vβ families in the donor anti–third- party MLC and posttransplantation samples divided by total skewed anti–third-party MLC; +, skewed spectratype; −, no skewing; NO, no overlapping skewing (anti–third-party MLC and post sample skewed in different peak); and ND, not determined.
Time series analysis of the responding T-cell repertoires in the transplant recipients
Noting that the reconstitution of the T-cell repertoire posttransplantation is a dynamic and evolving process, we extended our studies to look at the CDR3-size usage in the T-cell repertoire of patients over several months. Vβ family spectratype analysis of additionally obtained posttransplantation peripheral blood samples from 13 of the patients was used to to examine the stability of the overlapping repertoires. Stability was defined as the observation of overlapping spectratypes (skewed or not skewed) at 2 consecutive time points, in cases with at least 2 consecutive time points with resolvable spectratypes. The results demonstrated that even over the course of several months, overall 81.8% (± 4.0%; range, 57%-100%; median 80%) of the evaluable Vβ families remained consistently overlapping (Table 6). These findings are highly supportive of the hypothesis that the in vitro responses are reflective of the in vivo responses.
Time series overlapping spectratype analysis of T-cell repertoires of the MLC and the posttransplantation patient samples
| Vβ . | Patient no. . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 3 . | 4 . | 5 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 17 . | |
| 1 | −/−/−* | −/− | −/N/−/−* | +/+/N/− | +/+/+/− | −/− | +/+/+ | −/N | +/+/+ | N | −/−/−/N/−* | +/+/+/N | −/−/−/−* |
| 2 | N | +/+ | +/−/−/+ | +/+/N/− | +/+/−/+ | −/−* | +/+/+ | −/N | −/N/− | +/+ | +/N/N/−/+ | −/−/−/− | +/−/+/+ |
| 3 | N | N/+ | N | N/+/−/N | −/−/−/−* | −/−* | +/+/− | +/+ | +/−/+ | +/+ | −/−/−/−/+* | −/+/+/+ | +/−/+/+ |
| 4 | +/+/+ | −/+ | +/+/+/+ | −/+/−/N | N | N/+ | +/+/N | +/+ | −/N/− | −/N | +/N/+/N/N | −/−/−/N | −/N/−/N |
| 5 | −/−/+ | N/− | +/−/+/+ | +/−/N/N | −/−/−/−* | +/+ | −/+/+ | N* | +/+/+ | +/+ | +/−/+/+/+ | −/−/−/− | −/−/−/−* |
| 6 | +/+/+ | N/− | +/+/+/− | +/+/+/+ | −/−/−/+ | −/−* | −/N/N | +/N | +/+/N | −/−* | +/N+/− | −/+/+/+ | −/−/+/−* |
| 7 | N | −/−* | N/−/−/−* | N/N/+/+ | +/+/+/− | N | −/−/−* | N | +/N/+ | −/N | +/+/+/ND/− | +/+/+/N | −/−/N/−* |
| 8 | −/−/−/* | +/+ | +/+/+/+ | N/−/+/N | N | +/− | +/+/+ | N | −/+/+ | +/+ | +/−/−/−/−* | N | +/+/+/+ |
| 9 | +/+/+ | +/+ | +/+/+/+ | N/N/−/+ | +/+/+/+ | −/− | +/+/+ | +/N | +/+/+ | +/+ | −/−/−/−/−* | +/+/+/+ | −/−/−/−* |
| 11 | N | N/− | N/+/+/+ | N/−/−/−* | +/+/+/+ | N | −/−/−* | −/+ | −/+/N | +/+ | N/N/+/N/+ | +/−/+/−* | +/+/N/− |
| 12 | N/+/N | N/+ | N | +/+/+/N | N | −/− | N | +/+ | N | N/− | +/N/+/N/+ | N/N/+/N | ND |
| 13 | +/+/+ | +/− | +/+/−/+ | +/+/+/N | −/+/+/N | +/+ | −/+/+ | N/+ | N/+/+ | +/+ | −/N/−/N/N* | N/+/+/+ | ND |
| 14 | +/+/+ | N | N | N/+/N/+ | +/−/−/+ | N/+ | +/+/+ | N | N | N | +/N/+/+/N | +/+/+/+ | N/+/+/+ |
| 15 | −/−/− | N/+ | +/+/+/+ | N/+/+/+ | +/+/+/+ | −/− | +/+/+ | N/+ | +/−/−* | +/+ | +/N/+/N/N | −/N/+/+ | +/+/+/+ |
| 16 | −/+/− | −/− | +/−/+/− | +/+/+/+ | −/−/−/−/* | N | +/+/N | +/+ | N | +/+ | −/−/−/−/−* | −/−/−/−* | +/+/+/− |
| 17 | −/+/+ | −/+ | N/−/−/− | N/+/−/+ | −/−/−/− | −/+ | +/−/−* | N | +/+/N | +/+ | +/−/+/−/+* | +/+/+/− | −/+/+/+ |
| 18 | N/N/+ | N | N/N/−/N | N/+/N/+ | N | N | +/+/− | N | −/N/− | N | N | +/−/−/N | −/−/−/−* |
| 19 | +/+/−* | −/+ | N | −/+/N/+ | +/N/+/+ | +/− | +/+/+ | N | +/+/+ | +/+ | −/N/+/+/N | −/+/−/+ | −/+/+/+ |
| 21 | +/+/N | N | N/+/+/+ | N/N/+/N | N | N | +/+/+ | N | N/N/+ | N | N | N− | N/N/+/N |
| 22 | N | N | N | N/+/N/N | N/N/−/N | N | +/+/− | N | N | N | N | +/N−+/+ | +/+/+/− |
| 23 | +/+/+ | +/+ | +/+/+/+ | N/N/−/N | +/−/−/+ | +/+ | +/−/− | −/N | N/−/− | +/+ | −/N/+/+/+ | −/+/−/− | +/+/−/+ |
| % | 89 | 57 | 91 | 67 | 80 | 75 | 100 | 80 | 80 | 100 | 63 | 83 | 100 |
| Vβ . | Patient no. . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 3 . | 4 . | 5 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 17 . | |
| 1 | −/−/−* | −/− | −/N/−/−* | +/+/N/− | +/+/+/− | −/− | +/+/+ | −/N | +/+/+ | N | −/−/−/N/−* | +/+/+/N | −/−/−/−* |
| 2 | N | +/+ | +/−/−/+ | +/+/N/− | +/+/−/+ | −/−* | +/+/+ | −/N | −/N/− | +/+ | +/N/N/−/+ | −/−/−/− | +/−/+/+ |
| 3 | N | N/+ | N | N/+/−/N | −/−/−/−* | −/−* | +/+/− | +/+ | +/−/+ | +/+ | −/−/−/−/+* | −/+/+/+ | +/−/+/+ |
| 4 | +/+/+ | −/+ | +/+/+/+ | −/+/−/N | N | N/+ | +/+/N | +/+ | −/N/− | −/N | +/N/+/N/N | −/−/−/N | −/N/−/N |
| 5 | −/−/+ | N/− | +/−/+/+ | +/−/N/N | −/−/−/−* | +/+ | −/+/+ | N* | +/+/+ | +/+ | +/−/+/+/+ | −/−/−/− | −/−/−/−* |
| 6 | +/+/+ | N/− | +/+/+/− | +/+/+/+ | −/−/−/+ | −/−* | −/N/N | +/N | +/+/N | −/−* | +/N+/− | −/+/+/+ | −/−/+/−* |
| 7 | N | −/−* | N/−/−/−* | N/N/+/+ | +/+/+/− | N | −/−/−* | N | +/N/+ | −/N | +/+/+/ND/− | +/+/+/N | −/−/N/−* |
| 8 | −/−/−/* | +/+ | +/+/+/+ | N/−/+/N | N | +/− | +/+/+ | N | −/+/+ | +/+ | +/−/−/−/−* | N | +/+/+/+ |
| 9 | +/+/+ | +/+ | +/+/+/+ | N/N/−/+ | +/+/+/+ | −/− | +/+/+ | +/N | +/+/+ | +/+ | −/−/−/−/−* | +/+/+/+ | −/−/−/−* |
| 11 | N | N/− | N/+/+/+ | N/−/−/−* | +/+/+/+ | N | −/−/−* | −/+ | −/+/N | +/+ | N/N/+/N/+ | +/−/+/−* | +/+/N/− |
| 12 | N/+/N | N/+ | N | +/+/+/N | N | −/− | N | +/+ | N | N/− | +/N/+/N/+ | N/N/+/N | ND |
| 13 | +/+/+ | +/− | +/+/−/+ | +/+/+/N | −/+/+/N | +/+ | −/+/+ | N/+ | N/+/+ | +/+ | −/N/−/N/N* | N/+/+/+ | ND |
| 14 | +/+/+ | N | N | N/+/N/+ | +/−/−/+ | N/+ | +/+/+ | N | N | N | +/N/+/+/N | +/+/+/+ | N/+/+/+ |
| 15 | −/−/− | N/+ | +/+/+/+ | N/+/+/+ | +/+/+/+ | −/− | +/+/+ | N/+ | +/−/−* | +/+ | +/N/+/N/N | −/N/+/+ | +/+/+/+ |
| 16 | −/+/− | −/− | +/−/+/− | +/+/+/+ | −/−/−/−/* | N | +/+/N | +/+ | N | +/+ | −/−/−/−/−* | −/−/−/−* | +/+/+/− |
| 17 | −/+/+ | −/+ | N/−/−/− | N/+/−/+ | −/−/−/− | −/+ | +/−/−* | N | +/+/N | +/+ | +/−/+/−/+* | +/+/+/− | −/+/+/+ |
| 18 | N/N/+ | N | N/N/−/N | N/+/N/+ | N | N | +/+/− | N | −/N/− | N | N | +/−/−/N | −/−/−/−* |
| 19 | +/+/−* | −/+ | N | −/+/N/+ | +/N/+/+ | +/− | +/+/+ | N | +/+/+ | +/+ | −/N/+/+/N | −/+/−/+ | −/+/+/+ |
| 21 | +/+/N | N | N/+/+/+ | N/N/+/N | N | N | +/+/+ | N | N/N/+ | N | N | N− | N/N/+/N |
| 22 | N | N | N | N/+/N/N | N/N/−/N | N | +/+/− | N | N | N | N | +/N−+/+ | +/+/+/− |
| 23 | +/+/+ | +/+ | +/+/+/+ | N/N/−/N | +/−/−/+ | +/+ | +/−/− | −/N | N/−/− | +/+ | −/N/+/+/+ | −/+/−/− | +/+/−/+ |
| % | 89 | 57 | 91 | 67 | 80 | 75 | 100 | 80 | 80 | 100 | 63 | 83 | 100 |
Posttransplantation time points are separated by a shill (/).
% indicates the overall percentage of time series overlapping Vβ family spectratypes between the MLC and patient posttransplantation sample; +, overlapping spectratype between the MLC and the posttransplantation sample; −, no overlap; and N, not determined
Multiple posttransplantation samples with overlapping skewing, not overlapping with the MLC.
Evaluation of risk
Risk, in this study, is defined as the depletion of a Vβ family that was reactive in the MLCs but not subsequently found to be expanded (skewed) in the patient after transplantation. The incidence of risk was evaluated in an effort to determine whether hypothetical manipulation of the donor inoculum to deplete alloreactive Vβ families, guided by the MLC spectratype analysis, would have resulted in a prevalence of unnecessary T-cell repertoire loss. The results of this analysis indicated that overall there was a 6.7% (± 1.3%) likelihood of risk occurring (Table 7), suggesting that the MLCs could be reliably used to manipulate the donor inoculum without causing undue risk.
Evaluation of risk
| Vβ . | Patient no. . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | |
| 1 | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | ND | − | − |
| 2 | ND | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| 3 | ND | ND | − | ND | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 4 | − | − | − | − | − | − | ND | − | − | − | − | + | − | − | − | ND | − | + |
| 5 | − | − | − | − | − | ND | − | − | − | ND | − | − | − | + | ND | ND | − | − |
| 6 | − | ND | + | − | − | − | + | − | − | − | − | − | − | − | − | ND | − | − |
| 7 | ND | − | − | − | − | − | − | ND | − | ND | − | − | − | − | ND | − | − | ND |
| 8 | − | − | − | − | − | − | ND | − | − | ND | − | − | − | ND | − | − | − | − |
| 9 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| 11 | ND | − | + | − | − | − | − | ND | − | − | − | − | − | − | − | ND | − | ND |
| 12 | − | ND | − | ND | − | + | ND | − | ND | − | ND | + | − | − | ND | ND | ND | − |
| 13 | − | − | − | − | − | ND | − | − | − | − | − | − | − | − | − | − | ND | − |
| 14 | − | − | ND | ND | − | − | − | − | − | ND | ND | ND | − | − | ND | − | − | − |
| 15 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − |
| 16 | + | − | + | − | − | − | − | ND | − | − | ND | − | − | − | + | − | − | − |
| 17 | − | − | − | − | − | − | + | − | − | ND | − | − | − | − | ND | − | − | − |
| 18 | − | − | ND | − | − | − | ND | ND | − | ND | + | ND | ND | − | ND | ND | − | ND |
| 19 | − | + | − | ND | − | − | − | − | − | ND | − | − | − | − | − | ND | − | + |
| 21 | − | − | ND | − | − | − | ND | ND | − | ND | − | ND | ND | ND | ND | − | − | ND |
| 22 | ND | ND | ND | ND | − | + | − | ND | − | ND | ND | ND | ND | − | ND | ND | − | + |
| 23 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Risk, % | 6 | 6 | 17 | 0 | 5 | 11 | 12 | 0 | 0 | 0 | 6 | 13 | 0 | 11 | 8 | 9 | 0 | 17 |
| Vβ . | Patient no. . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | |
| 1 | − | − | − | − | − | − | − | − | − | − | − | ND | − | − | − | ND | − | − |
| 2 | ND | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| 3 | ND | ND | − | ND | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 4 | − | − | − | − | − | − | ND | − | − | − | − | + | − | − | − | ND | − | + |
| 5 | − | − | − | − | − | ND | − | − | − | ND | − | − | − | + | ND | ND | − | − |
| 6 | − | ND | + | − | − | − | + | − | − | − | − | − | − | − | − | ND | − | − |
| 7 | ND | − | − | − | − | − | − | ND | − | ND | − | − | − | − | ND | − | − | ND |
| 8 | − | − | − | − | − | − | ND | − | − | ND | − | − | − | ND | − | − | − | − |
| 9 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| 11 | ND | − | + | − | − | − | − | ND | − | − | − | − | − | − | − | ND | − | ND |
| 12 | − | ND | − | ND | − | + | ND | − | ND | − | ND | + | − | − | ND | ND | ND | − |
| 13 | − | − | − | − | − | ND | − | − | − | − | − | − | − | − | − | − | ND | − |
| 14 | − | − | ND | ND | − | − | − | − | − | ND | ND | ND | − | − | ND | − | − | − |
| 15 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ND | − | − |
| 16 | + | − | + | − | − | − | − | ND | − | − | ND | − | − | − | + | − | − | − |
| 17 | − | − | − | − | − | − | + | − | − | ND | − | − | − | − | ND | − | − | − |
| 18 | − | − | ND | − | − | − | ND | ND | − | ND | + | ND | ND | − | ND | ND | − | ND |
| 19 | − | + | − | ND | − | − | − | − | − | ND | − | − | − | − | − | ND | − | + |
| 21 | − | − | ND | − | − | − | ND | ND | − | ND | − | ND | ND | ND | ND | − | − | ND |
| 22 | ND | ND | ND | ND | − | + | − | ND | − | ND | ND | ND | ND | − | ND | ND | − | + |
| 23 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Risk, % | 6 | 6 | 17 | 0 | 5 | 11 | 12 | 0 | 0 | 0 | 6 | 13 | 0 | 11 | 8 | 9 | 0 | 17 |
+ indicates risk as defined by the theoretical depletion of a Vβ family not expanded in the patient posttransplantation but expanded in the MLC; −, no risk, as defined by (1) no depletion of a Vβ family not expanded in MLC or (2) the depletion of a Vβ family expanded in the patient posttransplantation and the MLC; and ND, not determined.
Discussion
The ability to effectively implement the widespread use of allogeneic BMT in the clinical setting for the treatment of malignancies is grounded primarily in the separation of GVHD from GVT effects. The continued identification of specific tumor-associated antigens should certainly help to facilitate this goal. However, the ability to apply the knowledge gained from this process to the clinical situation will be impacted by the fact that antigen recognition by T cells is HLA-restricted and even single nucleotide polymorphisms (SNPs) can have a huge impact on T-cell responses. A modality that has the potential to permit a transplantation strategy to be individualized to specific donor-patient responses should lead to improvements in BMT. In this regard, the use of in vitro cultures combined with a TCR Vβ CDR3 use analysis system that could predict in vivo responses is a powerful tool.
Determining alloreactive T-cell Vβ families via the approach outlined above affords the advantage of not having to identify the specific antigens involved, nor is it dependent on any specific miHA disparity, such as HLA-1–restricted HA1 miHA.33 Here, we demonstrate that overall, 68% of the in vitro–derived resolvable Vβ families exhibit spectratypes overlapping with those of the in vivo Vβ families. This includes T-cell families that are responding to antigens as well as those that are not. Most significantly, even when we looked only at the alloreactive Vβ family skewings in the MLCs, we still observed 65% overlapping skewings with the posttransplantation samples. The importance of this overlap is underscored by the observation that similar MLCs generated against third-party stimulation from 2 donor samples only resulted in 29% and 20% overlapping skewing, less then half of the overlap observed between the donor antihost. MLCs and the posttransplantation samples from these specific donor/patient pairs. Of the other 32% nonmatching spectratypes, only 7.8% of the Vβ families exhibited T-cell responses in vitro that were completely absent in vivo. These responses could represent low-frequency responses that might be diluted in situ. This set of results represents the worst-case scenario with regard to manipulation of the donor inoculum, given that at the time of transplantation, manipulations would be designed to deplete alloreactive T-cell Vβ families based on the results of the MLC spectratype analysis, obviously without having the benefit of the retrospective posttransplantation analysis. Even under these circumstances, the potential for risk (7.8%) is relatively small and is outweighed by the 68% overlap. This factor also compares favorably with the relatively high risk of developing acute or chronic GVHD and/or leukemic relapse in patients receiving an unmanipulated transplant.43-46 Furthermore, in terms of the breadth of a T-cell response to an opportunistic infectious agent, most foreign proteins can elicit multiple T-cell specificities and thus would unlikely be dependent upon any single Vβ family response. So the real chance of unnecessary removal of any given Vβ family would be minimal.
The remaining 24% of nonoverlapping Vβ family skewings between the MLCs and the patient posttransplantation samples do not, a priori, represent a potential danger to the patient. Vβ family skewing in the patient sample after transplantation that was not observed in the MLC could be evidence of tumor-associated antigen–specific GVT responses. Equally as likely, these nonoverlapping skewings could represent responses to infectious agents. One way to further dissect these observed responses will be by coupling the analysis of the antihost responses with the complementary analysis of the antitumor responses. To this end, we have started to collect primary tumor samples from multiple myeloma and leukemia patients who are anticipated to undergo allogeneic BMT. We will use the Vβ spectratype approach to also analyze in vitro–generated tumor responses once the donors for these patients are identified and we obtain informed consent.
The time series repertoire analysis of the patient samples after transplantation is of great interest, demonstrating a high fidelity of overlap in the T-cell repertoire. The importance of this fidelity is underscored by the fact that overlap is being observed during the process of increased repertoire reconstitution. An argument could be made that the overlap and observed skewing at earlier time points was an artifact related to the small absolute numbers of T cells in the patient after transplantation. However, as the absolute numbers of T cells increased over several months' time, the overlap remained intact and the alloreactive skewing was consistent, arguing against the likelihood of these observations being related to the smaller T-cell numbers.
Another striking observation is that for several patients (patients 5, 9, 11, 14, and 17) the expansion (skewing) of overlapping Vβ families was evident before the clinical diagnosis of GVHD was determined. This suggested that, at a minimum, use of the combined Vβ spectratype analysis of the patient after transplantation and the in vitro culture system could be used to direct preemptive risk adapted therapy for the treatment of GVHD. Taken together, the results demonstrated a robust overlap between the in vitro MLC and the patient posttransplantation spectratype analyses. The ability to predict the involvement of particular T-cell Vβ families in the alloreactive responses potentially involved in the development of clinical GVHD, coupled with the similar analysis of the GVL-reactive T-cell repertoire could allow for manipulation of the donor cell inoculum in a patient-specific manner to optimize BMT outcomes.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Angela Bucciarelli for her input on the statistical considerations of this paper.
This work was supported in part by the National Institutes of Health (Bethesda, MD) RO1 HL075622 and an award from the Amy Strelzer Manasevit Research Program of the National Marrow Donor Program (Minneapolis, MN) to T.M.F.
National Institutes of Health
Authorship
Contribution: T.M.F. designed the research, analyzed the data, and wrote the manuscript; K.G., S.A.B., and J.Z. performed research and analyzed data; J.F.H., N.F., M.D., and S.D.R. contributed patient samples and data; and R.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thea M. Friedman, Cancer Center, Hackensack University Medical Center, 30 Prospect Avenue, Hackensack, NJ 07601; e-mail: tfriedman@humed.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal