Thrombomodulin (TM), a widely expressing glycoprotein originally identified in vascular endothelium, is an important cofactor in the protein C anticoagulant system. TM appears to exhibit anti-inflammatory ability through both protein C–dependent and –independent pathways. We presently have demonstrated that recombinant N-terminal lectinlike domain of TM (rTMD1) functions as a protective agent against sepsis caused by Gram-negative bacterial infections. rTMD1 caused agglutination of Escherichia coli and Klebsiella pneumoniae and enhanced the macrophage phagocytosis of these Gram-negative bacteria. Moreover, rTMD1 bound to the Klebsiella pneumoniae and lipopolysaccharide (LPS) by specifically interacting with Lewis Y antigen. rTMD1 inhibited LPS-induced inflammatory mediator production via interference with CD14 and LPS binding. Furthermore, rTMD1 modulated LPS-induced mitogen-activated protein kinase and nuclear factor-κB signaling pathway activations and inducible nitric oxide synthase expression in macrophages. Administration of rTMD1 protected the host by suppressing inflammatory responses induced by LPS and Gram-negative bacteria, and enhanced LPS and bacterial clearance in sepsis. Thus, rTMD1 can be used to defend against bacterial infection and inhibit LPS-induced inflammatory responses, suggesting that rTMD1 may be valuable in the treatment of severe inflammation in sepsis, especially in Gram-negative bacterial infections.
Introduction
Septic shock syndrome resulting from excessive host immune responses induced by infectious organisms is a leading cause of death in hospitalized patients.1,-3 Pathophysiologic changes in sepsis involve the pathogen-induced uncontrolled release from immune cells, particularly monocytes and macrophages, of proinflammatory mediators.4 Gram-negative bacterial infection is one of the major causes of systemic bacterial sepsis.5 Lipopolysaccharide (LPS), a constituent of the Gram-negative outer membrane, is the leading cause of sepsis. LPS induces a rapid increase of proinflammatory mediators, leading to lethal systemic tissue damage and multiple organ failure, which mimics the inflammatory responses of septic syndrome.6 In mammals, membrane-bound CD14 and toll-like receptor 4 (TLR4)–MD-2 participate in cellular recognition of LPS.7 Binding of LPS to TLR4 triggers the activation of members of the mitogen-activated protein kinase (MAPK) pathway including p38, p42/p44 extracellular signal-regulated kinase (ERK1/2), and c-Jun N-terminal kinase (JNK).8 In resting unstimulated cells, nuclear factor-κB (NF-κB), a heterodimeric complex composed of 50- and 65-kDa (p50/p65) protein subunits,9 retains as an inactive complex bound to inhibitory κBα (IκBα) in the cytoplasm. While the cells are under proinflammatory stimulation by LPS, phosphorylation and degradation of IκBα permit NF-κB nuclear translocation and promote the expression of inflammatory genes including inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), and others.9
Thrombomodulin (TM) is a 557 amino acid type I glycosylated transmembrane protein10 with an NH2-terminal lectinlike region (domain 1; D1) followed by 6 epidermal growth factor (EGF)–like structures (domain 2; D2), an O-glycosylation site–rich domain (domain 3; D3), a transmembrane domain (domain 4; D4), and a cytoplasmic tail domain (domain 5; D5). TM domain 2 (TMD2) EGF-like structures are responsible for the anticoagulant activity of TM via the alteration of thrombin substrate specificity. TMD2-thrombin complex sequentially activates anticoagulant protein C inactivating procoagulant cofactors Va and VIIIa.11 TM expression also occurs in keratinocytes,12 polymorphonuclear neutrophils (PMNs),13 monocytes,14 and endothelial cells,15 indicating additional functions of TM besides anticoagulation.16 Indeed TM domains function as an adhesion molecule,17 an angiogenic factor,18 and an anti-inflammatory agent through protein C–dependent and –independent mechanisms.16,19 Recently, anti-inflammatory activity of TM domain 1 (TMD1) was implied by observing that mice with a deleted TM lectinlike domain (TMLeD/LeD) become more sensitive to LPS challenge through the suppressed expression of adhesion molecules via NFκB and MAPK signaling pathways.20 Moreover, mice with a mutation in the TM gene (TMpro/pro) strongly reduce the capacity to generate activated protein C, an anti-inflammatory agent in treatment of sepsis.21 Mice harboring the latter mutation display an unchanged pulmonary immune response induced by respiratory pathogens and LPS, suggesting the importance of TMD1 in bacteria and LPS-induced inflammatory responses.22 Furthermore, TMD1 sequesters high-mobility group-B1 (HMGB1) protein, a late cytokine mediator of lethal endotoxemia and sepsis, by interfering the binding of HMGB1 to receptor for the advanced glycation end product.23 TMD1 also interferes with complement activation and protects against arthritis.24
Presently, we have demonstrated that recombinant TMD1 (rTMD1) binds to LPS, which induces agglutination and enhances bacteria phagocytosis by macrophages. Moreover, rTMD1 has an anti-inflammatory role in the early phase of systemic inflammation in Gram-negative–mediated sepsis. rTMD1 specifically interacts with bacteria carrying smooth-type LPS such as Klebsiella pneumoniae, an important Gram-negative pathogen,25 and attenuates LPS- and K pneumoniae–induced inflammatory responses and lethality by binding to LPS, which blocks the downstream signal transduction. Thus, TMD1 may have an important protective function in neutralization of LPS and a therapeutic value in the treatment of septic shock syndrome and other inflammatory diseases.
Methods
Preparation of recombinant TM domain (rTMD) proteins using both Pichia and mammalian protein expression systems
The pPICZαA and pCR3-EK vectors (Invitrogen, San Diego, CA) were used for expression and secretion of human rTMD proteins containing 6× His tag and c-Myc epitope for purification and for protein detection in Pichia pastoris and human embryonic kidney 293 mammalian protein expression systems. An enterokinase cutting site between the rTMD sequence and His/c-Myc tag allowed subsequent removal of the tag sequence. The purified rTMD proteins were examined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. A nontagged rTMD1 was prepared by incubation of rTMD1 with enterokinase. Further details are provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cells and cell culture
Human leukemia monocytic THP-1 cells and the murine macrophage cell line RAW 264.7 were obtained from Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). BCRC's recommendations of cell culture conditions were followed. For differentiation, THP-1 cells were plated in medium containing 10 nM phorbol 12- myristate 13-acetate (Sigma-Aldrich, St Louis, MO) and allowed to adhere for 18 hours.
Inflammatory mediator assays
TNF-α in conditioned medium or mouse serum was measured by an enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Accumulation of nitrite in the medium or mouse serum was determined by a colorimetric assay using Griess reagent (Sigma-Aldrich). Blood urea nitrogen (BUN) and creatinine were measured with a Roche D&P modular (Roche Diagnostic Systems, Branchburg, NJ). Assays were performed according to the manufacturer's protocol.
Western blotting
rTMD1 (0-50 μg/mL [0-2.18 nmol/mL]) preincubated without or with Escherichia coli LPS (LPS O111:B4, Sigma-Aldrich) in the absence or presence of Lewis Y (Ley; Dextra Laboratories, Reading, United Kingdom) for 30 minutes was used to stimulate THP-1 or RAW 264.7 cells. Incubation period included 15 minutes for pIκB and IκB with antibodies (B-9 and C-21; Santa Cruz Biotechnology, Santa Cruz, CA, unless otherwise noted), 20 minutes for p38 (C-20) and pp38 (D-8), 30 minutes for ERK1/2 (K-23), pERK1/2 (E-4), lamin B2 (E-3; Zymed Laboratories, San Francisco, CA), NF-κB p50 (C-19), and NF-κB p65 nuclear translocation (C-20), and 24 hours for iNOS expression using a specific antibody (N-20). (Numbers in parentheses are antibody clone numbers.)
Animals and systemic sepsis models
Male FVB mice (8-10 weeks old) were used to model sepsis in vivo. The Institutional Animal Care and Use Committee of the National Cheng Kung University approved procedures. In the chronic endotoxemia model, various concentrations of rTMD1 or rTMD proteins in equimolar amounts were administered by tail intravenous injection and LPS (20 mg/kg) or K pneumoniae (5 × 102 CFU/mouse, BCRC) was administered by intraperitoneal injection. After 6 or 12 hours, mice were killed by pentobarbital anesthesia (50 mg/kg intraperitoneally). Sera were collected and assayed for TNF-α, NO, BUN, and creatinine, and lung and kidney tissues were removed, fixed with formalin, and embedded in paraffin for histochemical examination. In the lethal sepsis model, rTMD1 (2 mg/kg [87.3 nM/kg]) was intravenously administered at 0, 6, 12, and 24 hours after intraperitoneal administration of LPS (40 mg/kg). The half-life of rTMD1 in the circulation was determined by intravenous injection of rTMD1 (10 mg/kg [436.64 nM/kg]) and serum samples were collected at various time intervals. The levels of rTMD1 in the collected sera were determined by a sandwich ELISA which used anti–c-Myc monoclonal antibody and TM-H300 as capture and detection antibodies (both from Santa Cruz Biotechnology), respectively. For the lethal bacteremia model, rTMD1 (10 mg/kg) was administered before intraperitoneal injection of K pneumoniae (5 × 103 CFU/mouse). Mortality was monitored every 6 to 12 hours until all mice in either experimental group died. Experimental procedure of determining LPS half-life is as follows. LPS (20 mg/kg) was intraperitoneally administered to male FVB mice without or with rTMD1 (10 mg/kg intravenously). Serum samples were collected at various time intervals and the amount of LPS was determined by Limulus amebocyte lysate test (Associates of Cape Cod, E Falmouth, MA). A further experiment was performed to determine whether rTMD1 affects bacterial clearance. K pneumoniae (5 × 102 CFU/mouse) was intraperitoneally administered to male FVB mice without or with rTMD1 (10 mg/kg intravenously), and blood samples were collected at various time intervals. Outgrowth of K pneumoniae was quantified by plating serial dilutions of blood samples on blood agar plates and enumerating colonies after overnight incubation at 37°C.
K pneumoniae and LPS binding assays
K pneumoniae (3 × 105 CFU/well), E coli O111:B4 smooth-type LPS (5 μg/well), or bovine serum albumin (BSA, 5 μg/well) in 100 μL bicarbonate buffer (pH 9.6) were coated onto wells of high-binding microtiter plate (Corning Costar, Cambridge, MA). Nonspecific binding was blocked with binding buffer (20 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 5 mM CaCl2) containing 50 mg/mL BSA. Various concentrations of rTMD proteins without or with CD14 (10 μg/mL; R&D Systems) in binding buffer containing 1 mg/mL BSA were added to wells and incubated for 2 hours. In some experiments, 0.2 M mannose, 5 mM EDTA, or Ley were included in the binding buffer to compete with LPS and rTMD1 binding. Antibody against the c-Myc epitope of the rTMD proteins was added to wells and incubated for 2 hours, and peroxidase-labeled secondary antibody followed. Binding of rTMD proteins was detected by measuring absorbance at 450 nm.
Effect of rTMD1 on bacterial agglutination
K pneumoniae and E coli DH5α (BCRC) were washed twice in 0.1 M sodium bicarbonate buffer (pH 9.6) and incubated with 0.1 mg/mL fluorescein isothiocyanate (FITC) at 37°C in the dark. Each of 20 μg/mL of rTMD1, nontagged rTMD1, mammalian rTMD1, and recombinant TM domains 2 and 3 (rTMD23) was coincubated with FITC-labeled bacteria (1 × 105 CFU/mL) in buffer (20 mM Tris, pH 7.4, 0.15 M NaCl, 5 mM CaCl2, 1 mg/mL BSA) with or without 0.2 M mannose or 5 mM EDTA. Samples were individually placed on a glass slide and fluorescently labeled bacteria were observed microscopically to evaluate agglutination.26
THP-1 phagocytosis assay
FITC-labeled K pneumoniae (1 × 105 CFU/well) were preincubated with rTMD1 at 37°C for 30 minutes before adding to differentiated THP-1 cells. After incubation for 2 hours the THP-1 cells were washed twice with ice-cold PBS before fluorescence microscopy, or were further trypsinized, resuspended in PBS, and analyzed in a fluorescence-activated cell sorter (FACS; Becton Dickinson, San Jose, CA) to measure THP-1 cell intracellular fluorescence. Green fluorescence data from 10 000 events (cells) per condition collected at 530 nm on a log scale were analyzed with CellQuest software (Becton Dickinson).27
rTMD1 ligand analysis
Interaction of biotin-polyacrylamide (biotin-PAA) sugars (GlycoTech, Rockville, MD) and rTMD1 was assayed on a PerkinElmer Envision instrument using the AlphaScreen program.28 Details are provided in Document S1.
Analysis of Lewis antigens in E coli LPS O111:B4
E coli O111:B4 LPS (5 μg/well) was coated and blocked as described in “K pneumoniae and LPS binding assays.” Antibodies (5 μg/mL; Abcam) against Lewis a (Lea), Lewis b (Leb), Lewis X (Lex), and Ley were added to detect Lewis antigens in LPS. Details are provided in Document S1.
Statistical analyses
Survival data were analyzed by log-rank test. Data are expressed as the mean plus or minus the standard deviation (SD). Statistical significance was analyzed by unpaired Student t test. Differences between more than 2 groups were compared by one-way analysis of variance or 2-way analysis of variance and following Bonferroni post hoc test, with values of P less than .05 considered statistically significant.
Results
rTMD1 treatment reduces LPS-induced inflammatory mediator production in macrophages
To test whether TMD1 has anti-inflammatory property, we prepared rTMD proteins using both Pichia pastoris and mammalian protein expression systems. The rTMD1 proteins obtained from both systems had similar molecular mass with glycosylation modification, which was about 35 kDa and as assayed by silver staining and Western blotting (Figure S1A,B). We first tested the anti-inflammatory effect of rTMD proteins in RAW 264.7 and THP-1 cells stimulated with LPS. Pichia-expressed rTMD1 but not recombinant TMD2 containing EGF-like domain 1, 2, 3 (rTMD2/EGF123) dose-dependently inhibited TNF-α production in RAW 264.7 cells stimulated with LPS (Figure 1A); similar results were obtained in THP-1 cells (data not shown). Likewise, only rTMD1 could effectively inhibit NO production in RAW 264.7 cells (Figure 1B); similar results were obtained using mammalian-expressed rTMD1 (Figure 1A,B). To test whether the reduction of LPS-induced inflammatory mediator by rTMD1 results from LPS-rTMD1 binding, various concentrations of LPS and rTMD1 were used to test the interaction. In the absence of rTMD1, LPS dose-dependently increased TNF-α production (Figure 1C) and rTMD1 dose-dependently inhibited LPS-induced TNF-α production (Figure 1C), which is consistent with the hypothesis that LPS-rTMD1 binding results in the reduction effect of rTMD1 on the inflammatory mediator production by the cells.
Effects of rTMD proteins on LPS-induced inflammatory mediator production and LPS-induced signaling. (A,B) rTMD proteins from Pichia and mammalian protein expression systems were preincubated with LPS before adding to RAW 264.7 cells. After a 6-hour incubation, culture media were collected for the measurement of (A) TNF-α and (B) NO. mTMD1 represents rTMD1 from mammalian protein expression system. Values are the mean plus or minus SD (n = 3). A and B, **P < .01 and ***P < .001 compared with the LPS-treated cultures; ###P < .001 compared with the PBS group. (C) Various amounts of LPS were used to induce the TNF-α production in RAW264.7 cells, and the effects of rTMD1 were determined. Values are the mean plus or minus SD (n = 4). *P < .05, **P < .01, and ***P < .001 compared with the LPS-treated group. Western blotting was used to assay LPS-induced phosphorylation and degradation of IκBα (D), nuclear translocation of NF-κB p50 and p65 in nuclear fractions (E), ERK1/2 and p38 phosphorylation (F), and iNOS expression (G). All results are typical of those obtained in at least 3 independent experiments.
Effects of rTMD proteins on LPS-induced inflammatory mediator production and LPS-induced signaling. (A,B) rTMD proteins from Pichia and mammalian protein expression systems were preincubated with LPS before adding to RAW 264.7 cells. After a 6-hour incubation, culture media were collected for the measurement of (A) TNF-α and (B) NO. mTMD1 represents rTMD1 from mammalian protein expression system. Values are the mean plus or minus SD (n = 3). A and B, **P < .01 and ***P < .001 compared with the LPS-treated cultures; ###P < .001 compared with the PBS group. (C) Various amounts of LPS were used to induce the TNF-α production in RAW264.7 cells, and the effects of rTMD1 were determined. Values are the mean plus or minus SD (n = 4). *P < .05, **P < .01, and ***P < .001 compared with the LPS-treated group. Western blotting was used to assay LPS-induced phosphorylation and degradation of IκBα (D), nuclear translocation of NF-κB p50 and p65 in nuclear fractions (E), ERK1/2 and p38 phosphorylation (F), and iNOS expression (G). All results are typical of those obtained in at least 3 independent experiments.
rTMD1 blocks the LPS-induced signaling pathways
Phosphorylation and degradation of IκBα occurred in RAW 264.7 cells stimulated with LPS (100 ng/mL). The effect was totally reversed by rTMD1 (50 μg/mL [2.18 nmol/mL]; Figure 1D). The LPS-induced nuclear translocation of NF-κB was also inhibited in a dose-dependent fashion by rTMD1 (Figure 1E). LPS-induced phosphorylation of ERK1/2 and p38 was also inhibited by rTMD1 (Figure 1F). Similar effects of rTMD1 on activation of signal transduction pathway were observed in THP-1 cells (data not shown). iNOS induction in the RAW 264.7 cells by LPS was also inhibited by rTMD1 (Figure 1G).
rTMD1 reduces cytokine release, attenuates lung and renal injury, improves survival in experimental sepsis, and enhances bacterial LPS clearance
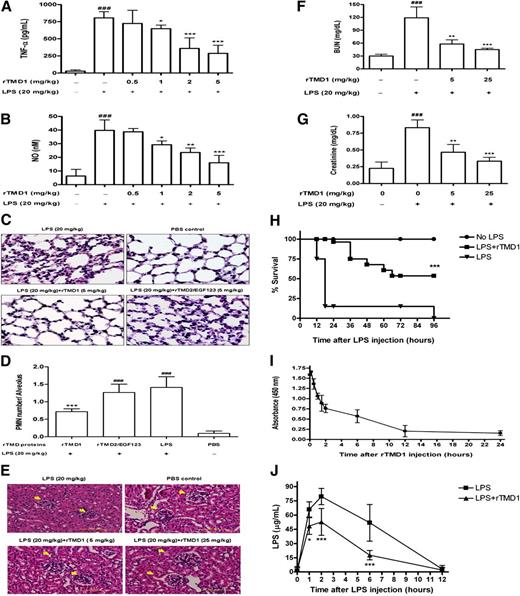
Because rTMD1 could inhibit LPS-induced inflammatory mediator productions and signaling pathways, we proposed that rTMD1 might function as a therapeutic agent to reduce the inflammatory response and lethality induced by LPS in vivo. TNF-α and NO levels were increased in mice 6 hours after intraperitoneal administration of 20 mg/kg LPS, relative to control mice. Mice receiving an intravenous injection of rTMD1 (1-5 mg/kg [43.66-218.3 nmol/kg]) had significantly decreasing levels of TNF-α and NO (Figure 2A,B). PMN infiltration was evident in lung sections in LPS-treated mice 6 hours after administration of LPS (Figure 2C,D). rTMD1 injection significantly inhibited pulmonary accumulation of PMNs 6 hours after LPS administration (Figure 2C,D). However, rTMD2/EGF123 had no significant effect on LPS-induced inflammatory responses and could serve as a negative control of yeast recombinant protein (Figure 2C,D). Similarly, LPS caused severe glomerular injury of the kidney. The extent of glomerulonephritis was significantly reduced in rTMD1-treated mice (Figure 2E). The increase of BUN and serum creatinine 12 hours after administration of LPS was consistent with the functional failure of the kidney. Mice treated with rTMD1 and then challenged with LPS had markedly reduced BUN and creatinine levels comparing with LPS-treated mice (Figure 2F,G). To study whether this effect protects against lethality, we treated mice with 4 intravenously administered doses of rTMD1 (2 mg/kg) or PBS and observed the mice until all mice in either experimental group died. rTMD1 treatment possessed significant protection against lethality and improved survival during endotoxemia (rTMD1-treated group survival, 100%; PBS-treated group survival, 10%, 1 day after LPS challenge; and rTMD1-treated group survival, 60%; PBS-treated group survival, 0%, 4 days after LPS challenge; Figure 2H), suggesting that rTMD1 may have therapeutic potential. The injected rTMD1 was detectable within 12 hours after each intravenous injection with a 3- to 4-hour half-life as assayed by ELISA (Figure 2I). The injected LPS (20 mg/kg) reached a maximum level 2 hours after intraperitoneal administration and was cleared from the circulation with an approximately 6- to 8-hour half-life (Figure 2J). rTMD1 administration significantly enhanced LPS clearance (Figure 2J). In addition, a rabbit polyclonal antibody against rTMD1 (TMD1 Ab) was prepared and characterized (Figure S2A,B). Pretreatment of rTMD1 with TMD1 Ab reversed the rTMD1's effect on the suppression of LPS-induced TNF-α release (Figure S2C,D), suggesting that the anti-inflammatory effect of rTMD1 on the inflammatory mediator production is rTMD1-specific.
rTMD1 reduces LPS-induced inflammatory response and lethality, attenuates LPS-induced pulmonary accumulation of PMNs and renal injury, and enhances LPS clearance in vivo. (A,B) rTMD1 was intravenously administered before intraperitoneal injection of LPS (20 mg/kg). Sera were collected 6 hours after administration of LPS for assay of (A) TNF-α and (B) NO production. Values are the mean plus or minus SD (n = 10). *P < .05, **P < .01, ***P < .001 compared with the LPS-treated group; ###P < .001 compared with the PBS-treated group. (C) Representative microscopic images of hematoxylin-and-eosin–stained sections of lung are shown. (D) The number of infiltrated PMNs in each alveolus was observed by light microscope (original magnification ×630). The number of PMNs was counted from 4 randomly fields per slide on each experimental mouse and normalized to fields of alveolus numbers on each slide. Values are the mean plus or minus SD (n = 10). ***P < .001 compared with the LPS-treated group; ###P < .001 compared with the PBS-treated group. These graphs represent the results from 3 independent experiments. (E-G) rTMD1 (5 mg/kg and 25 mg/kg) was intravenously administered before LPS (20 mg/kg) was intraperitoneally injected into mice. After 12 hours, mice were killed and kidney tissues were removed. Kidney sections were observed by histologic hematoxylin-and-eosin staining. Representative microscopic images are shown. (E) rTMD1 suppression of renal injury. Arrowheads indicate the site of the glomerulus (scale bars represent 50 μm). rTMD1 decreased the levels of renal injury markers BUN (F) and creatinine (G) in mice sera. For each experimental group, n = 4; ###P < .001 compared with the LPS-untreated control mice; **P < .01, ***P < .001 compared with the LPS-treated control mice. These graphs represent the results from 3 independent experiments. (H) Mice received LPS (40 mg/kg) and rTMD1 (4 intravenous doses of 2 mg/kg at 0, 6, 12, and 24 hours after LPS injection). Survival was determined. For each experimental group, n = 20. ***P < .001 compared with the LPS-treated group. (I) Clearance of rTMD1 in circulation. The half-life of rTMD1 in the circulation was determined by intravenous injection of rTMD1 (10 mg/kg) and the levels of rTMD1 in serum samples were measured by a sandwich ELISA using anti–c-Myc and TM-H300 as capture and detection antibodies, respectively. Values are the mean plus or minus SD (n = 5). (J) Clearance of LPS in circulation without or with rTMD1 treatment. LPS (20 mg/kg) was intraperitoneally administered to male FVB mice without or with rTMD1 (10 mg/kg; intravenously), and serum samples were collected at various time intervals and the amount of LPS was determined by the Limulus amebocyte lysate test. Values are the mean plus or minus SD. For each time interval group, n = 5; *P < .05 and ***P < .001 compared with the LPS-treated mice.
rTMD1 reduces LPS-induced inflammatory response and lethality, attenuates LPS-induced pulmonary accumulation of PMNs and renal injury, and enhances LPS clearance in vivo. (A,B) rTMD1 was intravenously administered before intraperitoneal injection of LPS (20 mg/kg). Sera were collected 6 hours after administration of LPS for assay of (A) TNF-α and (B) NO production. Values are the mean plus or minus SD (n = 10). *P < .05, **P < .01, ***P < .001 compared with the LPS-treated group; ###P < .001 compared with the PBS-treated group. (C) Representative microscopic images of hematoxylin-and-eosin–stained sections of lung are shown. (D) The number of infiltrated PMNs in each alveolus was observed by light microscope (original magnification ×630). The number of PMNs was counted from 4 randomly fields per slide on each experimental mouse and normalized to fields of alveolus numbers on each slide. Values are the mean plus or minus SD (n = 10). ***P < .001 compared with the LPS-treated group; ###P < .001 compared with the PBS-treated group. These graphs represent the results from 3 independent experiments. (E-G) rTMD1 (5 mg/kg and 25 mg/kg) was intravenously administered before LPS (20 mg/kg) was intraperitoneally injected into mice. After 12 hours, mice were killed and kidney tissues were removed. Kidney sections were observed by histologic hematoxylin-and-eosin staining. Representative microscopic images are shown. (E) rTMD1 suppression of renal injury. Arrowheads indicate the site of the glomerulus (scale bars represent 50 μm). rTMD1 decreased the levels of renal injury markers BUN (F) and creatinine (G) in mice sera. For each experimental group, n = 4; ###P < .001 compared with the LPS-untreated control mice; **P < .01, ***P < .001 compared with the LPS-treated control mice. These graphs represent the results from 3 independent experiments. (H) Mice received LPS (40 mg/kg) and rTMD1 (4 intravenous doses of 2 mg/kg at 0, 6, 12, and 24 hours after LPS injection). Survival was determined. For each experimental group, n = 20. ***P < .001 compared with the LPS-treated group. (I) Clearance of rTMD1 in circulation. The half-life of rTMD1 in the circulation was determined by intravenous injection of rTMD1 (10 mg/kg) and the levels of rTMD1 in serum samples were measured by a sandwich ELISA using anti–c-Myc and TM-H300 as capture and detection antibodies, respectively. Values are the mean plus or minus SD (n = 5). (J) Clearance of LPS in circulation without or with rTMD1 treatment. LPS (20 mg/kg) was intraperitoneally administered to male FVB mice without or with rTMD1 (10 mg/kg; intravenously), and serum samples were collected at various time intervals and the amount of LPS was determined by the Limulus amebocyte lysate test. Values are the mean plus or minus SD. For each time interval group, n = 5; *P < .05 and ***P < .001 compared with the LPS-treated mice.
rTMD1 reduces K pneumoniae-induced inflammatory responses and lethality and enhances bacterial clearance
To test whether rTMD1 protects against lethality induced by Gram-negative bacteria, we induced systemic sepsis in mice by injecting them intraperitoneally with K pneumoniae and treating them with rTMD1 (Figure 3A-C). TNF-α and NO levels were increased within 12 hours in mice receiving K pneumoniae (5 × 102 CFU). rTMD1 treatment (5-25 mg/kg; intravenously) effectively reduced the TNF-α and NO production (Figure 3A,B). For the survival experiment, all mice that received K pneumoniae (5 × 103 CFU) died within 18 hours, whereas 50% of the mice that received a single intravenous dose of rTMD1 (10 mg/kg) survived more than 24 hours (Figure 3C). To explore whether the effect of K pneumoniae–induced mortality results from rTMD1 promoted bacterial clearance, the amount of viable bacteria in the blood was determined. FVB mice were each injected intraperitoneally with K pneumoniae (5 × 102 CFU) without or with rTMD1 treatment (10 mg/kg; intravenously). rTMD1 significantly enhanced K pneumoniae clearance in circulation at 12, 24, and 36 hours after injection (Figure 3D). To demonstrate that rTMD1 has therapeutic potential, rTMD1 was infused after bacterial infection. In time course experiments, rTMD1 (10 mg/kg) was intravenously administered immediately (within a minute), 30, or 60 minutes after intraperitoneal administration of K pneumoniae. The result showed that rTMD1 could effectively suppress K pneumoniae–induced TNF-α release (Figure 3E) and enhance K pneumoniae clearance in circulation at 12, 24, and 36 hours after infection even at 30 or 60 minutes after treatment of rTMD1 (Figure 3F). Mice infected with K pneumoniae in the absence of rTMD1 started to die after 42 hours; however, the survival was improved in all rTMD1-treated groups.
rTMD1 reduces K pneumonae–induced inflammatory response and lethality, and enhances bacterial clearance. (A,B) rTMD1 was administered before injection of K pneumoniae (5 × 102 CFU/mouse) to mice. Sera were collected 12 hours after administration of K pneumoniae for assay of (A) TNF-α and (B) NO production. Values are the mean plus or minus SD (n = 10). **P < .01 and ***P < .001 compared with the K pneumoniae–treated group; ###P < .001 compared with the PBS-treated group. (C) rTMD1 (10 mg/kg) was administered before injection of K pneumoniae (5 × 103 CFU/mouse) to mice. Survival was determined. For each experimental group, n = 20. ***P < .001 compared with the K pneumoniae–treated group. These graphs represent the results from 3 independent experiments. (D) rTMD1 enhanced K pneumoniae clearance in circulation. FVB mice were intraperitoneally injected with K pneumoniae (5 × 102 CFU/mouse) without or with rTMD1 (10 mg/kg; intravenously). The blood samples from each group were collected at various time intervals and assayed for viable bacterial CFU counts. Values are the mean plus or minus SD. For each time interval group, n = 5; *P < .05 compared with the K pneumoniae–treated mice. (E,F) rTMD1 (10 mg/kg) was administered immediately, 30, or 60 minutes after injection of K pneumoniae (5 × 102 CFU/mouse) to mice. The serum levels of TNF-α (E) and K pneumoniae clearance (F) were determined. Values are the mean plus or minus SD. For each time interval group, n = 8-10; *P < .05 and ***P < .001 compared with the K pneumoniae–treated mice on each time interval group; ###P < .001 compared with the PBS-treated group. Similar results were obtained in 2 independent experiments.
rTMD1 reduces K pneumonae–induced inflammatory response and lethality, and enhances bacterial clearance. (A,B) rTMD1 was administered before injection of K pneumoniae (5 × 102 CFU/mouse) to mice. Sera were collected 12 hours after administration of K pneumoniae for assay of (A) TNF-α and (B) NO production. Values are the mean plus or minus SD (n = 10). **P < .01 and ***P < .001 compared with the K pneumoniae–treated group; ###P < .001 compared with the PBS-treated group. (C) rTMD1 (10 mg/kg) was administered before injection of K pneumoniae (5 × 103 CFU/mouse) to mice. Survival was determined. For each experimental group, n = 20. ***P < .001 compared with the K pneumoniae–treated group. These graphs represent the results from 3 independent experiments. (D) rTMD1 enhanced K pneumoniae clearance in circulation. FVB mice were intraperitoneally injected with K pneumoniae (5 × 102 CFU/mouse) without or with rTMD1 (10 mg/kg; intravenously). The blood samples from each group were collected at various time intervals and assayed for viable bacterial CFU counts. Values are the mean plus or minus SD. For each time interval group, n = 5; *P < .05 compared with the K pneumoniae–treated mice. (E,F) rTMD1 (10 mg/kg) was administered immediately, 30, or 60 minutes after injection of K pneumoniae (5 × 102 CFU/mouse) to mice. The serum levels of TNF-α (E) and K pneumoniae clearance (F) were determined. Values are the mean plus or minus SD. For each time interval group, n = 8-10; *P < .05 and ***P < .001 compared with the K pneumoniae–treated mice on each time interval group; ###P < .001 compared with the PBS-treated group. Similar results were obtained in 2 independent experiments.
Direct binding of rTMD1 with Gram-negative bacteria and LPS, and direct binding of membrane-bound TM with LPS
To test whether TMD1 is capable of specific binding to Gram-negative bacteria and LPS, rTMD1 and rTMD23 were used for binding with K pneumoniae, LPS, or BSA. rTMD1 but not rTMD23 could specifically bind to K pneumoniae (Figure 4A). Because rTMD1 could bind to Gram-negative bacteria, LPS of Gram-negative bacteria was assumed a potential candidate ligand of rTMD1. Appropriately, binding of rTMD1 and rTMD23 to LPS or BSA was measured. rTMD1 but not rTMD23 specifically bound to LPS (Figure 4B) and binding was inhibited by mannose and EDTA, suggesting that the TMD1 carbohydrate recognition domain interacted with LPS carbohydrate in a Ca2+-dependent manner (Figure 4C). From Figure 4B, the apparent dissociation constant (Kd) estimated from the 50% saturation of the binding of LPS with rTMD1 was about 1.6 × 10−6 M. We also demonstrated that LPS binds rTMD1 in a concentration-dependent manner with Kd ranging from 8.24 × 10−6 M to 9.01 × 10−8 M using surface plasmon resonance (SPR) assay (see Figure S3 for details). It was conceivable that the binding of rTMD1 to LPS might block the interaction of LPS with LPS-binding molecules, which blocks the LPS-induced inflammatory mediator productions and signaling pathways. As shown in Figure 4D, the binding of CD14 to LPS was inhibited by rTMD1 in a dose-dependent manner, but the binding of LPS binding protein to LPS was not inhibited by rTMD1 (data not shown). To explore whether the endogenous TMD1 can specifically interact with LPS as rTMD1does, endogenous membrane-bound TM14 and overexpressed full-length or lectinlike domain-deleted, membrane-bound TM17,29 were designed to test LPS binding capacity (Figure S4). The results showed that membrane-bound TM expressed on THP-1 cells could bind biotinylated LPS. In addition, full-length but not lectinlike domain-deleted, membrane-bound TM could bind biotinylated LPS. These results suggest that membrane-anchored TM could bind LPS via TM's N-terminal lectinlike domain.
Binding of rTMD proteins with K pneumoniae and LPS, and the blocking effect of rTMD1 on the binding of CD14 to LPS. (A) K pneumoniae or BSA. (B) E coli O111:B4 LPS or BSA. (A,B) K pneumoniae, LPS, or BSA was coated onto wells. Equimolar amounts of rTMD proteins were added to each well. The binding of rTMD proteins was detected. Values are the mean plus or minus SD (n = 6). ***P < .001 compared with the rTMD23-added group and BSA-coated group. (C) LPS was coated onto wells and incubated with rTMD1 (50 μg/mL) in binding buffer containing CaCl2 in the absence and presence of 0.2 M mannose or 5 mM EDTA. The results are expressed as the percentage of relative absorbance normalized with binding buffer group (100%). Values are the mean plus or minus SD (n = 6). ***P < .001 compared with the binding buffer group. The results shown are typical of those obtained in at least 3 independent experiments. (D) rTMD1 blockage of the binding of CD14 to LPS. LPS was coated onto wells and incubated with indicated concentrations of rTMD1 and CD14. The binding of CD14 to LPS was detected using CD14 antibody (M-305, Santa Cruz Biotechnology, Santa Cruz, CA). Values are the mean plus or minus SD (n = 4), **P < .01 and ***P < .001 compared with the group that received only CD14. Similar results were obtained in 3 independent experiments.
Binding of rTMD proteins with K pneumoniae and LPS, and the blocking effect of rTMD1 on the binding of CD14 to LPS. (A) K pneumoniae or BSA. (B) E coli O111:B4 LPS or BSA. (A,B) K pneumoniae, LPS, or BSA was coated onto wells. Equimolar amounts of rTMD proteins were added to each well. The binding of rTMD proteins was detected. Values are the mean plus or minus SD (n = 6). ***P < .001 compared with the rTMD23-added group and BSA-coated group. (C) LPS was coated onto wells and incubated with rTMD1 (50 μg/mL) in binding buffer containing CaCl2 in the absence and presence of 0.2 M mannose or 5 mM EDTA. The results are expressed as the percentage of relative absorbance normalized with binding buffer group (100%). Values are the mean plus or minus SD (n = 6). ***P < .001 compared with the binding buffer group. The results shown are typical of those obtained in at least 3 independent experiments. (D) rTMD1 blockage of the binding of CD14 to LPS. LPS was coated onto wells and incubated with indicated concentrations of rTMD1 and CD14. The binding of CD14 to LPS was detected using CD14 antibody (M-305, Santa Cruz Biotechnology, Santa Cruz, CA). Values are the mean plus or minus SD (n = 4), **P < .01 and ***P < .001 compared with the group that received only CD14. Similar results were obtained in 3 independent experiments.
rTMD1 induces agglutination of Gram-negative bacteria and enhances bacterial phagocytosis in THP-1 cells
Since TMD1 mediates cell-cell adhesion by binding to carbohydrate ligands17 and rTMD1 functions in clearance of LPS and K pneumoniae (Figures 2J and 3D, respectively), we postulated that rTMD1 may participate in binding and phagocytosis of bacteria if the carbohydrate moieties on the surface of the bacteria consist of ligands of TM. rTMD1 binding specificity was assayed by an agglutination reaction with FITC-labeled Gram-negative (K pneumoniae and E coli) bacteria. rTMD1 (20 μg/mL) induced marked agglutination of K pneumoniae and E coli DH5α (Figure 5A). Moreover, the agglutination activity was Ca2+-dependent and was attenuated by mannose and EDTA (Figure 5A). To rule out the possible interference effect of His/c-Myc tag in rTMD proteins, we prepared a nontagged rTMD1 to perform the test (Figure S1B). A nontagged rTMD1 showed the same effect as rTMD1, whereas tagged rTMD23 failed to produce agglutination; a similar result was obtained by using mammalian-expressed rTMD1 (Figure 5A). The effect of rTMD1 on the phagocytosis of FITC-labeled K pneumoniae by activated THP-1 cells was assayed. rTMD1 markedly increased the amount of bacterial phagocytosis by THP-1 cells (Figure 5B,C). Therefore, rTMD1 not only can interfere with the bacteria-induced inflammatory reaction, but also can induce agglutination of bacteria and promote phagocytosis by macrophages.
Effects of rTMD1 on bacterial agglutination and phagocytosis by THP-1 cells. (A) FITC-labeled K pneumoniae and E coli DH5α were incubated in buffer containing 5 mM CaCl2 and in the absence or presence of rTMD1 without or with 0.2 M mannose and 5 mM EDTA. The fluorescently labeled bacteria were observed using a fluorescence microscope to evaluate bacterial agglutination. Nontagged rTMD1 was prepared by incubation of rTMD1 with enterokinase and purified as described in Document S1. Mammalian expressed rTMD1 and an internal control with tagged rTMD23 were also included. Photomicrographs are representative of 3 independent experiments. (B) Representative photomicrograph showing the effect of rTMD1 on bacterial phagocytosis. Differentiated THP-1 cells were incubated with rTMD1-pretreated FITC-labeled K pneumoniae. The left panel shows the bright field image and the right panel shows the fluorescence photomicrographs (scale bars represent 200 μm). (C) FACS analysis of the samples from panel B. The graphs depict the level of FITC fluorescence (x-axis) versus the relative cell numbers (y-axis). These graphs represent the results from 3 independent experiments.
Effects of rTMD1 on bacterial agglutination and phagocytosis by THP-1 cells. (A) FITC-labeled K pneumoniae and E coli DH5α were incubated in buffer containing 5 mM CaCl2 and in the absence or presence of rTMD1 without or with 0.2 M mannose and 5 mM EDTA. The fluorescently labeled bacteria were observed using a fluorescence microscope to evaluate bacterial agglutination. Nontagged rTMD1 was prepared by incubation of rTMD1 with enterokinase and purified as described in Document S1. Mammalian expressed rTMD1 and an internal control with tagged rTMD23 were also included. Photomicrographs are representative of 3 independent experiments. (B) Representative photomicrograph showing the effect of rTMD1 on bacterial phagocytosis. Differentiated THP-1 cells were incubated with rTMD1-pretreated FITC-labeled K pneumoniae. The left panel shows the bright field image and the right panel shows the fluorescence photomicrographs (scale bars represent 200 μm). (C) FACS analysis of the samples from panel B. The graphs depict the level of FITC fluorescence (x-axis) versus the relative cell numbers (y-axis). These graphs represent the results from 3 independent experiments.
Identification of ligand specificity of rTMD1 and no competing effect of Ley on HMGB1-rTMD1 binding
To identify the ligand specificity of TMD1, a panel of carbohydrate ligands (Figure 6A) was tested for rTMD1 affinity using the AlphaScreen method. The result showed that Ley antigen was a specific ligand for either mammalian- or Pichia-expressed rTMD1 (Figure 6A). Ley specifically inhibited binding of rTMD1 with LPS in a dose-dependent manner (Figure 6B), indicating that the carbohydrate binding site of rTMD1 was involved in the interaction with LPS. ELISA demonstrated that E coli O111:B4 LPS contained Lex and Ley antigens rather than Lea and Leb antigens (Figure 6C), consistent with the binding specificity of rTMD1. Furthermore, Ley could dose-dependently neutralize the blocking effects of rTMD1 on LPS-induced ERK1/2 phosphorylation, IκBα degradation, and NF-κB p50 and p65 nuclear translocation (Figure 6D). To examine whether LPS/Ley-rTMD1 interactions interfere with HMGB1-rTMD1 interactions and thus whether the binding sites of LPS/Ley and HMGB1 overlap on TMD1, we performed an experiment to observe the effect of Ley on the binding of HMGB1 with rTMD1. The result showed that Ley could not abrogate the HMGB1 and rTMD1 interaction (Figure S5), suggesting that the structural domain of TMD1 mediating HMGB1 interaction does not overlap with LPS/Ley.
Analysis of rTMD1 ligand by AlphaScreen, inhibitory effect of Ley antigen on the binding of rTMD1 with LPS, and analysis of Lewis antigens in E coli LPS O111:B4. (A) AlphaScreen assay results. Values are the mean plus or minus SD (n = 4). Sugar binding specificity of mammalian-expressed rTMD1 is indicated by relative intensities (with reference to the highest absorbance unit). The sugar identities are designated by numbers as listed. ***P < .001 compared with the blank. The results were obtained from the average of 4 independent assays. Similar result was obtained using Pichia-expressed rTMD1. (B) Effect of Ley antigen on the binding of rTMD1 with LPS. LPS was coated onto wells and various concentrations of Ley and rTMD1 were added to each well. The binding of rTMD1 is expressed as the mean plus or minus SD (n = 4), *P < .05, **P < .01, and ***P < .001 compared with the rTMD1-only group. Similar results were obtained in 2 independent experiments. (C) Analysis of Lewis antigens in E coli LPS O111:B4. LPS (5 μg/well) was coated onto wells of high-binding microtiter plate. Lea, Leb, Lex, and Ley antibodies (5 μg/mL) were added to each well in binding buffer and incubated at 37°C for 2 hours followed by peroxidase-labeled secondary antibodies for 2 hours. The binding of Lewis antibodies to LPS was detected by measuring absorbance at 450 nm. Values are the mean plus or minus SD (n = 4). Similar results were obtained in 2 independent experiments. (D) Effect of Ley on the blocking effect of rTMD1 on LPS-induced signaling pathways. rTMD1 (20 μg/mL) and various concentrations of Ley were preincubated with LPS (100 ng/mL) before adding to cells. After incubation for 30 minutes, Western blot was used to assay LPS-induced ERK1/2 phosphorylation, degradation of IκBα in cytoplasmic fractions, and nuclear translocation of NF-κB p50 and p65 in nuclear fractions. Similar results were obtained in 2 independent experiments.
Analysis of rTMD1 ligand by AlphaScreen, inhibitory effect of Ley antigen on the binding of rTMD1 with LPS, and analysis of Lewis antigens in E coli LPS O111:B4. (A) AlphaScreen assay results. Values are the mean plus or minus SD (n = 4). Sugar binding specificity of mammalian-expressed rTMD1 is indicated by relative intensities (with reference to the highest absorbance unit). The sugar identities are designated by numbers as listed. ***P < .001 compared with the blank. The results were obtained from the average of 4 independent assays. Similar result was obtained using Pichia-expressed rTMD1. (B) Effect of Ley antigen on the binding of rTMD1 with LPS. LPS was coated onto wells and various concentrations of Ley and rTMD1 were added to each well. The binding of rTMD1 is expressed as the mean plus or minus SD (n = 4), *P < .05, **P < .01, and ***P < .001 compared with the rTMD1-only group. Similar results were obtained in 2 independent experiments. (C) Analysis of Lewis antigens in E coli LPS O111:B4. LPS (5 μg/well) was coated onto wells of high-binding microtiter plate. Lea, Leb, Lex, and Ley antibodies (5 μg/mL) were added to each well in binding buffer and incubated at 37°C for 2 hours followed by peroxidase-labeled secondary antibodies for 2 hours. The binding of Lewis antibodies to LPS was detected by measuring absorbance at 450 nm. Values are the mean plus or minus SD (n = 4). Similar results were obtained in 2 independent experiments. (D) Effect of Ley on the blocking effect of rTMD1 on LPS-induced signaling pathways. rTMD1 (20 μg/mL) and various concentrations of Ley were preincubated with LPS (100 ng/mL) before adding to cells. After incubation for 30 minutes, Western blot was used to assay LPS-induced ERK1/2 phosphorylation, degradation of IκBα in cytoplasmic fractions, and nuclear translocation of NF-κB p50 and p65 in nuclear fractions. Similar results were obtained in 2 independent experiments.
Discussion
TM expression is widely detected in a variety of cells, so it seems that the biologic function of TM is not merely limited in its well-characterized anticoagulant activity.16 Membrane-shed and rTMD proteins are important in attenuating inflammatory responses in endotoxin-induced cytokine production, tissue damage, and mortality.23,30,31 Transgenic mice (TMLeD/LeD) lacking the NH2-terminal lectin domain displayed augmented vulnerability to LPS challenge.20 HMGB1, which is a chromatin-binding protein and released by necrotic cells can bind to TMD1, which sequestered HMGB1 from binding to receptor for advanced glycation end product.23 Therefore, TMD1 provides protection against LPS-induced lethality. However, the direct interaction between TMD1 and LPS has not been demonstrated.
In this paper, we used different doses of LPS to perform in vitro and in vivo experiments. It is difficult to compare experiments with relative concentration (molarity) of rTMD1-mediated neutralization of LPS, because the real concentrations of rTMD1 and LPS in how much volume of mouse circulation is difficult to predict. The in vivo dosage of LPS (20-40 mg/kg) we used to induce acute symptoms in mice is similar to many previous reports.20,32 When LPS is parenterally administered to animals, a large proportion of injected LPS is initially found in the cell-free plasma with predominant binding to plasma proteins, including high density lipoproteins and apoproteins.33,34 It is expected that only a minor amount of injected LPS is available to act on the inflammation-responsive cells. In the in vitro system, we dealt with pure macrophage cell culture, which is very sensitive to LPS treatment. LPS concentration used in vitro would be much lower than the in vivo system. However, the difficulty of comparing in vitro and in vivo with relative concentrations of TMD1 and LPS still exists since the bioavailability of TMD1 and LPS is dynamic, as shown in Figure 2I,J.
The family of C-type lectins, through the interaction with carbohydrate recognition domains, participates in innate immune functions such as opsonization,26,35 complement activation,36 and leukocyte-endothelial cell adhesion.37 In a previous study, we demonstrated that TMD1 is a Ca2+-dependent functional lectin involved in cell-cell adhesion.17 The leukocyte endothelial adhesion molecules E- and P-selectin also consist of N-terminal lectin domains binding to Lea and Lex sequence-containing carbohydrate ligands.38,39 Furthermore, LPS of Helicobacter pylori isolates contains sequences related to Lex and Ley40,41 that can interact with selectins.42 These observations and our preliminary results prompted us to test whether LPS from Gram-negative bacteria contains ligands for rTMD1. Thus, we proposed that rTMD1 could bind to LPS, which would induce bacterial agglutination, since rTMD1 could be in a dimer form (Figure S6). Our results of this proposal clearly demonstrated that rTMD1 can specifically induce the agglutination of Gram-negative bacteria in the presence of Ca2+. In addition, rTMD1 dose-dependently enhances the phagocytosis of the K pneumoniae by macrophages. Both phenomena indicated that TMD1 may function as a natural opsonic moiety for innate immunity against Gram-negative bacteria.
The present results demonstrated that the carbohydrate recognition site in TMD1, but not the other extracellular domain TMD23, is responsible for binding with bacteria and LPS. We further demonstrated that rTMD1 significantly reduces LPS-induced production of TNF-α and NO in macrophages, suppresses LPS-induced MAPK and NF-κB signal pathways, inhibits LPS-increased iNOS expression (Figures 1,2), and blocks the interaction of CD14 with LPS (Figure 4D). Thus, rTMD1 blocks the interaction of LPS with its signaling receptor and then prevents widespread activation of inflammatory reaction in the early stage of sepsis. To compare the binding selectivity and strength of rTMD1 with other lectin-carbohydrate interactions, we established the approximate affinity for this interaction. From Figure 4B, the apparent Kd is about 1.6 × 10−6 M. Thus, the binding strength of rTMD1 with LPS is in the same order with the selectins and their natural ligands,43 implying that the binding of TMD1-LPS/Ley has physiologic relevance. rTMD1 not only opsonizes Gram-negative bacteria but also dampens LPS-induced inflammatory reactions. Previous studies reported that increased levels of membrane-shed TM fragments could be detected in circulation and urine,44 which has been widely accepted as an indicator in various diseases. Two forms of membrane-shed TM fragments (63/57 kDa and 35 kDa) derived from the N-terminal extracellular region of TM have been purified from urine.45 The 63/57 kDa fragment but not the 35 kDa fragment is active for protein C activation, suggesting that the fragment without protein C activation activity (35 kDa) might be membrane-shed TMD1. Many proteases, including rhomboids that could mediate TM shedding, have been reported.46 Furthermore, the mechanism of triggering protease-mediated TMD shedding remains unclear. A recent report showed that TM is shed in a metalloproteinase-dependent manner induced by proinflammatory factors.47 Furthermore, our recent data documented that lysophosphatidic acid induces TMD1 shedding in a metalloproteinase-dependent manner.29 These results indicated that membrane-shed soluble TMD1 could be generated during inflammatory processes. Membrane-shed TM fragments that are markedly increased in patients with severe sepsis48 should not be considered merely a marker of endothelial injury; rather, the TMD1-containing fragments in plasma may represent one of the natural protective mechanisms to be a decoy receptor and an anti-inflammatory agent especially in Gram-negative bacterial infection. Membrane-shed TMD1 might not present in enough amounts to exert anti-inflammatory function under pathologic conditions. Therefore, exogenous administration of rTMD1 may have therapeutic potential. We provided further evidence that membrane-bound TMD1could specifically bind LPS (Figure S4). However, the role of membrane-bound TMD1 in LPS-induced inflammatory response remains to be investigated.
TM in combination with thrombin can activate protein C, which in turn can suppress the inflammatory reaction.21,31 On the other hand, rTMD1 can bind to LPS and K pneumoniae, which can induce the agglutination and opsonization of bacteria, facilitate the phagocytosis of the bacteria particles by macrophages, enhance the LPS and K pneumoniae clearances, and reduce inflammatory reactions and lethality. Sepsis-associated acute organ dysfunction occurs in severe bacteremia. Treatment with rTMD1 preserves kidney function and alleviates PMN infiltration in lungs, which significantly reduces the mortality rate in LPS- and K pneumoniae–challenged mice. TMD1 can protect animal hosts from the deterioration caused by bacterial sepsis by different mechanisms. The interaction of TMD1 with HMGB1, a late mediator of endotoxin lethality,23 provides another function of TMD1 in modulation of inflammation. Since Ley could not abrogate the HMGB1 and rTMD1 interaction, a novel mechanism that the direct interaction of TMD1 with the Ley-like structure of LPS can sequester LPS from activation of signaling pathways at a very early stage of sepsis represents another possible role of TMD1 in the attenuation of inflammatory responses.
The binding of rTMD1 with K pneumoniae and LPS presently observed is mediated by rTMD1's carbohydrate recognition. We demonstrated that Ley is the most specific ligand for rTMD1 and can effectively compete with LPS for binding to rTMD1. TMD1 is also the first identified receptor for Ley. It is worthy to notice that TMD1 interferes with the adhesions of PMN–endothelial cell and monocyte–endothelial cell interactions.20,24 Our results further deduced that membrane-bound TMD1-Ley interaction may participate in the cell-cell adhesion in keratinocytes17 and in leukocyte-endothelial interaction,20,24 since both TM17 and Ley49,50 are on the surface of these cells.
In conclusion, the specific interaction of rTMD1 with Ley highlights a novel mechanism in modulating LPS-mediated inflammatory responses. The therapeutic potential of rTMD1 in treating acute Gram-negative septicemia should be noticed. Moreover, rTMD1 provides an alternative to treat sepsis without the side effect of bleeding that may encounter protein C–activating therapy like rTMD23, recombinant TM domains 1, 2, 3, or activated protein C.21 The identification of Ley as the specific ligand of rTMD1 also paves the way for investigating the roles of the TMD1-Ley couple in cell-cell interaction and their biologic functions in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Science Council, Executive Yuan (Taipei, Taiwan) grants NSC 95-2752-B-006-003-PAE, NSC 95-2752-B-006-004-PAE, and NSC 95-2752-B-006-005-PAE.
Authorship
Contribution: C.-S.S. designed and performed the research and wrote the paper; G.-Y.S. and H.-L.W. designed and wrote the paper and supervised the research; H.-M.H., Y.-C.K., K.-L.K., and C.-Y.M. prepared the recombinant proteins, performed studies, and took part in writing the corresponding sections of the paper; C.-H.K. and B.-I.C. contributed vital reagents and analytical tools; and C.-F.C., C.-H.L., and C.-H.W. performed the AlphaScreen assay.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hua-Lin Wu or Guey-Yueh Shi, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, 1 University Road, Tainan 701, Taiwan, Republic of China; e-mail: halnwu@mail.ncku.edu.tw or gyshi@mail.ncku.edu.tw.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal