Abstract
Cross-presentation is a crucial mechanism in tumoral and microbial immunity because it allows internalized cell associated or exogenous antigens (Ags) to be delivered into the major histocompatibility complex I pathway. This pathway is important for the development of CD8+ T-cell responses and for the induction of tolerance. In mice, cross-presentation is considered to be a unique property of CD8α+ conventional dendritic cells (DCs). Here we show that splenic plasmacytoid DCs (pDCs) efficiently capture exogenous Ags in vivo but are not able to cross-present these Ags at steady state. However, in vitro and in vivo stimulation by Toll-like receptor-7, or -9 or viruses licenses pDCs to cross-present soluble or particulate Ags by a transporter associated with antigen processing-dependent mechanism. Induction of cross-presentation confers to pDCs the ability to generate efficient effector CD8+ T-cell responses against exogenous Ags in vivo, showing that pDCs may play a crucial role in induction of adaptive immune responses against pathogens that do not infect tissues of hemopoietic origin. This study provides the first evidence for an in vivo role of splenic pDCs in Ag cross-presentation and T-cell cross-priming and suggests that pDCs may constitute an attractive target to boost the efficacy of vaccines based on cytotoxic T lymphocyte induction.
Introduction
Cross-presentation is a mechanism that involves the uptake and processing of exogenous antigens (Ags) within the major histocompatibility complex (MHC) class I pathway.1 This process differs from the classical MHC class I processing pathway in which MHC class I molecules present Ags that are synthesized within the cells. Cross-presentation occurs subsequent to transport of Ag from the endosome to cytosol and digestion by the proteasome and may concern different forms of exogenous Ags, such as soluble,2 particulate,3 or cell-associated Ags.4 However, alternative mechanisms have been described, and the cellular and molecular pathways that lead to the presentation of endocytosed proteins on MHC class I molecules are not yet fully defined.5 Cross-presentation is a crucial mechanism allowing induction of naive CD8+ T-cell responses against exogenous Ag and has been implicated in the elicitation of protective cytotoxic T lymphocyte (CTL) responses against tumors as well as in the generation of immune responses against clinically relevant pathogens that do not infect tissues of hemopoietic origin.6,7 In addition to cross-priming of naive CD8+ T cells, cross-presentation appears to be central to the maintenance of peripheral tolerance to self-Ag, called “cross-tolerance.”8,9
With very few exceptions,10,11 cross-presentation is restricted to dendritic cells (DCs)2,12 and macrophages13,14 but is not normally seen in other nucleated cells. DCs are considered the only Ag-presenting cell (APC) able to prime naive T cells and are thus involved in the induction of both adaptive immunity and tolerance. The outcome of T-cell responses depends on DC maturation state, as immature or semimature DCs have been described to induce tolerance.15 Immature DCs are present in the periphery where they sample Ags. They are characterized by high expression of receptors involved in endocytosis and phagocytosis.16 Maturation of DCs can be induced by pathogens or by inflammatory signals. Pathogens are recognized through pathogen-associated molecular patterns, which interact with pattern recognition receptors, including Toll-like receptors (TLRs), constitutively expressed by DCs.17,18 Maturation involves the migration of DCs to lymphoid organs, the activation of proteolytic activity for Ag processing, and the up-regulation of MHC and costimulatory molecules. After maturation, DCs prime naive T cells, leading to their differentiation into effector T cells.19
Several populations of DCs have been described, and it is unclear whether all these DC types can cross-present exogenous Ag. In mice, splenic DCs are constituted of a heterogeneous population characterized by the expression of CD11c and can be divided into several subsets according to the expression of various surface markers.20 Conventional DCs (cDCs), including CD8α+ (CD11b−) and CD8α− (CD11b+) cells, efficiently prime CD4+ and CD8+ T-cell responses.16 CD8α+ DCs has been described as the only cell type able to cross-present cellular and viral Ags.21,22 However, cross-presentation by CD8α− DCs can be inducible through triggering by FcR,23 lipopolysaccharide (LPS),24 or virus-like particles,25 suggesting that the capacity of this subset to cross-present depends on environmental factors or encountered pathogens.
Plasmacytoid DCs (pDCs) display a CD11clow B220+ phenotype and also express Ly6-C, CD45RA,26 as well as BST-2.27 Their TLR pattern expression is different from cDCs because they only express TLR-7 and TLR-9, whereas cDCs express almost all TLRs, including TLR-7 and TLR-9. These cells are principally characterized as type I interferon (IFN) producers after viral stimulation or TLR engagement. At steady state, they show a poor efficiency to induce alloreaction. Until recently, pDCs have been considered to have also a low capacity of phagocytosis and macropinocytosis.28 However, recent papers challenged this observation by demonstrating that both human29 and mouse30,31 pDCs are able to capture exogenous Ags efficiently. Furthermore, recent data suggest that pDCs may be directly involved in the induction of adaptive immunity both in humans and in mice. Indeed, human pDCs activate both CD4+ and CD8+ T cells in vitro after exposure to inactivated influenza A virus (IAV).32 In mice, pDCs promote Th1 CD4+ T-cell responses after stimulation through TLR-933 and prime CD8+ T cells when infected by cytomegalovirus.34 In vivo, mouse splenic pDCs loaded with an antigenic peptide and transferred to syngeneic recipients induce a CTL response after stimulation by IAV.35 pDCs are also capable to induce efficient CD8+ T-cell responses against endogenous Ag in vivo after either TLR-ligand (TLR-L) or viral stimulation.35,36 Altogether, the aforementioned studies demonstrate that activated pDCs display a full T-cell stimulation capacity after direct presentation of loaded antigenic peptide or of endogenous Ags. In contrast, the ability of mouse pDCs to capture, degrade, and cross-present exogenous Ags has not been thoroughly investigated. Moreover, it has been claimed that mouse pDCs are unable to cross-prime naive CD8+ T cells in vivo.36 However, recently, human blood pDCs were shown to perform cross-presentation in vitro.29 To clarify this question, it is thus important to assess whether murine pDCs could also cross-present exogenous Ags in vitro and, more importantly, in vivo under normal or pathologic conditions.
In this study, we thus analyze the ability of splenic pDCs to perform the different steps involved in the cross-presentation pathway. We show that, at steady state, splenic pDCs efficiently capture and degrade exogenous Ags both in vitro and in vivo but are unable to cross-present these Ags. However, TLR-L or viral stimulation induces efficient cross-presentation leading to in vivo cross-priming of naive CD8+ T cells by pDCs. Here we clearly demonstrate that splenic pDCs possess a specialized machinery to deliver different forms of Ag to the cross-presentation pathway and that this machinery is under the control of TLR activation. This study represents the first in vivo demonstration that pDCs are able to elicit CD8+ T-cell responses against exogenous Ags.
Methods
Mice
129sv (H-2b) and C57BL/6 CD45.1 (H-2b) mice were purchased from Charles River Laboratories (Les Oncins, France) and used when between 6 and 10 weeks old. Rag−/− OT-I T-cell receptor transgenic mice, specific for the Kb-restricted ovalbumin257-264 (OVA257-264) epitope, kindly provided by Sylvie Garcia (Pasteur Institute, Paris, France), were bred at the animal facilities of the Pasteur Institute. All mice were maintained under specific pathogen-free conditions. All animal work was approved by the institutional animal experimentation committee: Direction Départementale des Services Vétérinaires de la Préfecture de Police de Paris.
Culture medium
Complete medium consisted of RPMI 1640 (Invitrogen, Paisley, United Kingdom) containing L-alanyl-L-glutamine dipeptide (GlutaMAX, Invitrogen) supplemented with 10% fetal calf serum (Valeant Pharmaceuticals, Costa Mesa, CA), 5 × 10−5 M 2-mercaptoethanol, and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL; Invitrogen). Synthetic HL-1 medium was obtained from Lonza Walkersville (Walkersville, MD) and supplemented with 5 × 10−5 M 2-mercaptoethanol, anti-biotics, and GlutaMAX.
Proteins, peptides, and cell lines
The synthetic peptide SIINFEKL (OVA257-264), corresponding to the H-2Kb restricted CTL epitope of ovalbumin (OVA) was purchased from NeoMPS (Strasbourg, France). The OVA protein was obtained from Calbiochem (San Diego, CA) and contained less than 50 U/mg LPS; 1-μm beads (polystyrene microspheres) were coated with either peptide or OVA protein at 0.5 mg/mL by passive adsorption according to the manufacturer's procedures (Polysciences, Warrington, PA).
Molecular weight 40 K fluorescein isothiocyanate-dextran (Sigma-Aldrich, St Louis, MO), Fluoresbrite Yellow Green beads (Polysciences), or Red beads (Invitrogen) and OVA protein labeled with Alexa 488 (Invitrogen) were used in capture assays. DQ OVA (Invitrogen) was used to test OVA degradation. Lucifer Yellow and dimethyl amiloride (Sigma-Aldrich) were used to analyze micropinocytosis activity.
B3Z, a CD8+ T-cell hybridoma specific for the OVA257-264 epitope, was a generous gift from N. Shastri (University of California, Berkeley).
TLR-L and viruses
R848 (TLR-7 ligand) was purchased from Invivogen (Toulouse, France) and unmethylated cytosine-guanine (CpG) motifs (TLR-9 ligand-CpG 1826 5′-TCCATGACGTTCCTGACGTT-3′) were synthesized by Proligo (Paris, France). For in vivo experiments, CpG (100 μg) was mixed with 15 μL N-[1-(2,3-dioleoyloxy)propyl]-N, N, N-trimethyl ammonium methylsulfate (DOTAP; Roche Diagnostics, Mannheim, Germany) diluted in 35 μL phosphate-buffered saline (PBS), such that the final volume was 75 μL.
Flow cytometry
Isolated cells were resuspended as described35 and labeled with monoclonal antibodies (mAbs; indicated clone): anti-CD11c (HL-3), -B220 (RA3-6B2), -Ly6-C (AL-21), -CD45RA (14.8), -CD11b (M1/70), -CD8α (53-6.7), -Kb (AF6-88.5), -I-Ab (25-9-17), -CD40 (HM40-3), -CD80 (16-10A1), -CD86 (GL1), -CD3ϵ (145-2C11), -CD90.2 (53-2.1). All mAbs and isotype control Igs were purchased from BD Biosciences PharMingen (San Diego, CA). Anti–BST-2 was purchased from either Miltenyi Biotec (Bergisch-Gladbach, Germany; mPDCA-1 mAb, clone JF05-1C2.4.1) or AbCys (Paris, France; MIPC mAb, clone 120G8). H-2Kb-SIINFEKL-Pentamers (ProImmune, Oxford, United Kingdom) staining was done according to the same procedure. Events were acquired on a Cyan cytometer (Dako, Glostrup, Denmark) and analyzed using FlowJo Software (TreeStar, Ashland, OR).
Microscopy analysis
Purified pDCs from OVA-A488–injected mice were adhered onto poly-lysine–coated glass slides and then stained with Alexa 546–conjugated anti–BST-2 (120G8) and biotin-conjugated anti–CD11c Abs. Biotinylated Ab was revealed with Alexa 647-streptavidin, and nuclei were stained with Hoechst 33 342 (0.1 μg/mL). After one night in mounting medium (Fluoromount-G; Southern Biotechnology Associates, Birmingham, AL), BST-2+ cells were screened under a Zeiss Axiovert 200M microscope with an oil immersion objective (63× APOCHROMAT) and a Roper Scientific Coolsnap HQ camera (Roper Scientifics, Trenton, NJ). The fluorescent image was deconvoluted using Zeiss Axiovision 4.2 and Photoshop CS2 software (Adobe Systems, Mountain View, CA).
Preparation of splenic DC subsets
Splenocytes were recovered as described.35 Splenic pDCs were purified by staining cells with biotinylated anti–mPDCA-1 mAb, then selecting by Automacs using MACS-antibiotin (Miltenyi Biotec). Splenic cDCs were purified from the recovered negative fraction that was stained with MACS-anti-CD11c mAb. Sorted DC subsets were always more than 90% pure.
Ag-presentation assays
Stimulation of the B3Z T-cell hybridoma was monitored by measuring the amount of interleukin-2 (IL-2) released into the supernatant of 24-hour cultures in the presence of purified DC subsets in 96-well culture plates. Serial dilutions of purified pDCs, or cDCs, were incubated with B3Z hybridoma cells (105 cells/well) in the presence or absence of Ags (either beads or soluble OVA or OVA257-264 peptide) and TLR-L (CpG or R848). After 24 hours, plates were centrifuged and supernatants were frozen for at least 1 hour at −20°C. IL-2 was assayed as described35 using the IL-2–dependent CTL-L cell line (ATCC, Manassas, VA).
Stimulation of OT-I T cells was detected by monitoring their proliferation. Lymph node cells were isolated from Rag−/− OT-I transgenic mice and cultured in complete HL-1 synthetic medium. For in vitro experiments, purified DC subsets (104/well) were first incubated for 2 hours in 96-well culture plates with serial dilutions of Ags in the presence or absence of TLR-L (CpG or R848). After washing, 2 × 104 OT-I cells/well were added. At 72 hours later, OT-I cell proliferation was scored according to [3H]thymidine incorporation as described.35
For ex vivo experiments, serial dilution of purified DC subsets from injected mice was cocultured with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled OT-I cells. Briefly, OT-I cells were incubated for 15 minutes at room temperature in the presence of 1 μM CFSE. At 72 hours later, cells were analyzed by flow cytometry.
Adoptive transfer
129sv mice were intravenously injected with either PBS, OVA alone, or OVA combined with R848, CpG-DOTAP, BV, or IAV. Two hours later, pDCs from the treated mice were purified and adoptively transferred into CD45.1 recipient mice, which received 2 × 106 CFSE (5 μM)–labeled OT-I cells. One week later, splenocytes and lymph node (LN) cells were isolated and cells were incubated with GolgiPlug (BD Biosciences, San Jose, CA) in the presence or absence of OVA257-264 peptide (1 μg/mL) for 4 hours. The cell samples were then stained with anti-CD45.2, -CD45.1, -CD8α, -CD3ϵ, and -CD44 in PBS containing 1% fetal calf serum and 0.1% NaN3 for 15 minutes. For intracellular cytokine staining, cells were fixed and permeabilized, and then anti–IFN-γ (XMG1.2 clone) mAb or control isotype IgG2a (R35-95 clone) was added.
In vivo killing assay
Naive syngenic splenocytes were pulsed with OVA257-264 peptide (10 μg/mL; 30 minutes, 37°C), washed extensively, and labeled with a high concentration (1.25 μM) of CFSE (Invitrogen). The nonpulsed control population was labeled with a low concentration (0.125 μM) of CFSE. Both CFSEhigh- and CFSElow-labeled cells were mixed at a 1:1 ratio (5 × 106 cells of each population) and then injected intravenously into mice. The number of CFSE+ cells remaining in the spleen after 20 hours was determined by FACS. Specific lysis was calculated as follows: % specific lysis = 100 − [100 × (% CFSEhigh immunized mice/% CFSElow immunized mice)/(% CFSEhigh naive mouse/% CFSElow naive mouse)].
Statistical analysis
The Dunnett analysis of variance test was used for statistical analysis.
Results
Splenic pDCs capture and degrade exogenous Ags in vitro
To analyze the cross-presentation capacity of pDCs, we purified DCs from spleen of 129sv mice. Total splenocytes from 129sv mice contained 2% cDCs (CD11chi B220−) and 1% pDCs (CD11clow B220+; Figure 1A). Splenic pDCs expressed Ly-6C, CD45RA, and BST-2 (PDCA-1; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Almost all cDCs expressed CD11b, reflecting the low percentage of the CD8α+ population, as observed in most mouse strains except C57BL/6.38 pDCs isolated from either CpG- or R848-injected mice up-regulated costimulatory molecules, such as CD40 and CD80 and MHC class II molecules (Figure S1B). Only a slight increase was observed for CD86, whereas the expression of MHC class I molecules was not modified. To test cytokine production, DC subsets were purified. The purity of DC subsets was in all cases more than 90%. Most of the cells contaminating sorted pDCs were B cells (data not shown). Contamination by cDCs was always under 1.5% with less than 0.1% of CD8α+ cells (Figure S2A,B). Purified pDCs produced large amounts of IFN-α, IL-6, and IL-12 after stimulation (Figure S1C). These results clearly show that purified pDCs matured after TLR-stimulation and were functional.
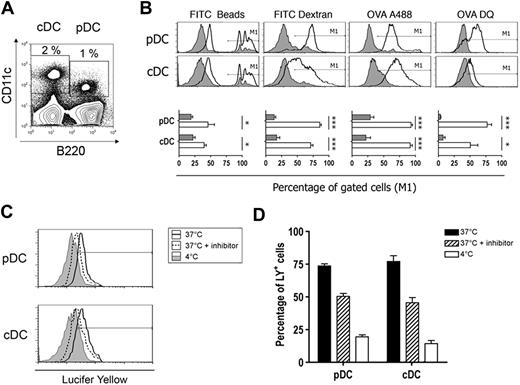
Splenic pDCs capture and degrade Ags efficiently. (A) Spleen cells were incubated for 1 hour in the presence of labeled Ags at 37°C in culture medium (CM) or at 4°C in CM containing 0.1% azide. Cells were stained with anti-CD11c and anti-B220 mAbs and analyzed by flow cytometry. DC subsets were gated as indicated. (B) Top panel: fluorescent intensity of labeled Ags was analyzed for each gated DC subset incubated at 37°C (thick line histogram) or at 4°C in CM containing 0.1% azide (filled histogram). Bottom panel: the percentage of cells gated in M1 that have captured Ag is plotted for each subset. Results represent cumulative data from 4 independent experiments and are expressed as mean percentages plus or minus SD. *P < .05, ***P < .001. (C) Spleen cells were incubated with Lucifer Yellow (0.4 mg/mL) in the presence (dotted line histogram) or absence (thick line histogram) of inhibitor (dimethyl amiloride, 100 μM) at 37°C or at 4°C (filled histogram), and fluorescent intensity was analyzed for both DC subsets. Lucifer Yellow+ cells were gated as shown. (D) Percentage of Lucifer Yellow+ cells in each condition is plotted. Results represent cumulative data from 4 independent experiments and are expressed as mean percentages plus or minus SD.
Splenic pDCs capture and degrade Ags efficiently. (A) Spleen cells were incubated for 1 hour in the presence of labeled Ags at 37°C in culture medium (CM) or at 4°C in CM containing 0.1% azide. Cells were stained with anti-CD11c and anti-B220 mAbs and analyzed by flow cytometry. DC subsets were gated as indicated. (B) Top panel: fluorescent intensity of labeled Ags was analyzed for each gated DC subset incubated at 37°C (thick line histogram) or at 4°C in CM containing 0.1% azide (filled histogram). Bottom panel: the percentage of cells gated in M1 that have captured Ag is plotted for each subset. Results represent cumulative data from 4 independent experiments and are expressed as mean percentages plus or minus SD. *P < .05, ***P < .001. (C) Spleen cells were incubated with Lucifer Yellow (0.4 mg/mL) in the presence (dotted line histogram) or absence (thick line histogram) of inhibitor (dimethyl amiloride, 100 μM) at 37°C or at 4°C (filled histogram), and fluorescent intensity was analyzed for both DC subsets. Lucifer Yellow+ cells were gated as shown. (D) Percentage of Lucifer Yellow+ cells in each condition is plotted. Results represent cumulative data from 4 independent experiments and are expressed as mean percentages plus or minus SD.
Because pDCs were initially considered to display poor phagocytic or endocytic capacities, we analyzed the ability of freshly isolated pDCs to uptake and degrade soluble and particulate Ags. DCs were incubated with various labeled Ags that are internalized by different pathways. Synthetic beads are taken up by phagocytosis,39 dextran polymers by endocytosis,40 and OVA by macropinocytosis.40 All these 3 Ags were efficiently captured by both pDC and cDC subsets (Figure 1B). The significant decrease of Ag uptake observed at 4°C in the presence of azide indicates that capture was an active mechanism. Macropinocytosis activity of pDCs was confirmed by Lucifer Yellow capture, which was specifically inhibited by dimethyl amiloride (Figure 1C,D).
In addition, OVA degradation after capture was assessed using DQ OVA, a self-quenched conjugate of OVA that exhibits bright green fluorescence on proteolytic degradation. DC subsets had strong DQ OVA degradation activities and pDCs were even more active than cDCs.
Overall, these findings demonstrate that splenic pDCs are able to capture Ags in vitro and exhibit ability to degrade the soluble OVA Ag.
Cross-presentation ability of pDCs is inducible
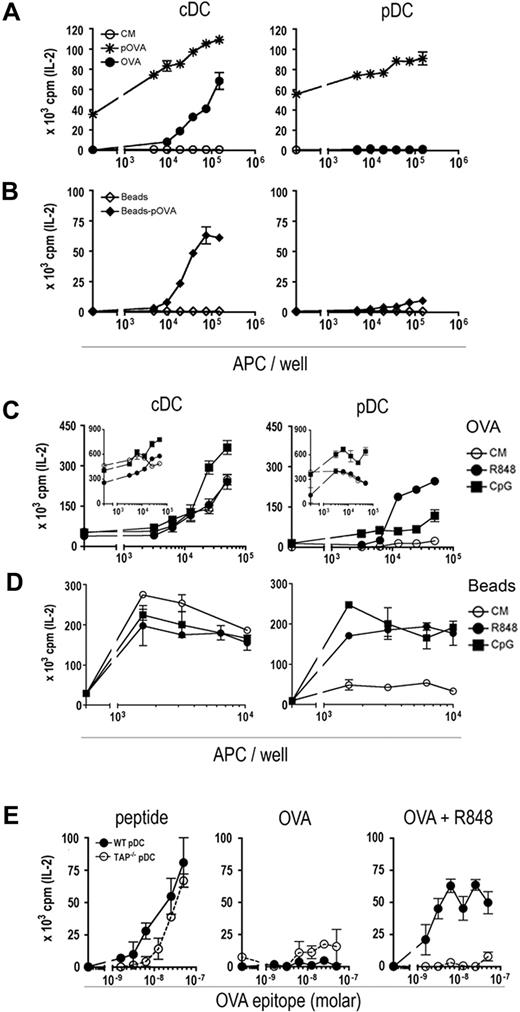
Purified pDC and cDC subsets from 129sv mice were incubated in the presence of OVA257-264 peptide, of soluble OVA, or of beads carrying the OVA257-264 peptide (Figure 2A,B). Cross-presentation was assessed by the stimulation of OVA257-264–specific B3Z hybridoma. It is noteworthy that B3Z stimulation is uniquely dependent on the presence of OVA257-264–H-2kb complexes and does not require costimulatory signaling. pDCs were not able to present OVA to B3Z cells despite their capacity to capture and degrade this soluble Ag (Figure 2A), whereas cDCs stimulated efficiently the B3Z hybridoma. Similar results were obtained with beads carrying the OVA257-264 peptide (Figure 2B). In contrast, both DC subsets, when loaded with the OVA257-264 peptide, stimulated B3Z hybridoma, showing their ability to present antigenic peptide. Thus, the absence of B3Z stimulation by OVA-loaded pDCs revealed that the OVA epitope was not presented at the cell surface in association with H-2Kb molecule. These results demonstrate that, at steady state, pDCs are not competent to cross-present particulate or soluble exogenous Ags.
Cross-presentation by pDCs is inducible. (A-D) Various concentrations of purified cDCs (left panels) or pDCs (right panels) were cultured for 24 hours with 105 OVA-specific B3Z cells. (A,B) Cells were incubated in the presence of: (A) culture medium alone (○, CM), OVA (●, 3 mg/mL), OVA257-264 peptide ( pOVA, 1 μg/mL), (B) control beads (◇, 107/mL) or beads-OVA257-264 (♦, 107/mL). (C,D) Cells were incubated in the presence of OVA (3 mg/mL; C, main graph), OVA257-264 peptide (1 μg/mL; C, inset) or beads-OVA257-264 (107/mL, D) and culture medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (E) 104-purified pDCs from TAP−/− or WT mice were incubated in the presence of serial dilution of OVA257-264 peptide (left), OVA alone (middle), or OVA and R848 (right) and 105 B3Z for 24 hours. Stimulation of B3Z was measured as the release of IL-2 into supernatants as assayed by CTLL-2 proliferation monitored by 3H-labeled thymidine incorporation. Results are expressed as mean cpm plus or minus SD for duplicate wells and are representative of 3 experiments.
pOVA, 1 μg/mL), (B) control beads (◇, 107/mL) or beads-OVA257-264 (♦, 107/mL). (C,D) Cells were incubated in the presence of OVA (3 mg/mL; C, main graph), OVA257-264 peptide (1 μg/mL; C, inset) or beads-OVA257-264 (107/mL, D) and culture medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (E) 104-purified pDCs from TAP−/− or WT mice were incubated in the presence of serial dilution of OVA257-264 peptide (left), OVA alone (middle), or OVA and R848 (right) and 105 B3Z for 24 hours. Stimulation of B3Z was measured as the release of IL-2 into supernatants as assayed by CTLL-2 proliferation monitored by 3H-labeled thymidine incorporation. Results are expressed as mean cpm plus or minus SD for duplicate wells and are representative of 3 experiments.
Cross-presentation by pDCs is inducible. (A-D) Various concentrations of purified cDCs (left panels) or pDCs (right panels) were cultured for 24 hours with 105 OVA-specific B3Z cells. (A,B) Cells were incubated in the presence of: (A) culture medium alone (○, CM), OVA (●, 3 mg/mL), OVA257-264 peptide ( pOVA, 1 μg/mL), (B) control beads (◇, 107/mL) or beads-OVA257-264 (♦, 107/mL). (C,D) Cells were incubated in the presence of OVA (3 mg/mL; C, main graph), OVA257-264 peptide (1 μg/mL; C, inset) or beads-OVA257-264 (107/mL, D) and culture medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (E) 104-purified pDCs from TAP−/− or WT mice were incubated in the presence of serial dilution of OVA257-264 peptide (left), OVA alone (middle), or OVA and R848 (right) and 105 B3Z for 24 hours. Stimulation of B3Z was measured as the release of IL-2 into supernatants as assayed by CTLL-2 proliferation monitored by 3H-labeled thymidine incorporation. Results are expressed as mean cpm plus or minus SD for duplicate wells and are representative of 3 experiments.
pOVA, 1 μg/mL), (B) control beads (◇, 107/mL) or beads-OVA257-264 (♦, 107/mL). (C,D) Cells were incubated in the presence of OVA (3 mg/mL; C, main graph), OVA257-264 peptide (1 μg/mL; C, inset) or beads-OVA257-264 (107/mL, D) and culture medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (E) 104-purified pDCs from TAP−/− or WT mice were incubated in the presence of serial dilution of OVA257-264 peptide (left), OVA alone (middle), or OVA and R848 (right) and 105 B3Z for 24 hours. Stimulation of B3Z was measured as the release of IL-2 into supernatants as assayed by CTLL-2 proliferation monitored by 3H-labeled thymidine incorporation. Results are expressed as mean cpm plus or minus SD for duplicate wells and are representative of 3 experiments.
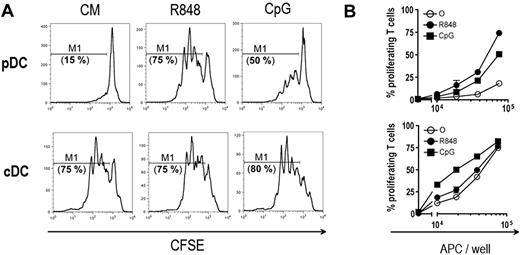
To test whether cross-presentation ability was inducible, pDCs and cDCs were cultured with OVA and B3Z cells in the presence of R848 or CpG TLR-L (Figure 2C). OVA cross-presentation by cDCs was slightly increased by stimulation with CpG but not with R848 (Figure 2C left panel). In contrast, R848-activated pDCs, incubated with OVA, induced a strong stimulation of B3Z cells (Figure 2C right panel). Other TLR-7 ligands, including Loxoribine and PolyU, also strongly induced OVA cross-presentation by splenic pDCs (Figure S3). pDC stimulation by CpG resulted in only a slight activation of B3Z. However, both R848 and CpG activation of pDCs incubated with beads carrying the OVA257-264 peptide induced a strong stimulation of B3Z, suggesting that these 2 signals induced efficient cross-presentation of this particulate Ag (Figure 2D). Importantly, pDC viability in culture was similar in the presence and in the absence of TLR ligands indicating that enhancement of cross-presentation was not the result of increased pDC survival after activation (data not shown). Induction of cross-presentation by pDCs was transporter associated with antigen processing (TAP) dependent because R848-activated TAP−/− pDCs displayed no OVA cross-presentation (Figure 2E). TAP deficiency did not affect peptide presentation to B3Z indicating that TAP−/− pDCs are as efficient as wild-type pDCs to stimulate B3Z. Altogether, these results demonstrate the capacity of pDCs to cross-present exogenous Ags through a TAP-dependent pathway, after TLR activation.
pDCs are able to cross-prime naive CD8+ T cells in vitro
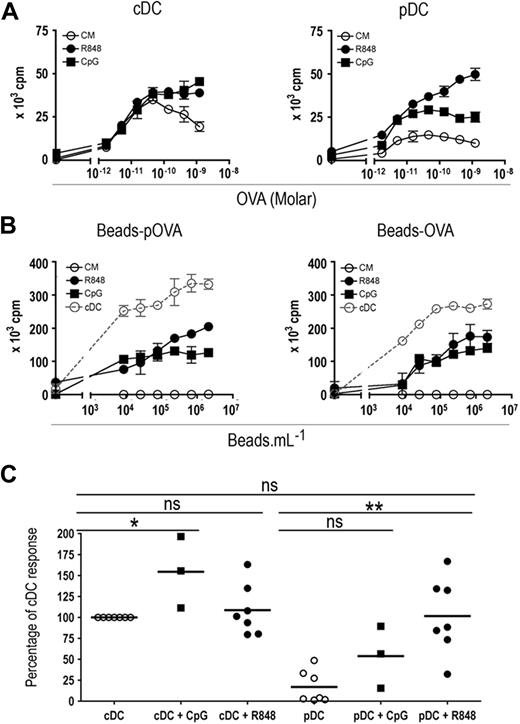
To analyze the ability of pDCs to cross-prime naive CD8+ T cells, we tested whether purified pDCs can stimulate naive OT-I T cells. OT-I T cells cultured with pDCs pulsed with OVA or beads carrying either the OVA257-264 peptide (beads-pOVA) or the OVA protein (beads-OVA) proliferated poorly (Figure 3A right panel, and B), in agreement with our results showing that pDCs are unable to cross-present exogenous Ags. Activation of pDCs by R848 and, to a lesser extent, by CpG led to efficient presentation of soluble or particulate OVA to OT-I cells, which proliferated actively (Figure 3A,B). Both activated and nonactivated cDCs presented soluble or particulate OVA to OT-I cells very efficiently (Figure 3A,B). Statistical analysis (Figure 3C) indicated that, after activation by R848, pDCs were as efficient as cDCs (either activated by R848 or not) to cross-present soluble OVA. Although the results were not significantly different for soluble OVA, CpG activation also induced cross-priming by pDCs. Importantly, neither activation by R848 or CpG nor coating of OVA on beads modified Ag capture by both DC subsets (Figure S4), indicating that the induction of cross-presentation ability was not the result of an increased capture of the Ags. Thus, after activation by TLR-L, pDCs become able to cross-present particulate or soluble exogenous Ags and to cross-prime naive CD8+ T cells in vitro.
pDCs cross-prime naive T cells. (A) A total of 104 purified splenic cDCs (left panel) or pDCs (right panel) were incubated for 2 hours in the presence of various concentrations of OVA with either medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (B) A total of 104 purified splenic cDCs (gray dotted line) or pDCs (black lines) were incubated for 2 hours in the presence of various concentrations of beads carrying the OVA257-264 peptide (beads-pOVA, left panel) or beads carrying OVA protein (beads-OVA, right panel) with either medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (A,B) Cells were washed twice and cocultured with 2 × 104 OT-I CD8+ transgenic T cells for 72 hours. T-cell proliferation was measured according to 3H-labeled thymidine incorporation during the final 6 hours of culture. Results are expressed as mean cpm plus or minus SD for duplicate wells. One representative experiment of 3 (with CpG) or 7 (with R848) is depicted. (C) For statistical analysis, cumulative data from 3 to 7 independent experiments are plotted. Each dot represents the maximum OT-I response obtained in the presence of a given DC subset and of various doses of OVA. Results are expressed as a percentage of OVA cross-presentation obtained with nonstimulated cDCs (cDC = 100%). ns indicates not significant. *P < .05, **P < .01.
pDCs cross-prime naive T cells. (A) A total of 104 purified splenic cDCs (left panel) or pDCs (right panel) were incubated for 2 hours in the presence of various concentrations of OVA with either medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (B) A total of 104 purified splenic cDCs (gray dotted line) or pDCs (black lines) were incubated for 2 hours in the presence of various concentrations of beads carrying the OVA257-264 peptide (beads-pOVA, left panel) or beads carrying OVA protein (beads-OVA, right panel) with either medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (A,B) Cells were washed twice and cocultured with 2 × 104 OT-I CD8+ transgenic T cells for 72 hours. T-cell proliferation was measured according to 3H-labeled thymidine incorporation during the final 6 hours of culture. Results are expressed as mean cpm plus or minus SD for duplicate wells. One representative experiment of 3 (with CpG) or 7 (with R848) is depicted. (C) For statistical analysis, cumulative data from 3 to 7 independent experiments are plotted. Each dot represents the maximum OT-I response obtained in the presence of a given DC subset and of various doses of OVA. Results are expressed as a percentage of OVA cross-presentation obtained with nonstimulated cDCs (cDC = 100%). ns indicates not significant. *P < .05, **P < .01.
pDCs capture Ag in vivo and cross-present it after further activation in vitro
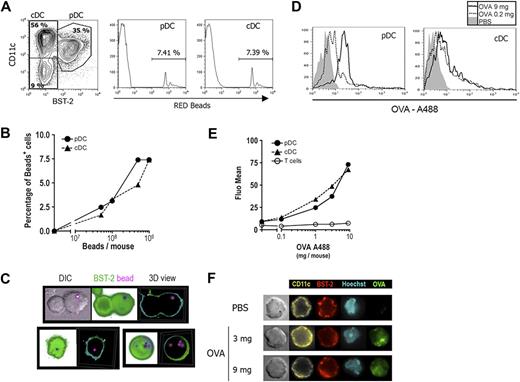
In vivo, pDCs are less represented than cDCs and are not similarly distributed within the spleen.41 It was thus important to determine whether pDCs efficiently capture exogenous Ags in vivo. Fluorescent beads or various doses of OVA-A488 were injected intravenously; and 2 hours later, fluorescence intensity was determined for both DC subsets. As shown in Figure 4, both cDCs and pDCs captured fluorescent beads efficiently (Figure 4A), and this efficacy was comparable after the injection of various bead numbers (Figure 4B). OVA-A488 was also very efficiently captured by pDCs (Figure 4D). The mean fluorescence of both DC subsets increased in a dose-dependent manner after the injection of various doses of OVA-A488 (Figure 4E). As expected, OVA was not captured by T cells.
pDCs capture and internalize Ag in vivo. (A,B) Mice were injected intravenously with various numbers of fluorescent red beads. Two hours later, CD11c+ cells enriched from spleen were stained with anti-CD11c and BST-2 (PDCA-1) mAbs and analyzed by flow cytometry. The percentage of cells that had captured beads was determined by gating red-positive cells for pDCs (CD11clow BST-2+ cells, left histogram) and cDCs (CD11chi BST-2− cells, right histogram). One representative experiment of 4 is depicted in each case. (A) Analysis of capture by DCs from the mouse injected with 109 beads is shown. (B) Percentage of bead-positive cells was determined among cDCs (▲,  ) and pDCs (●,
) and pDCs (●,  ) and plotted against the number of red beads injected per mouse. (C) Purified pDCs isolated from (109) red bead-injected mice were stained with anti-BST-2 (120G8, green) mAb and analyzed by fluorescent microscopy (original magnification ×630). Three-dimensional acquisition was done to obtain different slices of the cells. Representative DIC (differential interference contrast), shadow views (projection of each slice in the same image), and 3-dimensional reconstitutions are shown. (D,E) Mice were injected intravenously with various doses of OVA-A488. Two hours later, splenocytes were labeled with anti-CD11c, BST-2 (PDCA-1), and CD3ϵ mAbs and analyzed by flow cytometry. (D) Histograms show OVA-488 fluorescent intensity for both pDCs and cDCs (gated as in panel A) from mice injected with PBS (filled histogram) or 0.2 mg (thin histogram) or 9 mg (thick histogram) OVA. One representative experiment of 3 is depicted. (E) Mean OVA-A488 fluorescence was determined for T cells (○, black line), cDCs (▲,
) and plotted against the number of red beads injected per mouse. (C) Purified pDCs isolated from (109) red bead-injected mice were stained with anti-BST-2 (120G8, green) mAb and analyzed by fluorescent microscopy (original magnification ×630). Three-dimensional acquisition was done to obtain different slices of the cells. Representative DIC (differential interference contrast), shadow views (projection of each slice in the same image), and 3-dimensional reconstitutions are shown. (D,E) Mice were injected intravenously with various doses of OVA-A488. Two hours later, splenocytes were labeled with anti-CD11c, BST-2 (PDCA-1), and CD3ϵ mAbs and analyzed by flow cytometry. (D) Histograms show OVA-488 fluorescent intensity for both pDCs and cDCs (gated as in panel A) from mice injected with PBS (filled histogram) or 0.2 mg (thin histogram) or 9 mg (thick histogram) OVA. One representative experiment of 3 is depicted. (E) Mean OVA-A488 fluorescence was determined for T cells (○, black line), cDCs (▲,  ), and pDCs (●,
), and pDCs (●,  ) and is plotted against the dose of OVA-488 injected. T cells were gated as CD3ϵ+ cells. (F) Purified pDCs isolated from mice injected with PBS or 3 or 9 mg OVA-A488 (green) were stained with anti-BST-2 (120G8, red) and CD11c (yellow) mAbs and Hoechst 44 432 (light blue) and then analyzed by fluorescent microscopy (original magnification ×630). One representative experiment of 3 is depicted.
) and is plotted against the dose of OVA-488 injected. T cells were gated as CD3ϵ+ cells. (F) Purified pDCs isolated from mice injected with PBS or 3 or 9 mg OVA-A488 (green) were stained with anti-BST-2 (120G8, red) and CD11c (yellow) mAbs and Hoechst 44 432 (light blue) and then analyzed by fluorescent microscopy (original magnification ×630). One representative experiment of 3 is depicted.
pDCs capture and internalize Ag in vivo. (A,B) Mice were injected intravenously with various numbers of fluorescent red beads. Two hours later, CD11c+ cells enriched from spleen were stained with anti-CD11c and BST-2 (PDCA-1) mAbs and analyzed by flow cytometry. The percentage of cells that had captured beads was determined by gating red-positive cells for pDCs (CD11clow BST-2+ cells, left histogram) and cDCs (CD11chi BST-2− cells, right histogram). One representative experiment of 4 is depicted in each case. (A) Analysis of capture by DCs from the mouse injected with 109 beads is shown. (B) Percentage of bead-positive cells was determined among cDCs (▲,  ) and pDCs (●,
) and pDCs (●,  ) and plotted against the number of red beads injected per mouse. (C) Purified pDCs isolated from (109) red bead-injected mice were stained with anti-BST-2 (120G8, green) mAb and analyzed by fluorescent microscopy (original magnification ×630). Three-dimensional acquisition was done to obtain different slices of the cells. Representative DIC (differential interference contrast), shadow views (projection of each slice in the same image), and 3-dimensional reconstitutions are shown. (D,E) Mice were injected intravenously with various doses of OVA-A488. Two hours later, splenocytes were labeled with anti-CD11c, BST-2 (PDCA-1), and CD3ϵ mAbs and analyzed by flow cytometry. (D) Histograms show OVA-488 fluorescent intensity for both pDCs and cDCs (gated as in panel A) from mice injected with PBS (filled histogram) or 0.2 mg (thin histogram) or 9 mg (thick histogram) OVA. One representative experiment of 3 is depicted. (E) Mean OVA-A488 fluorescence was determined for T cells (○, black line), cDCs (▲,
) and plotted against the number of red beads injected per mouse. (C) Purified pDCs isolated from (109) red bead-injected mice were stained with anti-BST-2 (120G8, green) mAb and analyzed by fluorescent microscopy (original magnification ×630). Three-dimensional acquisition was done to obtain different slices of the cells. Representative DIC (differential interference contrast), shadow views (projection of each slice in the same image), and 3-dimensional reconstitutions are shown. (D,E) Mice were injected intravenously with various doses of OVA-A488. Two hours later, splenocytes were labeled with anti-CD11c, BST-2 (PDCA-1), and CD3ϵ mAbs and analyzed by flow cytometry. (D) Histograms show OVA-488 fluorescent intensity for both pDCs and cDCs (gated as in panel A) from mice injected with PBS (filled histogram) or 0.2 mg (thin histogram) or 9 mg (thick histogram) OVA. One representative experiment of 3 is depicted. (E) Mean OVA-A488 fluorescence was determined for T cells (○, black line), cDCs (▲,  ), and pDCs (●,
), and pDCs (●,  ) and is plotted against the dose of OVA-488 injected. T cells were gated as CD3ϵ+ cells. (F) Purified pDCs isolated from mice injected with PBS or 3 or 9 mg OVA-A488 (green) were stained with anti-BST-2 (120G8, red) and CD11c (yellow) mAbs and Hoechst 44 432 (light blue) and then analyzed by fluorescent microscopy (original magnification ×630). One representative experiment of 3 is depicted.
) and is plotted against the dose of OVA-488 injected. T cells were gated as CD3ϵ+ cells. (F) Purified pDCs isolated from mice injected with PBS or 3 or 9 mg OVA-A488 (green) were stained with anti-BST-2 (120G8, red) and CD11c (yellow) mAbs and Hoechst 44 432 (light blue) and then analyzed by fluorescent microscopy (original magnification ×630). One representative experiment of 3 is depicted.
To confirm that beads and OVA-A488 were internalized by pDCs after in vivo capture, these cells were purified and analyzed by fluorescence microscopy. Three-dimensional images of pDCs purified from bead-injected mice show beads surrounded by plasmatic membrane demonstrating that beads were internalized into the cells (Figure 4C). pDCs from mice injected with either 3 or 9 mg of OVA-A488 showed bright green fluorescence into the cells (Figures 4F, S5), whereas those purified from PBS-injected mice did not exhibit any green fluorescence (top panel). These results show that pDCs are as efficient as cDCs to take up and to internalize both particulate and soluble Ags in vivo.
We then analyzed the ex vivo capacity of pDCs and cDCs to cross-present OVA after in vivo capture. Thus, 129sv mice were injected with OVA; and 2 hours later, purified DC subsets were either left unstimulated or were activated with R848 or CpG in vitro and cultured with CFSE-labeled OT-I cells (Figure 5). Nonactivated pDCs did not stimulate OT-I cells, whereas after in vitro activation by R848, pDCs induced a strong OT-I cell proliferation. CpG activation also led to cross-presentation by pDCs, albeit in a lesser extent. As expected, unstimulated cDCs effectively cross-presented OVA to OT-I cells, and this activity was not enhanced by in vitro activation with either CpG or R848. These findings demonstrate that TLR triggering is required for efficient cross-presentation of in vivo captured soluble OVA by pDCs.
OVA captured in vivo is cross-presented by pDCs after in vitro stimulation. Mice were injected intravenously with OVA (9 mg). Two hours later, pDCs and cDCs were purified and various concentrations of both DC subsets were cocultured with either culture medium alone (CM), R848 (1 μg/mL), or CpG (10 μg/mL) and 2.5 × 104 CFSE-labeled OT-I T cells. CFSE profiles of OT-I T cells were analyzed by flow cytometry 72 hours later. (A) CFSE profiles obtained with 2 × 105 DCs are shown for both DC subsets and for each conditions as indicated. Proliferating OT-I T cells are gated in M1. (B) Percentages of gated proliferating T cells are plotted according to the number of pDCs (top panel) or cDCs (bottom panel) per well in culture medium (○, CM), or in the presence of R848 (●) or CpG (■). One representative experiment of 3 is depicted.
OVA captured in vivo is cross-presented by pDCs after in vitro stimulation. Mice were injected intravenously with OVA (9 mg). Two hours later, pDCs and cDCs were purified and various concentrations of both DC subsets were cocultured with either culture medium alone (CM), R848 (1 μg/mL), or CpG (10 μg/mL) and 2.5 × 104 CFSE-labeled OT-I T cells. CFSE profiles of OT-I T cells were analyzed by flow cytometry 72 hours later. (A) CFSE profiles obtained with 2 × 105 DCs are shown for both DC subsets and for each conditions as indicated. Proliferating OT-I T cells are gated in M1. (B) Percentages of gated proliferating T cells are plotted according to the number of pDCs (top panel) or cDCs (bottom panel) per well in culture medium (○, CM), or in the presence of R848 (●) or CpG (■). One representative experiment of 3 is depicted.
Activation by TLR-L licenses pDCs to cross-prime naive T cells in vivo
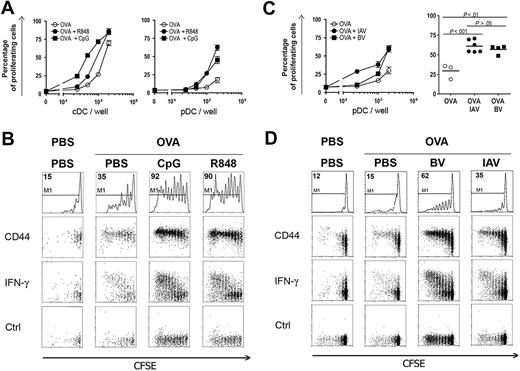
We further investigated the induction of cross-presentation by pDCs in vivo. Mice were injected with OVA protein and, 30 minutes later, they were given either R848 or CpG in DOTAP. pDCs and cDCs purified 2 hours later were cocultured with CFSE-labeled OT-I cells. A strong OT-I proliferation was observed with pDCs purified from mice injected with OVA together with either R848 or CpG in DOTAP (Figure 6A right panel). The cross-presentation induced by CpG was higher in vivo than after in vitro stimulation possibly because the CpG was complexed with DOTAP only for the in vivo experiments, allowing efficient delivery of CpG to endosomes. Induction of cross-presentation was obtained after in vivo administration of either CpG B or C, whereas only a low percentage of proliferating OT-I T cells was observed with pDCs purified from mice injected with the same dosage of CpG A (Figure S6). These results suggest that induction of cross-presentation could involve both NF-κB activation and IFN-α production.42 In contrast, in vivo cDC activation by CpG or R848 only slightly increased the cross-priming of OT-I T cells (Figure 6A left panel). Similar results were obtained for mice that received OVA and either CpG or R848 simultaneously. Furthermore, because pDC purification was based on the expression of BST-2 and that this marker is up-regulated by different cell types after activation, we checked whether in vivo treatment by CpG or R848 affected the purity of pDCs after purification. Figure S2C showed that the purity of pDCs from PBS- or TLR-L–injected mice was always higher than 97%. Thus, cross-presentation by purified pDCs after activation by TLR-L was not the result of cells contaminating this population.
pDCs cross-prime OT-I T cells in vivo. 129sv mice were injected intravenously with OVA (9 mg) and either PBS, 10 μg of R848, 100 μg CpG in DOTAP, 103 hemagglutinin units of heat-inactivated Influenza A virus (IAV), or 106 pfu of BV. A control group received only PBS. Two hours later, splenic DC subsets were purified by magnetic sorting and their capacity to cross-prime OT-I T cells in vitro (A,C) and in vivo (B,D) was assessed. (A,C) Various numbers of purified cDCs (A, left panel) and pDCs (A, right panel; C) were cultured with 2.5 × 104 CFSE-labeled OT-I T cells. OT-I T-cell proliferation was analyzed by flow cytometry 72 hours later. Proliferating T cells were assessed by CFSE dye dilution by OT-I T cells as described in Figure 5. Percentages of proliferating T cells are plotted against the number of DCs per well. (A) cDCs (left panel) and pDCs (right panel) were purified from mice injected with either OVA alone (○) or with R848 (●) or with CpG (■). (C) pDCs were purified from mice injected with either OVA alone (○) or with BV (●) or with IAV (■). Left panel: 1 representative experiment of 3 is depicted. Right panel: statistical analysis of OT-I T-cell proliferation, when cocultured with 2 × 105 purified pDCs, from 3 independent experiments, including 1 or 2 mice per group in each experiment. (B,D) Purified pDCs were injected into CD45.1 hosts that had received 2 × 106 CFSE-labeled OT-I T (CD45.2) cells 24 hours before. Seven days later, splenocytes from these mice were incubated 4 hours in the presence of brefeldin A with or without OVA257-264 peptide. Cells were stained using anti-CD45.1, CD45.2, CD3ϵ, and CD8α mAbs, and OT-I T cells were characterized as CD45.1−, CD45.2+, CD8α+, and CD3ϵ+ cell populations. (B) OT-I T-cell analysis from mice that received pDCs purified from mice injected with PBS or with OVA and PBS, CpG, or R848 as indicated. CD44 expression and intracellular expression of IFN-γ by OT-I T cells were assessed as described in “Adoptive transfer.” First row: CFSE profiles of OT-I T cells (percentages of OT-I T cells showing CFSE dye dilution and gated in M1 are indicated); second row: CD44 expression on OT-I T cells according to CFSE profile; third row: IFN-γ intracellular staining on OT-I T cells according to CFSE profile; last row: intracellular staining with control isotype. (D) CFSE profiles of OT-I T cells from mice that received pDCs purified from mice injected with PBS or with OVA and PBS, BV, or IAV. First row: percentages of OT-I T cells showing CFSE dye dilution and gated in M1. Second row: CD44 expression by OT-I cells according to CFSE profiles. Third row: IFN-γ intracellular staining on OT-I cells according to CFSE profile; last row: intracellular staining with control isotype. One representative experiment of 4 is depicted.
pDCs cross-prime OT-I T cells in vivo. 129sv mice were injected intravenously with OVA (9 mg) and either PBS, 10 μg of R848, 100 μg CpG in DOTAP, 103 hemagglutinin units of heat-inactivated Influenza A virus (IAV), or 106 pfu of BV. A control group received only PBS. Two hours later, splenic DC subsets were purified by magnetic sorting and their capacity to cross-prime OT-I T cells in vitro (A,C) and in vivo (B,D) was assessed. (A,C) Various numbers of purified cDCs (A, left panel) and pDCs (A, right panel; C) were cultured with 2.5 × 104 CFSE-labeled OT-I T cells. OT-I T-cell proliferation was analyzed by flow cytometry 72 hours later. Proliferating T cells were assessed by CFSE dye dilution by OT-I T cells as described in Figure 5. Percentages of proliferating T cells are plotted against the number of DCs per well. (A) cDCs (left panel) and pDCs (right panel) were purified from mice injected with either OVA alone (○) or with R848 (●) or with CpG (■). (C) pDCs were purified from mice injected with either OVA alone (○) or with BV (●) or with IAV (■). Left panel: 1 representative experiment of 3 is depicted. Right panel: statistical analysis of OT-I T-cell proliferation, when cocultured with 2 × 105 purified pDCs, from 3 independent experiments, including 1 or 2 mice per group in each experiment. (B,D) Purified pDCs were injected into CD45.1 hosts that had received 2 × 106 CFSE-labeled OT-I T (CD45.2) cells 24 hours before. Seven days later, splenocytes from these mice were incubated 4 hours in the presence of brefeldin A with or without OVA257-264 peptide. Cells were stained using anti-CD45.1, CD45.2, CD3ϵ, and CD8α mAbs, and OT-I T cells were characterized as CD45.1−, CD45.2+, CD8α+, and CD3ϵ+ cell populations. (B) OT-I T-cell analysis from mice that received pDCs purified from mice injected with PBS or with OVA and PBS, CpG, or R848 as indicated. CD44 expression and intracellular expression of IFN-γ by OT-I T cells were assessed as described in “Adoptive transfer.” First row: CFSE profiles of OT-I T cells (percentages of OT-I T cells showing CFSE dye dilution and gated in M1 are indicated); second row: CD44 expression on OT-I T cells according to CFSE profile; third row: IFN-γ intracellular staining on OT-I T cells according to CFSE profile; last row: intracellular staining with control isotype. (D) CFSE profiles of OT-I T cells from mice that received pDCs purified from mice injected with PBS or with OVA and PBS, BV, or IAV. First row: percentages of OT-I T cells showing CFSE dye dilution and gated in M1. Second row: CD44 expression by OT-I cells according to CFSE profiles. Third row: IFN-γ intracellular staining on OT-I cells according to CFSE profile; last row: intracellular staining with control isotype. One representative experiment of 4 is depicted.
We then conducted a similar experiment in which pDC loading and activation and OT-I cell priming entirely occurred in vivo. Purified pDCs from mice injected with either OVA alone or with OVA and TLR-L were adoptively transferred into CD45.1 hosts that had been given CFSE-labeled CD45.2+ OT-I cells. Seven days later, OT-I cells in the spleen were analyzed for their proliferation state, expression of CD44, and production of IFN-γ (Figure 6B). pDCs from PBS-treated mice did not induce any proliferation of OT-I cells, whereas a weak OT-I proliferation was obtained with pDCs from OVA-injected mice. pDCs from mice injected with OVA and either CpG in DOTAP or R848 induced strong in vivo proliferation of OT-I cells. This proliferation correlated with CD44 up-regulation and IFN-γ production, showing that OT-I cells were fully differentiated into effector cells (Figure 6B). OT-I T-cell responses from LN were similar to those obtained in the spleen (data not shown). Therefore, pDCs activated by TLR-L migrated to lymphoid organs after their transfer by intravenous route and cross-primed naive T cells in vivo both in spleen and LN.
Viral activation licenses pDCs to cross-prime naive T cells in vivo
pDCs sense viral infection through the interaction with TLRs. Because synthetic TLR-7 and TLR-9 ligands induce pDC cross-presentation, we then analyzed the cross-presentation ability of pDCs after stimulation with heat-inactivated IAV (HI-IAV) and live BV, which activate pDCs through interaction with TLR-743 and TLR-9,37,44 respectively. Mice were injected with the OVA protein and either 103 hemagglutinin units of HI-IAV or 109 pfu of BV; and 2 hours later, pDCs were purified and cocultured with CFSE-labeled OT-I T cells (Figure 6C). pDCs purified from mice injected with HI-IAV induced a strong proliferation of OT-I T cells. OT-I T-cell proliferation was also higher after BV stimulation than with pDCs purified from mice injected with the OVA protein alone. OT-I proliferation induced by virus-activated pDCs was statistically different from proliferation obtained with nonactivated pDCs, demonstrating that viral activation leads to cross-priming of naive T cells in vitro (Figure 6C right panel).
These results were confirmed in vivo by adoptive transfer of pDCs into CD45.1 mice previously injected with CFSE-labeled OT-I T cells. Under these conditions, 62% and 35% of OT-I T cells showed CFSE dilution with pDCs previously activated with BV or IAV, respectively (Figure 6D). In both cases, proliferating T cells up-regulated CD44 and produced IFN-γ, demonstrating that these cells were activated and differentiated into effector cells. No OT-I proliferation was observed with nonactivated pDCs. A similar OT-I T-cell response was observed in LN (data not shown). Altogether, these results demonstrate that viral stimulation leads to cross-priming of CD8+ naive T cells by pDCs in vivo both in spleen and LN.
Cross-priming of a primary CD8+ T-cell response by R848-activated pDCs
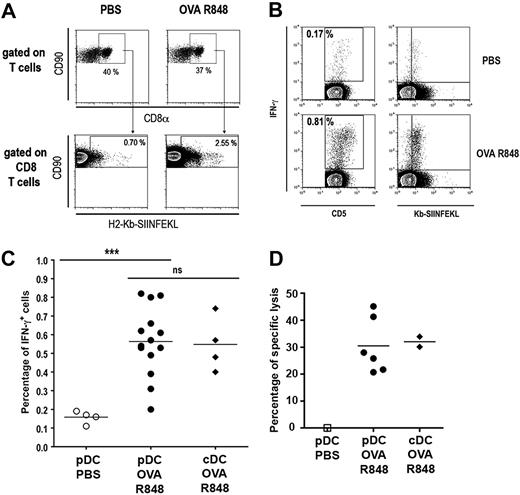
Because TLR-activated pDCs are able to cross-prime naive transgenic T cells specific for exogenous Ags, we analyzed their ability to induce a primary CD8+ T-cell response against soluble OVA in nontransgenic mice. Naive 129sv mice were immunized by 2 consecutive injections (at days 0 and 7) of pDCs purified from mice injected with PBS alone or with OVA and R848. Seven days after the second injection, the percentage of OVA257-264–specific CD8+ T cells (Kb-SIINFEKL-Pentamer+) was determined (Figure 7A). A 3.6-fold expansion of OVA257-264–specific CTL was observed representing 2.5% of total splenic CD8+ T cells compared with the 0.7% observed in mice immunized with pDCs purified from PBS-injected mice. This expansion was associated with IFN-γ production by specific T cells, as shown by costaining of CD8+ T cells by Kb-SIINFEKL-pentamer and intracellular IFN-γ after restimulation by the OVA peptide (Figure 7B). The percentage of IFN-γ+ cells observed after immunization with R848-activated pDCs pulsed with OVA was significantly higher than in the PBS-treated pDC group. Notably, this percentage did not differ from mice that received cDCs (Figure 7C). In addition, expansion of functional specific T cells correlates with the induction of OVA257-264–specific cytotoxic activity as shown by in vivo killing assay (Figure 7D) and in vitro cytotoxic assay (Figure S7). It is noteworthy that this cytotoxic activity was similar to the CTL activity induced by cDCs purified from the same mice, whereas pDCs purified from control mice did not show any CTL activity. These data demonstrate that TLR-activated pDCs cross-prime naive nontransgenic CD8+ T cells leading to specific in vivo proliferation of CD8+ T cells that displayed full lytic function in vivo confirming results obtained with OT-I T cells.
Cross-priming of a primary CD8+ T response by R848-activated pDCs. Naive 129sv mice were immunized by 2 consecutive intravenous injections (at days 0 and 7) of pDCs or cDCs purified from mice injected with either PBS alone or OVA (9 mg) and 10 μg R848. Spleen from immunized mice were analyzed 1 week after the last immunization. (A) Splenocytes were labeled with anti-CD90, anti-CD8α mAbs, and H2-Kb-SIINFEKL-pentamer and analyzed by flow cytometry. First row: CD8α+ CD90+cells were gated among total T cells. Second row: Kb-SIINFEKL-pentamer+ cells were gated among CD8+ T cells. Percentages of gated cells are indicated in each quadrant. (B,C) Splenocytes were restimulated with OVA peptide (1 μg/mL) for 24 hours and with brefeldin A for the last 4 hours. Cells were then labeled with anti-CD5, anti-CD8α mAbs, and H2-Kb-SIINFEKL-pentamer. Intracellular anti–IFN-γ staining was performed before analysis by flow cytometry. CD8α+ cells were gated among total T cells and percentage of IFN-γ+ within CD5+ T cells is shown. IFN-γ is plotted against H2-Kb-SIINFEKL-pentamer to visualize costaining (B). Pooled results showing percentages of IFN-γ+ cells among CD8+ T cells from 2 independent experiments are plotted (C). (D) Specific lysis of OVA257-264–loaded cells was assayed by in vivo killing. Results from individual mice are shown for each group.
Cross-priming of a primary CD8+ T response by R848-activated pDCs. Naive 129sv mice were immunized by 2 consecutive intravenous injections (at days 0 and 7) of pDCs or cDCs purified from mice injected with either PBS alone or OVA (9 mg) and 10 μg R848. Spleen from immunized mice were analyzed 1 week after the last immunization. (A) Splenocytes were labeled with anti-CD90, anti-CD8α mAbs, and H2-Kb-SIINFEKL-pentamer and analyzed by flow cytometry. First row: CD8α+ CD90+cells were gated among total T cells. Second row: Kb-SIINFEKL-pentamer+ cells were gated among CD8+ T cells. Percentages of gated cells are indicated in each quadrant. (B,C) Splenocytes were restimulated with OVA peptide (1 μg/mL) for 24 hours and with brefeldin A for the last 4 hours. Cells were then labeled with anti-CD5, anti-CD8α mAbs, and H2-Kb-SIINFEKL-pentamer. Intracellular anti–IFN-γ staining was performed before analysis by flow cytometry. CD8α+ cells were gated among total T cells and percentage of IFN-γ+ within CD5+ T cells is shown. IFN-γ is plotted against H2-Kb-SIINFEKL-pentamer to visualize costaining (B). Pooled results showing percentages of IFN-γ+ cells among CD8+ T cells from 2 independent experiments are plotted (C). (D) Specific lysis of OVA257-264–loaded cells was assayed by in vivo killing. Results from individual mice are shown for each group.
Discussion
Cross-presentation is the pathway by which exogenous Ags are routed for presentation by MHC class I molecules leading to activation of CD8+ T cells. This pathway is crucial for the development of cytotoxic CD8+ T-cell responses against tumors and pathogens that do not directly infect APCs, as well as for the induction of tolerance. In mice, it is generally considered that cross-presentation is a unique property of CD8α+ cDCs, leading to immunity or tolerance.22 However, some evidence indicates that other cells are able to cross-present exogenous Ags, such as activated CD8α− cDC,23–25 macrophages,13,14 B cells,10 endothelial cells,11 and neutrophils.45 Here we describe the capacity of cross-presentation of splenic pDCs, and we demonstrate that this capacity is regulated through TLR activation. Therefore, after TLR stimulation, pDCs cross-prime naive T cells, leading to their differentiation into functional effector cells in vivo.
Ag capture is the first step required for cross-presentation. Here we provide evidence that splenic pDCs efficiently capture soluble or particulate Ags via phagocytosis, endocytosis, or macropinocytosis and degrade internalized Ags. In vivo, pDCs capture Ags at least as well as cDCs, demonstrating that both populations are efficient on Ag uptake in vivo. However, Ags internalized by pDCs are cross-presented only after TLR activation and not at steady state. This mechanism is TAP dependent, suggesting that Ag escapes from the phagosome into the cytosol and that peptides steaming from proteasome digestion are transported into the ER for MHC I presentation. This pathway has been described for professional APCs, such as cDCs and macrophages,46 indicating that the mechanism leading to cross-presentation in pDCs is probably similar to those operating in cDCs.
Cross-presentation by cDCs is generally considered to be a constitutive property of these cells. It has been reported that only CD8α+ cDCs are able to cross-present Ags constitutively,21 and targeting Ag to CD8α+ cDCs with an anti–DEC-205 Ab leads to cross-presentation of this Ag.47 Cross-presentation by CD8α− cDCs, however, is inducible through triggering by FcR,23 LPS,24 or virus-like particles.25 Altogether, these observations strongly suggest that CD8α− cDCs and pDCs possess a specialized machinery to deliver exogenous Ags to the cross-presentation pathway; but contrary to CD8α+ cDCs, this machinery is under the control of factors induced by activation. The mechanism underlying the induction of cross-presentation is probably not linked to enhancement of Ag uptake because, at steady state, pDCs are able to capture exogenous Ags as efficiently as cDCs. Using bone marrow granulocyte-macrophage colony-stimulating factor–derived DCs, Datta et al48 showed that TLR-induced cross-presentation is independent of endosomal acidification and relies on Ag processing machinery. The fact that cross-presentation by pDCs is induced through TLR signaling and is TAP dependent indicates that these 2 cell populations could use a similar mechanism. Induction of cross-presentation by pDCs may depend on factors involved in Ag processing, such as proteasome, TAP, or MHC I molecules or on rerouting of endocytosed Ags into the cytosol. The exact mechanisms that lead to TLR-induced cross-presentation remain to be defined.
Conflicting results on the cross-presentation capacity of pDCs were obtained using Flt3L-derived pDCs (pBMDCs) generated in vitro. pBMDCs incubated with OVA protein primed naive OT-I T cells only after stimulation with a mixture of CpG, CD40L, and granulocyte-macrophage colony-stimulating factor.49 Others showed that CpG stimulation did not allow cross-priming of OT-I T cells by pBMDCs either in vitro50 or in vivo.36 Here we showed that in vitro stimulation of splenic pDCs with either CpG or R848 induces to the cross-presentation of exogenous Ags and cross-priming. These contrasting results could be the result of the source of pDCs because we used freshly isolated splenic pDCs instead of pBMDCs that are generated in the presence of Flt3L. Of note, Flt3L not only allows expansion of DCs but also influences DC-mediated immunogenicity in a variety of models.51–54 Furthermore, mouse Flt3L treatment leads to expansion of tolerogenic DCs.55 Thus, in these previous studies, Flt3L signaling could have altered or enhanced the functionality of pDCs during their development in culture.
In this study, we also demonstrate that in vivo pDCs cross-present soluble or particulate Ags either after activation with TLR-L or after viral stimulation and cross-prime specific naive T cells. We and others previously showed that pDCs need viral or TLR-L stimulation to activate CD8+ T cells against endogenous Ags or antigenic peptides.36,56 Here our data demonstrate that the activation state of pDCs is essential for both cross-presentation of exogenous Ags and in vivo cross-priming of T-cell responses. Thus, at steady state, pDCs are not able to cross-present exogenous Ags by MHC class I molecules in agreement with results obtained by Sapoznikov et al,31 showing that cross-presentation is absent in a mouse model lacking cDCs. However, in this model, T-cell responses were not analyzed after TLR-L or viral stimulation.
The activation state of DCs is crucial for the functional outcome of the DC–T-cell interaction because nonactivated DCs tolerize or delete T cells, whereas activation converts DCs to a stimulatory state that results in T-cell activation and memory.19 Our data show that pDCs do not participate to cross-tolerance at steady state because of their inability to cross-present Ags. Furthermore, TLR-L or viral activation induces pDC maturation and leads to the generation of effector CD8+ T cells against exogenous Ags as previously shown for endogenous Ags or antigenic peptides.36,56 Altogether, these data indicate that splenic pDCs do not induce tolerance but rather participate to immunity and particularly against viral infection, which activate pDCs through TLR-7 or TLR-9. However, we do not exclude that, under particular conditions or in certain anatomic localizations, pDCs may present tolerogenic functions.41
CD8α+ cDCs has been described as the main DC subset involved in the presentation of viral Ags.22 We report here that cross-presentation by pDCs is efficiently induced through TLR-7 activation, a molecule not expressed by the CD8α+ cDC subset. This suggests that pDCs could play a particular role in immunity against single-strand RNA virus infection, and especially in the CD8+ T-cell responses. Consistent with this notion, we show that stimulation by IAV, which activates pDCs through TLR-7,43 leads to the induction of cross-presentation by pDCs. However, viral induction of cross-presentation by pDCs is not restricted to TLR-7. Indeed, BV, which activates pDCs through TLR-9 expressed by all DC subsets,37,44 also induces cross-presentation by pDCs.
In conclusion, we provide here the first in vivo evidence of the role of splenic pDCs in Ag cross-presentation and naive T-cell cross-priming. The fact that human pDCs were recently described to cross-present in vitro exogenous vaccines, such as lipopeptide or viral Ag from apoptotic cells,29 suggests that pDCs could represent a key DC subset in the induction of adaptive responses against certain infections both in human and mice. pDCs thus could represent an important target to boost the efficacy of vaccines based on CTL induction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Albert, L. Maljessi, and R. Lo-Man for helpful discussions and comments on the manuscript and Marie Rojas for her excellent technical help.
This work was supported by grants from Institut Pasteur (PIC-PTR 278), Ligue Nationale Contre le Cancer (Equipe Labellisée), and European Community (Theravac Project; C.L.). J.M. was supported by fellowships from the French government and G.M. by Foundation pour la Recherche Médicale.
Authorship
Contribution: J.M. designed and performed the research and wrote the paper; G.M. and G.S. performed preliminary experiments not included in the paper; N.E. provided influenza viruses; and G.D. and C.L. directed the work and contributed to the design of the project and to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claude Leclerc, Institut Pasteur, Unité de Régulation Immunitaire et Vaccinologie, Inserm, U883, Paris F-75015, France; e-mail: cleclerc@pasteur.fr; or Gilles Dadaglio, Institut Pasteur, Unité de Régulation Immunitaire et Vaccinologie, Inserm, U883, Paris F-75015, France; e-mail: gdadag@pasteur.fr.
References
Author notes
*G.D. and C.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal