Abstract
Exhaustion of virus-specific T cells may play an important role in the pathophysiology of chronic viral infections. Here, we analyzed telomere length and telomerase activity in HIV-1–specific CD8+ T cells from progressors or controllers to determine underlying molecular pathways of T-cell exhaustion and senescence. Telomere lengths of HIV-1–specific CD8+ T cells from progressors were significantly shorter compared with autologous cytomegalovirus (CMV)/Epstein-Barr virus (EBV)–specific CD8+ T cells or bulk CD8+ T cells, while telomere lengths from controllers significantly exceeded those of autologous bulk CD8+ T cells and reached a similar level as HIV-1–specific CD8+ T cells collected during primary HIV-1 infection. Telomere length stabilization in controllers corresponded to high levels of constitutive telomerase activity, which was associated with preservation of cytotoxic and proliferative properties. Conversely, limited constitutive telomerase activity was observed in HIV-1–specific CD8+ T cells from progressors, although an increase in both telomere length and telomerase activity was achieved in antigenic-peptide–stimulated cells from progressors after blocking the PD-1/PD ligand 1 (PD-L1) pathway. Collectively, these data suggest a causal role of telomere shortening for the functional deficiencies of HIV-1–specific CD8+ T cells in chronic progressive infection, while high constitutive telomerase activities appears to contribute to maintenance of polyfunctional HIV-1–specific CD8+ T cells from HIV-1 controllers.
Introduction
Spontaneous control of HIV-1 viremia is achieved in a small proportion of infected individuals called HIV controllers. The relative overrepresentation of specific MHC class I alleles such as HLA-B57 and HLA-B27 in this specific patient population1,2 suggests that low-level viremia in these persons is at least partially mediated by HIV-1–specific CD8+ T cells. However, progressive viremia in advanced stages of infection typically occurs in the presence of strong, broadly diversified, and polyclonal HIV-1–specific CD8+ T-cell populations3–5 with preserved recognition of the autologous virus6 and a similar immunodominance pattern as described in controllers.7,8 This paradoxic finding has recently been related to a defective functional profile of HIV-1–specific CD8+ T cells in chronic progressive infection, which have preserved IFN-γ secretion, but lack ex vivo antigen-specific proliferative activities. In contrast, HIV-1–specific CD8+ T cells collected from HIV-1 controllers were found to have strong ex vivo proliferative activities, which was associated with increased perforin expression and superior cytotoxic properties.3
Although cellular exhaustion and accelerated immune senescence has been repeatedly suggested as key mechanisms in HIV immunopathogenesis,9–13 aging of HIV-1–specific T cells as well as regulatory molecular processes governing their senescence have never been analyzed. Moreover, how alterations in immune senescence are involved in the development of HIV-1–specific T-cell dysfunction in progressive HIV-1 infection or in the maintenance of polyfunctional HIV-1–specific T cells in HIV controllers is currently unknown. In addition, it is currently unclear whether progressive senescence of HIV-1–specific T cells is an irreversible process, or whether aging of these cells can actively be manipulated for immunotherapeutic purposes.
In the present study, we conducted a detailed analysis of the telomere length and telomerase activity in HIV-1–specific CD8+ T cells. Telomeres represent distal hexameric chromosomal DNA segments that progressively shorten during cellular divisions and hence represent key molecular markers of cell aging.14,15 The lengths of telomeres can be actively enhanced by telomerase, an RNA-dependent DNA polymerase that synthesizes telomeric repeats by its catalytic component, telomerase reverse transcriptase (hTERT).16,17 This enzyme is expressed in highly selected cell types such as tissue and hematopoietic stem cells, as well as certain subsets of T and B lymphocytes during antigenic stimulation or activation.18,19 Up-regulating the activity of this enzyme appears to be the predominant biological mechanism that actively protects cells against replicative senescence and might influence the functional profile of these cells as well. Here, we demonstrate that HIV-1–specific CD8+ T cells from HIV-1 controllers have long telomeres and high levels of constitutive telomerase activity, while telomere shortening with limited telomerase activity was observed in HIV-1–specific CD8+ T cells from progressors. Interestingly, an active augmentation of telomerase activity and telomere length in HIV-1–specific CD8+ T cells from progressors was achieved by exposing antigenic-peptide–stimulated cells to monoclonal antibodies blocking PD ligand 1 (PD-L1). These data suggest that constitutive telomerase activity protects against telomere erosion and replicative senescence in HIV-1–specific CD8+ T cells from controllers, while progressive telomere shortening contributes to functional defects of HIV-1–specific CD8+ T cells from progressors.
Methods
Patients
Patients with HIV-1 were recruited from the Massachusetts General Hospital in Boston and gave written consent in accordance with the Declaration of Helsinki to participate in the study. Patients with early HIV-1 infection were defined as having a negative HIV-1 enzyme-linked immunosorbent assay (ELISA) test, or a positive HIV-1 ELISA test with concomitant incomplete Western blot (< 3 bands). HIV-1 controllers had viral loads of fewer than 5000 copies/mL and were infected for more than 13 years in the absence of antiretroviral therapy. Progressors were defined as being infected for more than 19 months and having a viral load of more than 30 000 copies/mL with no exposure to highly active antiretroviral therapy (HAART). The study was approved by the Massachusetts General Hospital Institutional Review Board.
Sorting of antigen-specific CD8+ T cells
Peripheral blood mononuclear cells (PBMCs) were stained with HIV-1–, cytomegalovirus (CMV)–, or Epstein-Barr virus (EBV)–specific tetramers/pentamers for 20 minutes at room temperature, followed by 15 minutes of surface staining with CD3 and CD8 antibodies, and processed to live cell sorting at 70 pounds per square inch using a 10-color fluorescence-activated cell sorter (FACS) ARIA instrument (Becton Dickinson, San Jose, CA). Live sorting was carried out in an appropriate and specifically designated biosafety hood (Baker Hood; Baker, Sanford, ME) according to a National Institutes of Health (NIH)–approved biosafety sorting protocol.
Telomere measurement by real-time quantitative PCR
Real-time quantitative polymerase chain reaction (RQ-PCR) procedures were carried out for the measurement of telomere length (T) in live-sorted HIV-1–specific CD8+ T cells and control cell populations as described previously.20,21 The single-copy gene 36B4 was used as a housekeeping gene (S). Telomere length of each sample was expressed as the relative T/S ratio after being standardized to the single-copy gene of the same sample as well as the mean T/S ratio for all samples on the same plate reflecting the well-to-well variation. The average of relative T/S ratios from at least 2 independent experiments was calculated for each sample.
RQ-TRAP assay
Telomerase activity in 1 000 live-sorted HIV-1–specific CD8+ T cells or control cell populations was assessed by using a SYBR Green (Applied Biosystems, Foster City, CA) real-time quantitative telomeric repeat amplification protocol (RQ-TRAP) assay as previously described.22 The threshold cycle value (Ct) of each sample was compared with standard curves generated from serial dilutions of telomerase-positive 293T cell extracts (5000, 1000, 200, 40, and 8 cells). Standards and negative controls with heat-inactivated samples and lysis buffer only were assayed on each plate. Each sample was analyzed at least in 2 independent assays. Telomerase activity was calculated relative to 293T cells and expressed as relative telomerase activity (RTA = Ct (293T)/Ct (sample)). The PCR products were visualized by gel electrophoresis.
Proliferation assay
Ex vivo proliferation assays were performed as previously described.23,24 In indicated experiments, PBMCs were initially exposed to a PD-L1–blocking antibody25 for 16 hours in the presence of antigenic peptides (0.2 μg/mL) and washed once before continuing incubation. On day 6, cells were harvested, washed with phosphate-buffered saline (PBS), and stained with monoclonal CD8 antibodies (Becton Dickinson) and, if indicated, MHC class I tetramers/pentamers refolded with the studied epitopic HIV-1 peptides. Cells were then washed and subjected to cell sorting.
Cytotoxicity assay
Cytotoxicity assays with fluorogenic caspase-3 substrates were performed as previously published,26 using the Cytoxilux cytotoxicity kit (OncoImmunin, Gaithersburg, MD). Target cells mixed with effector cells but lacking previous loading with HIV-1–specific peptides as well as peptide-labeled target cells without subsequent exposure to effector PBMCs were used as controls. The proportion of target cells dying of MHC class I–restricted cytolysis was calculated by subtracting the proportion of caspase-3–positive target cells in the control samples from the total proportion of caspase-3–positive target cells labeled with a specific viral peptide.
Statistics
Data are indicated as medians and ranges or means and standard deviations. Linear correlations were calculated using standard Pearson correlation coefficients. Differences between nominal data were tested for statistical significance by use of paired or unpaired Student t tests, as appropriate, and a P value less than .05 was considered significant.
Results
Telomere length stabilization in HIV-1–specific CD8+ T cells during long-term nonprogressive HIV-1 infection
Accelerated cellular senescence has been proposed as a critical mechanism contributing to dysfunctional immune responses in HIV-1 infection; however, telomere length as a key molecular marker of cellular senescence has never been assessed in these cells in a comparative population study involving patients with differential disease progression.13 To investigate the cellular senescence profile of HIV-1–specific CD8+ T cells during various stages of HIV-1 infection, we sorted tetramer+ HIV-1–specific CD8+ T cells from individuals with acute/primary, chronic progressive, and chronic nonprogressive HIV-1 infection and subsequently performed an RQ-PCR to assess their telomere length. Autologous total CD8+ T-cell populations and EBV- or CMV-specific CD8+ T cells were also sorted and used as reference populations. The demographic and clinical characteristics of the study cohorts are shown in Table 1; of note, there was no difference in the median age of individuals between the patient populations, but controllers had approximately 4- to 5-fold longer durations of known HIV-1 disease courses and approximately 2 000-fold lower median viral loads compared with progressors. The targeted epitopes as well as the magnitude of the analyzed CD8+ T-cell populations are indicated in Table 1 and Figure 1A, respectively.
Clinical and demographic characteristics of study patients
| . | No. of patients . | Age, y . | Time since diagnosis, mo . | CD4 cell count/ μL . | Viral load, copies/mL . | HIV-1 epitope tested . | EBV/CMV epitope tested . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | A2IV9 . | A2SL9 . | A3QK10 . | B8EI8 . | B8FL8 . | B27KK10 . | B57KF11 . | B57TW10 . | No. . | A2-NV9 (CMV) . | B8-FL8 (EBV) . | ||||||
| Progressor | 13 | 46 | 36 | 526 | 143 000 | 14 | 0 | 5 | 1 | 4 | 1 | 2 | 1 | 0 | 11 | 7 | 4 |
| Controller | 9 | 43 | 182.5 | 596 | 82 | 14 | 2 | 3 | 0 | 5 | 4 | 0 | 0 | 0 | 9 | 4 | 5 |
| Acute | 6 | 43.5 | 2 | 747 | 196 300 | 7 | 0 | 0 | 0 | 0 | 4 | 1 | 1 | 1 | 0 | 0 | 0 |
| . | No. of patients . | Age, y . | Time since diagnosis, mo . | CD4 cell count/ μL . | Viral load, copies/mL . | HIV-1 epitope tested . | EBV/CMV epitope tested . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | A2IV9 . | A2SL9 . | A3QK10 . | B8EI8 . | B8FL8 . | B27KK10 . | B57KF11 . | B57TW10 . | No. . | A2-NV9 (CMV) . | B8-FL8 (EBV) . | ||||||
| Progressor | 13 | 46 | 36 | 526 | 143 000 | 14 | 0 | 5 | 1 | 4 | 1 | 2 | 1 | 0 | 11 | 7 | 4 |
| Controller | 9 | 43 | 182.5 | 596 | 82 | 14 | 2 | 3 | 0 | 5 | 4 | 0 | 0 | 0 | 9 | 4 | 5 |
| Acute | 6 | 43.5 | 2 | 747 | 196 300 | 7 | 0 | 0 | 0 | 0 | 4 | 1 | 1 | 1 | 0 | 0 | 0 |
Assessment of telomere length in HIV-1–specific, CMV/EBV-specific, and bulk CD8+ T cells from different patient populations with HIV-1. (A) Proportion of studied antigen-specific tetramer+ CD8+ T cells in total CD8+ T-cell populations from individuals with chronic progressive, chronic long-term nonprogressive, or acute HIV-1 infection, as determined by flow cytometry. Horizontal bars reflect medians. (B) Telomere length of HIV-1–specific, CMV/EBV-specific, and autologous bulk CD8+ T cells from the corresponding individuals. Horizontal bars reflect means.
Assessment of telomere length in HIV-1–specific, CMV/EBV-specific, and bulk CD8+ T cells from different patient populations with HIV-1. (A) Proportion of studied antigen-specific tetramer+ CD8+ T cells in total CD8+ T-cell populations from individuals with chronic progressive, chronic long-term nonprogressive, or acute HIV-1 infection, as determined by flow cytometry. Horizontal bars reflect medians. (B) Telomere length of HIV-1–specific, CMV/EBV-specific, and autologous bulk CD8+ T cells from the corresponding individuals. Horizontal bars reflect means.
Between a total of 13 individuals with chronic progressive infection and 9 individuals with long-term spontaneous viral control, we found no significant differences in telomere length in total, unselected CD8+ T cells (P = .55). However, when comparing isolated HIV-1–specific CD8+ T-cell populations targeting multiple immunodominant HIV-1 cytotoxic T lymphocyte (CTL) epitopes, we observed significantly longer telomeres in HIV-1–specific CD8+ T cells from controllers compared with progressors (P < .001). In addition, the average telomere length of HIV-1–specific CD8+ T cells from individuals with chronic progressive infection was significantly lower compared with the corresponding bulk CD8+ T cells sorted from the same study population (P = .009), while the telomeres of HIV-1–specific CD8+ T cells from controllers were significantly longer than those from the respective bulk CD8+ T cells isolated from that study cohort (P = .004; Figure 1B). Using 2 cell lines (293T and K562) with known telomere lengths as references,21 we calculated the absolute telomere lengths of HIV-1–specific CD8+ T cells from progressors to reach a median of 5.8 kb (range, 4.8-6.4 kb). In contrast, HIV-1–specific CD8+ T cells from controllers has a median absolute telomere length of 7.4 kb (range, 6.3-12.9 kb; P < .001). For comparative purposes, we also performed telomere length analysis in autologous EBV- and CMV-specific T cells in the respective study subjects. While telomere lengths of EBV- and CMV-specific CD8+ T cells were not different between HIV-1 controllers and progressors (P = .77), they were significantly longer compared with HIV-1–specific CD8+ T cells from progressors (P = .02) but tended to be shorter compared with HIV-1–specific CD8+ T cells from controllers, although not significantly so (P = .2; Figure 1B). In addition, telomere lengths of HIV-1–specific CD8+ T cells recruited during acute or early HIV-1 infection had similar telomere lengths as those from controllers, although the latter had likely been recruited much earlier, but were significantly longer than the telomere lengths from individuals with chronic progressive infection (Figure 1B). Overall, these data indicate a correlation between telomere length of HIV-1–specific CD8+ T cells and the level of HIV-1 viremia in chronic infection, with, compared with bulk CD8+ T cells, a below-average telomere length of HIV-1–specific CD8+ T cells in progressive infection and an above-average telomere length in long-term nonprogressive infection.
Up-regulation of telomerase activity in HIV-1–specific CD8+ T cells from HIV-1 controllers
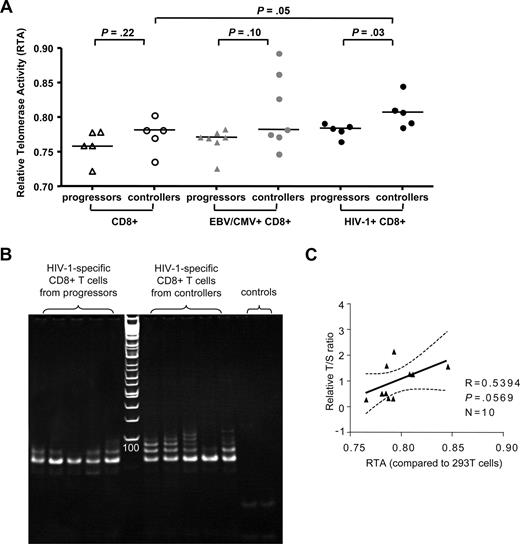
The fact that telomere lengths in HIV-1–specific CD8+ T cells from HIV-1 controllers were significantly higher compared with bulk CD8+ T cells from the same individuals suggests an active regulatory process leading to telomere elongation in these cells. Telomerase, an enzyme that is exclusively expressed in stem cells and lymphocytes, can actively extend telomere lengths of lymphocytes,27 but so far telomerase activity has never been assessed in HIV-1–specific CD8+ T cells. To test whether telomerase activity is constitutively up-regulated in HIV-1–specific CD8+ T cells during long-term nonprogressive infection, we obtained total protein extractions from freshly sorted HIV-1–specific CD8+ T cells without prior antigenic stimulation from individuals with either long-term nonprogressive or chronic progressive infection and subsequently analyzed telomerase activity using a functional assay based on ex vivo elongation of telomere ends on a substrate primer following addition of the protein extracts. For reference purposes, corresponding populations of bulk CD8+ T cells from progressors and controllers, as well as CMV- or EBV-specific CD8+ T cells, were similarly tested for telomerase activity. Overall, constitutive telomerase activity did not significantly differ between bulk CD8+ T cells or CMV/EBV-specific CD8+ T cells from controllers and progressors. Yet, in HIV-1–specific CD8+ T cells from controllers, constitutive, antigen-independent telomerase activity was stronger compared with autologous bulk CD8+ T cells (P = .05) or HIV-1–specific CD8+ T cells from progressors (P = .03). In contrast, HIV-1–specific CD8+ T cells from progressors did not significantly differ from autologous bulk CD8+ T cells or CMV/EBV-specific CD8+ T cells from either one of the 2 study groups (Figure 2). Interestingly, the correlation between telomerase activity and telomere length of HIV-1–specific CD8+ T cells was only moderately pronounced (r = .54, P = .06; Figure 2C), suggesting that telomerase activity does not represent the only mechanism involved in the regulation of telomere length in these cells. Overall, these data suggest a selective up-regulation of constitutive telomerase activity in HIV-1–specific CD8+ T cells from HIV-1 controllers, while telomerase activity in progressors was limited.
Comparative analysis of telomerase activity in HIV-1–specific CD8+ T-cell populations from individuals with progressive or controlled HIV-1. (A) Relative telomerase activity (RTA) of HIV-1–specific CD8+ T cells, as well as corresponding CMV/EBV-specific and bulk CD8+ T cells, from HIV progressors or controllers measured by RQ-TRAP. Horizontal bars reflect medians. (B) Visualization of telomeric extension of substrate primers by RQ-TRAP following exposure to protein extractions collected from the indicated sorted HIV-1–specific CD8+ T-cell populations from progressors or controllers. Left panel to the ladder reflect samples from progressors; right panel reflect samples from controllers; the last 2 lanes reflect the heat-inactivated samples and lysis buffer only control. (C) Correlation between telomere length and telomerase activity of the analyzed HIV-1–specific CD8+ T cells.
Comparative analysis of telomerase activity in HIV-1–specific CD8+ T-cell populations from individuals with progressive or controlled HIV-1. (A) Relative telomerase activity (RTA) of HIV-1–specific CD8+ T cells, as well as corresponding CMV/EBV-specific and bulk CD8+ T cells, from HIV progressors or controllers measured by RQ-TRAP. Horizontal bars reflect medians. (B) Visualization of telomeric extension of substrate primers by RQ-TRAP following exposure to protein extractions collected from the indicated sorted HIV-1–specific CD8+ T-cell populations from progressors or controllers. Left panel to the ladder reflect samples from progressors; right panel reflect samples from controllers; the last 2 lanes reflect the heat-inactivated samples and lysis buffer only control. (C) Correlation between telomere length and telomerase activity of the analyzed HIV-1–specific CD8+ T cells.
Telomere length stabilization is associated with strong proliferative and cytotoxic activities of HIV-1–specific CD8+ T cells
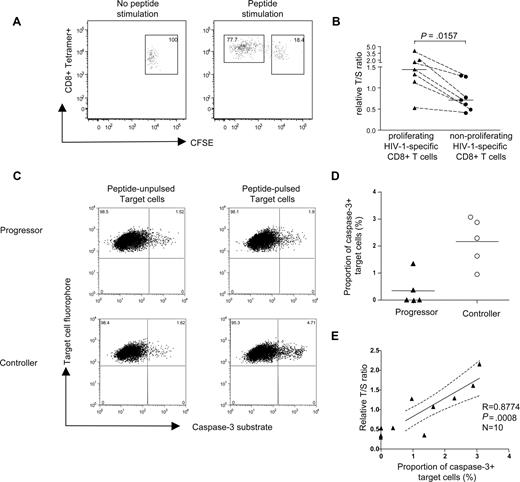
A series of studies have shown that HIV-1–specific CD8+ T cells from chronic progressive infection have a critical defect in ex vivo proliferative capacities compared with both HIV-1–specific CD8+ T cells during primary or chronic long-term nonprogressive infection.3,23 Telomere shortening has previously been shown to limit proliferative capacities of cells and might therefore represent the key molecular correlate of the proliferative deficiency and replicative senescence observed in HIV-1–specific CD8+ T cells from individuals with chronic progressive infection.28 To test this, we performed an intraindividual comparison between the telomere lengths of proliferating and nonproliferating HIV-1–specific CD8+ T cells recognizing the same CTL epitope. PBMCs from donors with HIV-1 with detectable proliferative CD8+ T-cell responses were labeled with CFSE and subsequently stimulated with a pool of overlapping peptides corresponding to selected HIV-1 gene products; after 6 days in culture, we selectively sorted tetramer+ CD8+ T cells that were either CFSEbright (nonproliferating) or CFSEdim (proliferating; Figure 3A). In a total of 7 HIV-1–specific CD8+ T-cell populations from 7 study persons, we found a significantly higher telomere length of proliferating cells versus nonproliferating cells (Figure 3A,B), indicating that telomere length stabilization correlates with preserved proliferative capacity of HIV-1–specific CD8+ T cells.
Correlation between telomere length/telomerase activity of HIV-1–specific CD8+ T cells and their proliferative and cytotoxic activity. (A) Representative dot plots indicating gating of proliferating (CFSEdim) and nonproliferating (CFSEbright) tetramer+ HIV-1–specific CD8+ T cells. Numbers in quadrants reflect proportion of tetramer+ CD8+ T cells. (B) Comparison of telomere lengths between proliferating and corresponding nonproliferating tetramer+ HIV-1–specific CD8+ T cells. Horizontal bars reflect medians. (C) Representative dot plots reflecting caspase-3 expression in peptide-pulsed target cells following exposure to HIV-1–specific CD8+ T cells from individuals with progressive or controlled viremia. Numbers in quadrants reflect proportion of target cells. (D) Proportion of caspase-3–positive target cells after coincubation with PBMCs from HIV-1 controllers (n = 5) or HIV-1 progressors (n = 5). Horizontal bars reflect means. (E) Correlation between cytotoxic activity of HIV-1–specific CD8+ T cells (measured by proportion of caspase-3–positive target cells) and corresponding telomere length.
Correlation between telomere length/telomerase activity of HIV-1–specific CD8+ T cells and their proliferative and cytotoxic activity. (A) Representative dot plots indicating gating of proliferating (CFSEdim) and nonproliferating (CFSEbright) tetramer+ HIV-1–specific CD8+ T cells. Numbers in quadrants reflect proportion of tetramer+ CD8+ T cells. (B) Comparison of telomere lengths between proliferating and corresponding nonproliferating tetramer+ HIV-1–specific CD8+ T cells. Horizontal bars reflect medians. (C) Representative dot plots reflecting caspase-3 expression in peptide-pulsed target cells following exposure to HIV-1–specific CD8+ T cells from individuals with progressive or controlled viremia. Numbers in quadrants reflect proportion of target cells. (D) Proportion of caspase-3–positive target cells after coincubation with PBMCs from HIV-1 controllers (n = 5) or HIV-1 progressors (n = 5). Horizontal bars reflect means. (E) Correlation between cytotoxic activity of HIV-1–specific CD8+ T cells (measured by proportion of caspase-3–positive target cells) and corresponding telomere length.
Since proliferative capacity has been linked to perforin expression and cytotoxic effects in previous studies, we next tested whether telomere length stabilization in HIV-1–specific CD8+ T cells from individuals with long-term nonprogressive infection is also associated with enhanced cytotoxic activities. To analyze this, we used a direct, highly sensitive cytotoxicity assay to correlate cytotoxic properties of HIV-1–specific CD8+ T cells with the corresponding telomere length of these cells. For these studies, 5 individuals from our original progressor cohort and 5 additional individuals from our controller cohort were selected (mean relative T/S ratio of corresponding HIV-1–specific CD8+ T-cell populations from progressors vs controllers: 0.41 vs 1.48; P = .003). Thawed bulk PBMCs were added to single HLA-matched heterologous B-cell lymphoma (BCL) cell line pulsed with the peptide of interest in the presence of caspase-3 substrates that emitted fluorescence signals in dying target cells. Results from these assays indicated that cytotoxic activities of HIV-1–specific CD8+ T cells with preserved telomere length had significantly higher cytotoxic activities compared with HIV-1–specific CD8+ T cells with shortened telomeres (Figure 3C,D). Moreover, we observed a significant correlation between lymphocellular telomere length and corresponding cytotoxic activity (Figure 3E). Collectively, these data indicate that key lymphocellular effector functions of HIV-1–specific CD8+ T cells are critically associated with telomere length stabilization.
Blockade of PD-1/PD-L1 interactions leads to elongation of telomere length and up-regulation of telomerase activity
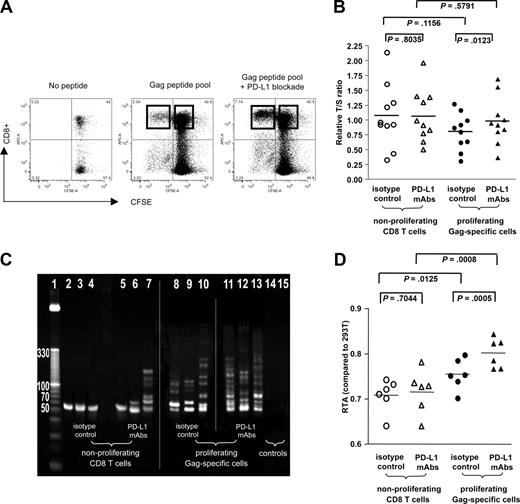
Previous studies have shown that functional defects of HIV-1–specific CD8+ T cells, in particular their antigen-specific proliferative activity, can be reversed in vitro by blockade of the inhibitory PD-1/PD-L1 pathway;25,29 however, molecular mechanisms underlying this restoration of proliferative T-cell activity are unclear. Given the observed strong correlation between telomere length and telomerase activity on the one hand and proliferative capacity of HIV-1–specific CD8+ T cells on the other, we hypothesized that a main mechanism responsible for the reconstitution of T-cell proliferation resulting from PD-1/PD-L1 blockade involves up-regulation of telomerase activity with subsequent elongation of telomere length. To test this hypothesis, we stimulated CFSE-labeled PBMCs from HIV-1 progressors with a pool of overlapping HIV-1 gag peptides in the presence or absence of PD-L1–blocking antibodies and sorted proliferating, CFSEdim gag-specific CD8+ T cells as well as nonproliferating, CFSEbright bulk CD8+ T cells after 6 days of culture. In agreement with previous studies,25,27 we observed a significant increase in the proportion of proliferating CD8+ T cells following antigenic stimulation in the presence of PD-L1–blocking antibodies (median of 4.58%; range, 0.47%-26.05% vs 7.68%; range, 1.34%-27.55%; P = .009; Figure 4A). Although PD-L1 blockade had no significant impact on telomere lengths of bulk CD8+ T cells, we observed that it resulted in a significant elongation of the telomere lengths of proliferating gag-specific CD8+ T cells when compared with corresponding proliferating gag-specific CD8+ T cells cultured without PD-L1 blockade (P = .01; Figure 4B). When assessing the corresponding telomerase function of these cell populations, we noticed that after 6 days in culture, gag-specific CD8+ T cells generally had stronger telomerase activities than bulk CD8+ T-cell populations, likely resulting from the fact that these cells had been stimulated with antigenic peptides, which is known to cause telomerase up-regulation. Moreover, corresponding to the enhancement of telomere lengths by PD-L1 blockade described above, telomerase activities in gag-specific CD8+ T cells was significantly augmented when these cells were stimulated with antigen in the presence of PD-L1 antibodies compared with identical cells cultured without PD-L1 blocking antibodies (P < .001; Figure 4C,D). This effect of PD-L1 blockade on telomere length and telomerase activity occurred in HIV-1–specific CD8+ T-cell populations with a wide range of different baseline telomere lengths (4.84-9.02 kb), indicating that PD-L1 blockade can reconstitute telomere lengths in lymphocytes with different degrees of exhaustion or senescence. To test whether the observed telomerase up-regulation by PD-L1 blockade depends on simultaneous antigenic T-cell receptor (TCR) stimulation or can also occur without such stimulus, we analyzed telomerase activity and telomere lengths of tet+ HIV-1–specific CD8+ T cells from progressors (n = 5) that were incubated with PD-L1–blocking antibodies without concomitant TCR stimulation; however, neither telomere lengths (P = .4) nor telomerase activity (P = .55) of these cells were significantly affected by PD-L1–blocking antibodies when compared with untreated cells. Taken together, these results show that PD-L1 blockade can actively increase telomere length and telomerase activity of HIV-1–specific CD8+ T cells in the context of simultaneous TCR stimulation and imply that PD-1/PD-L1 interactions may have an important role for the manipulation of aging processes and the replicative lifespan of lymphocytes.
Augmentation of telomere length and telomerase activity of HIV-1–specific CD8+ T cells after antibody-mediated PD-L1 blockade. (A) Dot plots reflecting proliferative activity of HIV-1–specific CD8+ T cells in the presence or absence of PD-L1–blocking antibodies. CFSEdim proliferating HIV-1–specific CD8+ T cells after antigenic stimulation and bulk CFSEbright CD8+ T cells were sorted according to the gates indicated. Numbers in quadrants reflect proportion of gated lymphocytes. (B) Telomere length of HIV-1–specific CD8+ T cells proliferating in the presence or absence of PD-L1–blocking antibodies as well as of the corresponding nonproliferating bulk CD8+ T cells. (C,D) Telomerase activity of bulk CD8+ T cells and sorted HIV-1–specific CD8+ T cells that were stimulated with antigenic peptides in the presence or absence of PD-L1–blocking antibodies, as determined by RQ-TRAP with subsequent analysis by gel electrophoresis (panel C; vertical lines have been inserted to indicate repositioned gel lanes) and after normalization to a reference cell line as described in “Methods” (D). Horizontal bars in panels B and D reflect means.
Augmentation of telomere length and telomerase activity of HIV-1–specific CD8+ T cells after antibody-mediated PD-L1 blockade. (A) Dot plots reflecting proliferative activity of HIV-1–specific CD8+ T cells in the presence or absence of PD-L1–blocking antibodies. CFSEdim proliferating HIV-1–specific CD8+ T cells after antigenic stimulation and bulk CFSEbright CD8+ T cells were sorted according to the gates indicated. Numbers in quadrants reflect proportion of gated lymphocytes. (B) Telomere length of HIV-1–specific CD8+ T cells proliferating in the presence or absence of PD-L1–blocking antibodies as well as of the corresponding nonproliferating bulk CD8+ T cells. (C,D) Telomerase activity of bulk CD8+ T cells and sorted HIV-1–specific CD8+ T cells that were stimulated with antigenic peptides in the presence or absence of PD-L1–blocking antibodies, as determined by RQ-TRAP with subsequent analysis by gel electrophoresis (panel C; vertical lines have been inserted to indicate repositioned gel lanes) and after normalization to a reference cell line as described in “Methods” (D). Horizontal bars in panels B and D reflect means.
Discussion
Although accelerated immune senescence has been repeatedly suggested as a critical component in HIV-1 pathogenesis,11,12,30 the aging profile of HIV-1–specific CD8+ T cells, which play a major role in the host's immune response against HIV-1, has never been investigated. Here, we analyzed HIV-1–specific CD8+ T cells at the level of telomere length and telomerase activity, which represent the principal molecular pathway regulating somatic cell proliferation and cell aging, and might similarly have critical influence on the functional profile of lymphocytes. Our data indicate significant telomere shortening with limited telomerase enzymatic activity in HIV-1–specific CD8+ T cells from progressors, while HIV-1 controllers, despite substantially prolonged durations of HIV-1 disease history, had HIV-1–specific CD8+ T cells whose telomeres were substantially longer than those of autologous bulk CD8+ T cells and also tended to be longer than those of lymphocytes recognizing CMV or EBV, although not significantly so. Moreover, we noted an antigenic peptide–independent, constitutive up-regulation of telomerase in HIV-1–specific CD8+ T cells from controllers, which clearly exceeded telomerase activity in corresponding cells from progressors and was associated with maintenance of key lymphocellular effector functions. These data suggest a causal role of telomere shortening and cellular senescence for dysfunction of HIV-1–specific CD8+ T cells in progressors and similarly indicate an active protective mechanism against cellular exhaustion in HIV-1–specific CD8+ T cells from controllers by up-regulation of the enzymatic activity of telomerase. Finally, we observed that in the context of simultaneous TCR stimulation, blockade of the PD-1/PD-L1 pathway resulted in active reversibility of telomere erosion and an increase of telomerase activity in HIV-1–specific CD8+ T cells from progressors, indicating that signaling through this pathway can critically affect the process of cell aging and the overall cellular replicative lifespan.
Although our data clearly demonstrate a substantial reduction in telomere length of HIV-1–specific CD8+ T cells from progressors, none of the CD8+ T-cell populations analyzed had a telomere length of less than 4 kb, which is considered as the threshold for finite and nonreversible replicative senescence.14,31 Yet, it is important to realize that the analyzed HIV-1–specific CD8+ T-cell populations consist of multiple different clones,32,33 which were likely recruited at different stages of the infection and thus had different telomere lengths; the measured telomere length of the entire population therefore only represents the average of the telomere lengths of the different T-cell clones contributing to the entire cell population. Given that this average telomere length of HIV-1–specific CD8+ T cells in progressors was very low (median of 5.8 kb), our data suggest that HIV-1–specific CD8+ T-cell populations in chronic progressive infection consist of cell clones that are either already in the state of “replicative senescence” or in the process of advanced exhaustion approaching this condition. Thus, these data support a scenario in which constant high-level viremia in chronic progressive HIV-1 infection leads to a high clonal turnover with continuous deletion and de novo generation of rapidly exhausting HIV-1–specific CD8+ T-cell clones with progressive telomere shortening and resulting deficiencies of lymphocellular effector functions.
Our analysis of HIV-1–specific CD8+ T cells from controllers indicates that these cells have relatively long telomere lengths with a strong expression of telomerase, resembling a pattern that is typically observed in undifferentiated and naive cells that recently emigrated from the thymus.28,34 Indeed, the telomere lengths of these cells were similar to the telomere lengths of HIV-1–specific CD8+ T cells collected during acute infection, which had just been recruited. However, previous studies of HIV-1–specific CD8+ T cells in long-term nonprogressive infection have shown that these cells by no means exhibit the undifferentiated phenotype of immature cells, but instead typically show a terminally differentiated phenotype, in contrast to HIV-1–specific CD8+ T cells from progressors, which predominantly have a more immature “effector memory” phenotype.35 This paradoxic observation of a terminally differentiated phenotype in combination with sustained telomere length and telomerase activity that is reminiscent of young, undifferentiated cells appears to be contradictory to the conventional view that final T-cell differentiation is associated with replicative senescence,36 and suggest that HIV-1–specific CD8+ T cells from controllers follow a different pattern of differentiation compared with HIV-1–specific CD8+ T cells from progressors. Based on previous studies in a murine model of acute versus chronic lymphocytic choriomeningitis virus (LCMV) infection,37 it is conceivable that HIV-1–specific CD8+ T cells from controllers represent self-regenerating cells that primarily depend on homeostatic cytokines such as IL-15 and IL-7 for their persistence, which have been shown to allow for cellular proliferation without simultaneous loss of telomere length or telomere erosion.38,39 Indeed, up-regulation of IL-7 and IL-15 receptors have recently been reported on HIV-1–specific CD8+ T-cell clones from controllers, which corresponded to long-term persistence of these cells and protection against clonal elimination.33 Moreover, as a major finding of this study, we found that HIV-1–specific CD8+ T cells from controllers had high levels of baseline telomerase activity even without prior stimulation with antigen, which might explain why these cells do not appear to rely on chronic antigenic stimulation for their in vivo persistence. In fact, HIV-1–specific CD8+ T cells from controllers are typically maintained in the presence of extremely low or even undetectable HIV-1 antigen concentrations, and the constitutively high activity of telomerase in conjunction with an antigenic peptide–independent, homeostatic pattern of cell turnover might allow for the continuous persistence and proliferation of these cells without loss of telomere length. In contrast, HIV-1–specific CD8+ T cells from progressors tend to rapidly disappear after CTL escape mutations40 or following exposure to HAART,41 suggesting that their maintenance and differentiation is critically mediated by chronic antigenic stimulation, which apparently leads to progressive telomere erosion as well as limitations in their ability to up-regulate telomerase activity and ultimately results in lymphocellular exhaustion. Clearly, detailed molecular mechanisms that allow for the antigen-independent maintenance and differentiation of HIV-1–specific CD8+ T cells from controllers with simultaneous up-regulation of constitutive, antigen-independent telomerase activity and resulting protection against telomere loss need to be further investigated. Moreover, given that we only observed a relatively weak association between telomerase activities and telomere lengths, it is likely that telomerase activity is not the only mechanism affecting telomere homeostasis in HIV-1–specific CD8+ T cells; future studies will therefore be necessary to determine which roles other factors, such as the shelterin complex of nucleoproteins, which protects telomeric DNA,42 play in the regulation of telomere homeostasis of these cells.
As an important finding of this study, we observed that telomerase activity of HIV-1–specific CD8+ T cells can be actively increased by blocking the inhibitory PD-1/PD-L1 pathway. Recent studies have shown that blockade of PD-1/PD-L1 can result in the augmentation of proliferative activities of HIV-1–specific CD8+ T cells, although it was unclear if this effect is simply related to increased cell turnover that might accelerate aging and senescence of HIV-1–specific T cells,43 or to an opposite mechanism that involves an increase of the replicative lifespan and a reversion of cellular aging processes. Our data suggest that PD-1 pathway blockade does not simply affect G0/G1 cell-cycle checkpoints and the frequency of cells entering mitosis, but actively enhances telomerase activity and telomere length and in this way fundamentally alters the life expectancy and the ability of HIV-1–specific CD8+ T-cell clones to persist, self-renew, and expand during the course of the infection. However, it should be noted that we also observed preserved telomere length in cells for which intermediate or high PD-1 expression has been reported previously, such as CMV/EBV-specific CD8+ T cells; therefore, it appears that PD-1 expression by itself does not necessarily reflect cellular exhaustion or immune senescence, but could also be influenced by T-cell differentiation or activation.44 Mechanistically, it will be important to determine if up-regulation of telomerase activity after PD-L1 blockade is due to induction of higher levels of telomerase protein expression, or if interruption of the PD-1/PD-L1 interactions stimulates telomerase activity by nontranscriptional means involving alterations of specific signaling cascades, such as phosphorylation of Akt, which has recently been identified as a critical regulator of telomerase activity.45 Finally, recent data suggested general limitations of the replicative lifespan of pathogen-specific memory cells, which in elderly individuals can lead to a higher susceptibility to infections to which they have previously been immune.31 An active extension of telomere length and life expectancy of antigen-specific T cells by means of pharmacologic PD-1 blockade might therefore not only provide a promising strategy for enhancing immune control against HIV-1, but also for the maintenance and homeostasis of a variety of other antigen-specific T cells in elderly persons.
In conclusion, we report a constitutive, antigen-independent up-regulation of telomerase activity in HIV-1–specific CD8+ T cells from controllers, which was associated with stabilization of telomere length and effective execution of lymphocellular effector functions. In contrast, HIV-1–specific CD8+ T cells from progressors showed a pattern of telomere erosion and replicative senescence associated with functional deficiencies and limited constitutive telomerase activity, although both telomere lengths and telomerase activities were actively increased by blocking the PD-1/PD-L1 pathway in these cells. This study, which provides the first comprehensive assessment of telomere length and telomerase activity of antigen-specific T cells in a human model of chronic viral infection, contributes importantly to the mechanistic understanding of the long-term, antigen-independent persistence of highly-functional HIV-1–specific CD8+ T cells in controlled HIV-1 infection as well as of the corresponding emergence of functionally defective and exhausted HIV-1–specific CD8+ T cells in chronic progressive infection. Moreover, our observation of an active reversibility of cellular aging processes by blockade of the PD-1/PD-L1 pathway will have implications for the active manipulation of the generation, homeostasis, and functionality of antigen-specific T-cell responses in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the National Institutes of Health to X.G.Y. (AI068499, AI078799) and G.J.F. (AI56299). X.G.Y. is a recipient of a Harvard University Research Enabling Grant, the Doris Duke Clinical Scientist Development Award, and the Claflin Distinguished Scholar Award.
National Institutes of Health
Authorship
Contribution: M.L. and X.G.Y. designed the study; performed proliferation, cytotoxicity, and cell-sorting experiments; analyzed data; and wrote the manuscript. D.M. and T.D.C. performed and analyzed qPCR experiments. T.D.C., K.L.W., and J.H. assisted in flow cytometry experiments. M.T.W. performed cell-sorting experiments. F.P., A.T., and E.S.R. contributed PBMC specimens. G.J.F. produced the PD-L1–blocking antibody. B.D.W. contributed PBMC specimens and critically reviewed the manuscript.
Conflict-of-interest disclosure: G.J.F. has patents and royalty payments in the field of PD-1/PD-1 ligands. The remaining authors declare no competing financial interests.
Correspondence: Xu Yu, Partners AIDS Research Center, Massachusetts General Hospital, Boston, MA 02129; e-mail: xyu@partners.org.
References
Author notes
*M.L. and D.M. share the first authorship on this article.