CD40 has a rich history as a prominent immune cell activator.1 CD40 is best known as the key mediator of B lymphocyte immunoglobulin class switch. It also serves as a major activator of antigen-presenting cells, such as macrophages and dendritic cells. More recently, it was discovered that CD40 is expressed on many types of human cells including tissue structural cells, such as fibroblasts, epithelial cells, and endothelial cells.2,3 On these cells, like classic immune cells, CD40 also serves as a major mediator of cell activation. For example, on endothelial cells, engagement of CD40 incites the production of adhesion molecules, cytokines, chemokines, and inflammatory mediators important for wound healing.3,4
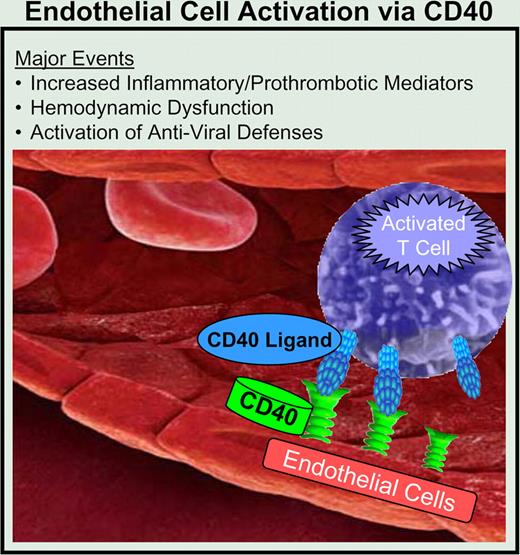
Interaction of activated T cells and endothelial cells via the CD40. CD40L pathway leads to endothelial cell activation. In addition to the known proinflammatory mediators induced by this pathway, many potential new targets were also identified. Also discovered were mediators of hemodynamic dysfunction and coordinated enhancement of innate antiviral defenses.
Interaction of activated T cells and endothelial cells via the CD40. CD40L pathway leads to endothelial cell activation. In addition to the known proinflammatory mediators induced by this pathway, many potential new targets were also identified. Also discovered were mediators of hemodynamic dysfunction and coordinated enhancement of innate antiviral defenses.
The natural ligand for CD40 is called CD40 ligand (CD40L; officially termed CD154) and can be expressed, after activation, on the surface of several cell types, including T lymphocytes and platelets.1,4,5 There is much interest in endothelial cell activation by CD40 ligand due to the potential consequences of this event, such as atherosclerotic progression, chronic inflammation, and organ rejection. Previously, blocking CD40 ligand was a therapeutic target in certain inflammatory diseases and in organ rejection as a pathway to inducing immune tolerance. However, due to human platelet expression of CD40L, thromboembolic events occurred in early clinical trials, setting back CD40L as an immune system target. However, these unanticipated side effects resulted in CD40 being vigorously pursued as a means to attenuate unwanted endothelial and other cell activation.
In this issue of Blood, Pluvinet et al focus on the down-stream events triggered by T-cell delivery of CD40L to several types of human endothelial cells. Two complementary approaches were used to find and validate the down-stream consequences of CD40 signaling in endothelial cells. First, they used a human T-cell line that highly expressed CD40L to activate CD40-bearing human endothelial cells. Using genome wide transcriptional profiling, they identified many new genes not thought to be regulated by the CD40-CD40L pathway. Some of these were further validated using other methods, such as Northern blotting, Western blotting, immunohistochemistry, and a preclinical animal model of organ rejection. A second strength of their approach was the use of RNAi to knock down expression of CD40 in endothelial cells, further proving the importance of CD40 as a master switch that sets in motion a host of coordinated events.
Their work on CD40 signaling revealed the rapid and global response of endothelial cells after activation by T cells, including coordinated kinase and transcription factor changes impacting on inflammation, cell motility, and vascular tone. Two major findings of particular interest are highlighted in their article. The first involves a vasoactive peptide called apelin,6 which was highly down-regulated in endothelial cells after CD40-CD40L interaction. Apelin appears to play an important role as a blood vessel dilator and may have cardioprotective effects. The authors also postulate that down-regulation of endothelial apelin in an inflamed transplanted kidney is a major factor in dysregulation of fluid homeostasis associated with renal transplant rejection. The second highlighted finding is that CD40-stimulated endothelial cells up-regulated genes involved in host defense against viruses. These genes include those involved in detecting viral RNA, such as TLR3, and in further activation of endothelial cells by up-regulating the IFNγ receptor. IFNγ binding to its receptor up-regulates CD40 expression, constituting an amplification pathway. Also of interest was the finding that certain proteins that permit viral entry were down-regulated by CD40. These include CXCR4, a chemokine receptor important for HIV entry into cells. These coordinated events appear to amplify innate immunity via the T-cell arm of adaptive immunity.
The work of Pluvinet et al provides much new information on human endothelial cell activation by CD40. Many of the genes and pathways identified herein require further validation. Moreover, endothelial cells are increasingly recognized as being diverse and differ from organ to organ and even within a single organ.7 Endothelial cell activation via delivery of CD40L from T cells may differ from other sources, such as platelets or microparticles. Therefore, it is important to discover the role of CD40L-bearing cells in different diseases. These unknowns aside, the door is open to tackling the new exciting targets of down-stream CD40 signaling in endothelial cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal