Abstract
Hematopoietic stem and progenitor cells were previously found to express Toll-like receptors (TLRs), suggesting that bacterial/viral products may influence blood cell formation. We now show that common lymphoid progenitors (CLPs) from mice with active HSV-1 infection are biased to dendritic cell (DC) differentiation, and the phenomenon is largely TLR9 dependent. Similarly, CLPs from mice treated with the TLR9 ligand CpG ODN had little ability to generate CD19+ B lineage cells and had augmented competence to generate DCs. TNFα mediates the depletion of late-stage lymphoid progenitors from bone marrow in many inflammatory conditions, but redirection of lymphopoiesis occurred in TNFα−/− mice treated with CpG ODN. Increased numbers of DCs with a lymphoid past were identified in Ig gene recombination substrate reporter mice treated with CpG ODN. TLR9 is highly expressed on lymphoid progenitors, and culture studies revealed that those receptors, rather than inflammatory cytokines, accounted for the production of several types of functional DCs. Common myeloid progenitors are normally a good source of DCs, but this potential was reduced by TLR9 ligation. Thus, alternate differentiation pathways may be used to produce innate effector cells in health and disease.
Introduction
Hematopoietic stem cells (HSCs) give rise to progenitors with potential to produce blood cell types with remarkably stable characteristics. Although this process is tightly controlled, recent findings suggest that hematopoiesis is dynamic and also responsive to environmental factors.1 The loss of differentiation options is gradual, and T lymphocytes, natural killer (NK) cells, and dendritic cells (DCs) can each be made from multiple progenitors under experimental circumstances.1,2 Indeed, apparently similar DCs arise from distinct myeloid or lymphoid progenitors.3 This new perspective raises the possibility that choices are made between multiple pathways to replenish effectors of the immune system. Thus, it is important to learn what normal and disease conditions favor particular differentiation routes
Several major categories of DCs have been found in murine bone marrow (BM). Conventional DCs (cDCs) are competent to present antigens, whereas plasmacytoid dendritic cells (pDCs) are potent producers of type I interferon.3 The pDCs are divisible into 2 subtypes (pDC1 and pDC2) on the basis of RAG-1 expression and patterns of cytokine production.4 Under experimental conditions, DCs are produced from stem cells, as well as lymphoid and myeloid progenitors.3–5 Flk-2/flt-3 ligand and the associated Stat3 signaling pathway are important for DC differentiation; consequently, efficient progenitors bear the Flk-2/flt-3 receptor.3 In our experience, the highest yields of pDCs are obtained from the primitive Linx−c-KithiSca-1+ (LSK) fraction of murine BM.4 Two recent reports identified a Lin−Flt3+c-Kitlo-CD115+ pro-DC population capable of generating pDCs and at least 2 categories of DCs.6,7 However, greater yields of DCs were produced from more primitive progenitors, and some of those are already restricted to particular DC pathways.7 Common lymphoid progenitors (CLPs) represent the main pathway to B lineage cells and include most progenitors destined for the NK lineage.8,9 CLPs also appear to make some contribution to DC production.10 It is unclear if DCs have various origins, depending on environmental conditions and demands.
A subset of BM with hybrid characteristics of NK cells and DCs was recently discovered4,11,12 and designated interferon-producing killer dendritic cells (IKDCs). Like NK cells, IKDCs are developmentally dependent on Id-2, independent of Notch signals and thrive in interleukin-15 (IL-15).11,13 Whereas IKDCs can be made from activated NK cells,14 IKDC regeneration from transplanted progenitors did not closely parallel the formation of NK cells.13 Recent studies concluded that IKDCs are like NK cells in being restricted to type 2 interferon secretion.15,16 Thus, IKDCs appear to represent specialized NK cells, and it remains to be seen whether they have any unique functions.
Additional perspective on environmental cues for differentiation has come from the discovery that stem and progenitor cells express functional Toll-like receptors (TLRs 2 and 4), and TLR signals alter lympho-hematopoiesis.17 HSCs were stimulated to enter cycle and acquire lineage markers by exposure to the TLR4 ligand lipopolysaccharide (LPS). LPS also reduced the differentiation requirements for myeloid progenitors and caused lymphoid progenitors to produce cDCs. This new mechanism represents a potential means for pathogen products to signal the rapid generation of innate immune cells within the BM or other tissues.18 However, its physiologic relevance in infectious diseases had not been explored.
TLRs participate in pathologic changes associated with herpes virus infections.19–21 We have now found that lymphoid progenitors assume other fates after HSV-1 inoculation of normal but not TLR9-deficient mice. Thus, unique differentiation pathways can be used to generate cells of the innate immune system in response to pathogen products.
Methods
Mice
All procedures were approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute institutional animal care and use committees. RAG-1/GFP knock-in mice have been described.22 Heterozygous F1 RAG-1/GFP mice were generated at the OMRF Laboratory Animal Resource Center (LARC). C57BL6 (B6; CD45.2 alloantigen), B6-Thy1.1, and B6-SJL (CD45.1 alloantigen) mice (Jackson Laboratory, Bar Harbor, ME) were bred and maintained in the LARC. B6-Thy1.1 mice were crossed with B6-RAG-1/GFP knock-in mice to produce animals expressing Thy1.1 and RAG-1/GFP. TNFα−/−, MyD88−/−, and TLR9−/− mice were maintained in the LARC. STAT5b-CA transgenic mice were previously described.23 H2-SVEX V(D)J recombination reporter mice (C57BL6) have been characterized elsewhere.24,25
Isolation of cell populations and flow cytometry
For gene expression assays, BM cells from B6-RAG1/GFP-Thy1.1 mice were enriched by negative selection using purified monoclonal antibodies (mAbs) anti-Gr1 (RB6-8C5), anti-CD11b/Mac-1 (M1/70), anti-CD19 (1D3), anti-CD45RA (14.8), and anti-TER119, followed by the BioMag goat anti–rat IgG system (Qiagen, Valencia, CA). After staining with biotin-antilineage markers [Gr-1, CD11b/Mac-1, CD19, CD45R/B220 (RA3/6B2), TER119, CD3α (17A2), CD8α (53–6.7), and pan-NK (DX-5)], and streptavidin-R613, lin−GFP+/− populations were separated using a FACSAria Flow Cytometer/Sorter (BD Biosciences, San Jose, CA). Lin−GFP− sorted cells were stained with APC-anti-c-kit (2B8), phycoerythrin (PE)-Cy5-anti-Sca1 (D7, eBioscience, San Diego, CA), fluorescein isothiocyanate-anti Thy1.1 (OX-7), and PE-anti CD34 (RAM34), whereas Lin−GFP+ cells were stained with APC-anti-c-kit, PE-Cy5-anti-Sca1, and PE-anti-IL-7Rα (A7R34, eBioscience). HSCs were double sorted as Lin−GFP−c-kithiSca1+Thy1.1lo, multipotent progenitors (MPPs) as Lin−GFP−c-kithiSca1+Thy1.1−, common myeloid progenitors (CMPs) as Lin−GFP−c-kit+Sca1−Thy1.1−CD34+, megakaryocyte-erythroid progenitors as Lin−GFP−c-kit+Sca1−Thy1.1−CD34−, early lymphoid progenitors as Lin−GFP+c-kithiSca1+IL-7Rα−, and CLPs as Lin−GFP+c-kitloSca1+IL-7Rα+. Isolation of CLPs from RAG1/GFP was as Lin−GFP+IL-7Rα+c-kitloSca1+. CLPs for culture were obtained from B6, TNF−/−, and TLR9−/− mice as Lin−IL-7Rα+c-kitloSca1+, after staining with fluorescein isothiocyanate-anti-lin markers, APC-anti-c-kit, PE-Cy5-anti-Sca1, and PE-anti-IL-7Rα. pDCs were sorted from B6 BM as B220+CD19−CD11cloLy6C+. Purity of each population was confirmed by postsorting analyses. All antibodies came from BD Biosciences PharMingen (San Diego, CA) unless otherwise stated. BM cells of cytosine-phosphate-guanosine (CpG)–treated, BM-transferred, or infected mice were harvested, and frequencies of B220+CD43+CD24+CD19+ pro-B, B220+CD43−IgM−CD19+ pre-B, B220+CD43−IgM+CD19+ B cells, as well as B220+CD19−CD11c+Ly6C+ pDC, B220+CD19−CD11c+Ly6C− NK-like IKDCs and B220−CD19−CD11c+CD11b+ cDCs were determined by flow cytometry on a FACSCalibur (BD Biosciences) using the CellQuest software. From lymphoid cultures, the B220+CD19+CD11c−CD11b− cells corresponded to B cells, whereas B220+CD19−CD11c+CD11b− fraction contained a mix of pDCs and NK-like IKDCs, and B220−CD19−CD11c+CD11b+ was considered to be cDCs. In addition, myeloid cells were defined as B220−CD19−CD11b+Gr-1+ and NK cells were defined as B220+/−CD19−NK1.1+CD11c−.
Reverse-transcribed polymerase chain reaction analysis of gene expression
mRNAs were isolated from sorted cells using MicroPoly(A) pure (Ambion, Austin, TX) and converted to cDNA with Moloney murine leukemia virus reverse transcriptase (RT) (Invitrogen, Carlsbad, CA). The polymerase chain reaction (PCR) was conducted using ampli-Taq DNA polymerase (Takara, Kyoto, Japan) and TaqStart antibody (Clontech, Mountain View, CA). Anti-Taq Ab was inactivated at 95°C for 7 minutes before amplification of 30 seconds at 94°C, 30 secondsd at 56°C, and 45 seconds at 72°C. Primers for TLR9 and IL-7R genes were described.4
CpG-stimulation and cytokine production
CLPs were cultured for the indicated times in complete X-VIVO15 serum-free culture medium9 with phosphorothiolated CpG-containing oligonucleotide (CpG-ODN) 1826 (0.6 μg/mL; InvivoGen, San Diego, CA). Cells were washed twice and placed again in culture for 8 days.
To evaluate in vivo changes after CpG treatment, mice were given a single intraperitoneal injection with 100 μg/200 μL CpG-ODN 1826, or endotoxin-free water (LAL water, control), followed by harvesting of BM cells at 48 hours. Sera were assayed using enzyme-linked immunosorbent assay (ELISA) kits for IFNα (PBL, Piscataway, NJ), IFNγ, Flt3-L (FL), and TNFα (R&D Systems, Minneapolis, MN). IFNα, IFNγ, and IL-12 production by sorted DC populations was determined by ELISA.
Lymphoid cultures
Sorted cells were cultured in X-VIVO15 medium (Lonza Walkersville, Walkersville, MD) containing 1% detoxified bovine serum albumin (StemCell Technologies, Vancouver, BC), 5 × 10−5 M 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, 100 ng/mL FL, 20 ng/mL stem cell factor, and 1 ng/mL IL-7,9 at 37°C and 5% CO2 for 8 days. The same culture was used for determining the effects of cytokine production on neighboring cells after CpG stimulation. CLPs from both TLR9−/− (CD45.2) and wild-type (CD45.1) were isolated, mixed, and stimulated with CpG.
IL-7 response assay
The intracellular phosphorylation of STAT5 induced by IL-7 was detected by flow cytometry as described.26 Purified CLPs were stimulated with CpG for 24 hours in serum-free, stromal cell-free, cytokine-free culture. After washing and 20 minutes of resting at 37°C to quiet endogenous signaling, the cells were incubated in either medium alone or with 25 ng/mL IL-7 (R&D Systems) for an additional 20 minutes. Cells were fixed (Fix & Perm Kit, Invitrogen) before permeabilization and intracellular staining with PE-conjugated anti-phospho-STAT5 Ab (BD Biosciences PharMingen) or the appropriate isotype control. Analyses were performed in an LSR II using the FACSDiva software (BD Biosciences).
HSV-1 infection
C57BL/6 mice (6-10 weeks of age) were anesthetized by intraperitoneal injection with xylazine (2 mg/mL; 6.6 mg/kg) and ketamine (30 mg/mL; 100 mg/kg). Corneas were subsequently scarified with a 25⅝-gauge needle, and tear films were blotted before topical application of HSV-1 strain McKrae in a 3-μL volume of RPMI 1640 (1000 plaque-forming units (pfu)/cornea). Virus stocks were maintained at −80°C at 1.1 × 109 pfu/mL. Seven days after infection, mice were anesthetized and perfused with 20 mL phosphate-buffered saline, pH 7.4. BM was removed from femurs after perfusion. Animal treatment was consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85-23, revised 1996). All procedures were approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute institutional animal care and use committees.
Statistics
The Prism, version 3.02, software (GraphPad, San Diego, CA) was used for statistical analysis. Intergroup comparisons were performed with the unpaired t test. P values were 2-tailed and considered significant if less than 0.05.
Results
B lymphoid progenitors in mice with acute HSV-1 infection are directed to produce dendritic cells
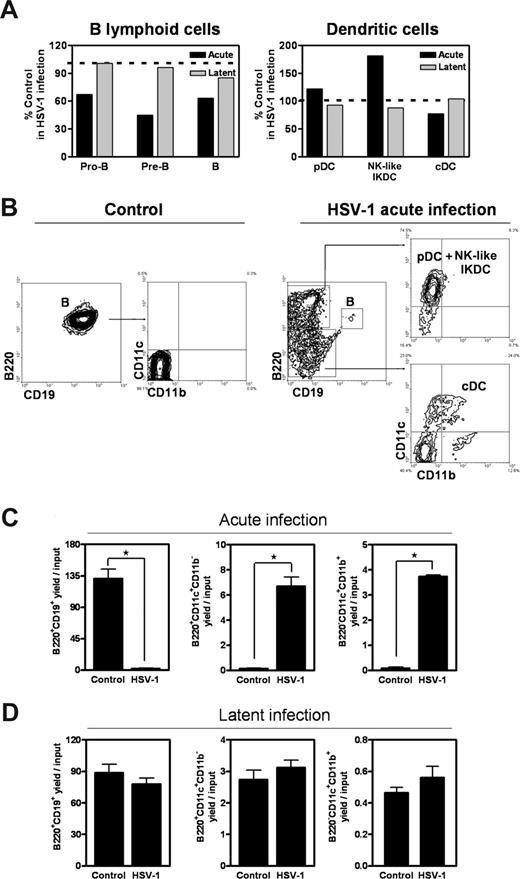
Our previous findings obtained with 2 TLR ligands and purified progenitors raised questions concerning the in vivo relevance to host defense, and a series of studies implicated TLR family molecules during HSV-1 infection.17,19–21 Therefore, a dose of 1000 pfu of the human pathogen HSV-1 was delivered to mice by corneal scarification. Late stages, ie, pro-B, pre-B, and B cells, were all reduced in marrow of these acutely infected mice, whereas pDC and NK-like IKDC populations were increased, and cDCs slightly declined (Figure 1A). Animals with latent infection resulting from 350 pfu HSV-1 survived at least 30 days, at which time no remarkable alterations were found in their BM.
Production of DCs from lymphoid progenitors in HSV-1–infected mice. (A) Total numbers of pro-B/large pre-B, small pre-B, B, pDCs, IKDCs, and cDCs were enumerated from femurs of mice after 7 days of acute infection or 30 days of latent HSV-1 infection. Percentage control values represent BM cellularity in each condition relative to BM cellularity in uninfected controls. The results are representative of 3 independent experiments. CLPs were double sorted from HSV-1–infected and control mice and placed in lymphoid cultures for 8 days. (B) The indicated flow cytometry gates were used to discriminate B220+CD19−CD11c+CD11b− pDC/NK-like IKDCs and B220−CD19−CD11c+CD11b+ cDCs. (C,D) Total numbers of recovered cells of each type after acute and latent infection were calculated and expressed as yields per input of common lymphoid progenitor (*significant difference, P < .05 by t test). Data are representative of 3 independent experiments. Error bars represent SEM.
Production of DCs from lymphoid progenitors in HSV-1–infected mice. (A) Total numbers of pro-B/large pre-B, small pre-B, B, pDCs, IKDCs, and cDCs were enumerated from femurs of mice after 7 days of acute infection or 30 days of latent HSV-1 infection. Percentage control values represent BM cellularity in each condition relative to BM cellularity in uninfected controls. The results are representative of 3 independent experiments. CLPs were double sorted from HSV-1–infected and control mice and placed in lymphoid cultures for 8 days. (B) The indicated flow cytometry gates were used to discriminate B220+CD19−CD11c+CD11b− pDC/NK-like IKDCs and B220−CD19−CD11c+CD11b+ cDCs. (C,D) Total numbers of recovered cells of each type after acute and latent infection were calculated and expressed as yields per input of common lymphoid progenitor (*significant difference, P < .05 by t test). Data are representative of 3 independent experiments. Error bars represent SEM.
Lin−IL-7Rα+c-KitLoSca-1+ CLPs persisted in HSV-1–infected mice, and they were tested for differentiation potential in lymphoid cultures (Figure 1B). The B lymphoid potential was reduced more than 95% in cells from acutely infected mice, whereas formation of B220+/−CD19−CD11c+CD11b+/− DCs was strongly favored. This was also apparent in terms of yield per input lymphoid progenitor (Figure 1C), but no abnormalities were found in CLPs taken from animals with latent infections (Figure 1D and data not shown).
The same disease model was used with TLR9−/− gene targeted mice. Lymphoid cells and DCs in BM of mutant animals were refractory to acute infection (Figure 2A). Furthermore, direction of CLPs to dendritic fates was negligible when the TLR9 receptor was absent (Figure 2B). Thus, B lymphopoiesis is diminished, and DC production is augmented during acute HSV-1 infection. This phenomenon is mediated by TLR9.
Lymphoid progenitors from TLR-deficient mice are not primed to become DCs during HSV-1 infection. (A) Total numbers of pre-B and B cells were enumerated in BM of TLR+/+ and TLR−/− mice on day 7 of acute infection. Data are means plus or minus SEM (*significant difference, P < .05). CLPs were highly purified from HSV-1–infected and control mice and placed in lymphoid cultures for 8 days. (B) The indicated flow cytometry gates were used to discriminate B220+CD19−CD11c+CD11b− pDCs + NK-like IKDCs and B220−CD19−CD11c+CD11b+ cDCs. Data are representative of 2 independent experiments with 3 replicates each.
Lymphoid progenitors from TLR-deficient mice are not primed to become DCs during HSV-1 infection. (A) Total numbers of pre-B and B cells were enumerated in BM of TLR+/+ and TLR−/− mice on day 7 of acute infection. Data are means plus or minus SEM (*significant difference, P < .05). CLPs were highly purified from HSV-1–infected and control mice and placed in lymphoid cultures for 8 days. (B) The indicated flow cytometry gates were used to discriminate B220+CD19−CD11c+CD11b− pDCs + NK-like IKDCs and B220−CD19−CD11c+CD11b+ cDCs. Data are representative of 2 independent experiments with 3 replicates each.
CpG treatment simulates herpes infection, and TNFα is not required to bias lymphoid progenitors to dendritic cell production
Because many herpes-mediated changes have been attributed to TLR9, we asked if treatment with a ligand for this receptor would influence lymphopoiesis. Flow cytometry was used to evaluate Lin−Sca-1+ BM cells recovered 48 hours after intraperitoneal injection of CpG (Figure 3A). Sca-1, a member of the interferon regulated Ly-6 gene family, increased on Lin− cells during inflammation.27 Consequently, we did not use Sca-1 exclusively to enumerate progenitors in this circumstance. Neither the density of IL-7Rα at the cell surface nor the expression of its RNA message was significantly changed on Lin−IL-7Rα+Sca-1+c-kitlo CLPs. Furthermore, CLPs were present in normal frequency in CpG-treated animals (Figure 3A,B). However, CpG treatment slightly reduced numbers of pro-B/large pre-B cells defined as B220+CD19+CD43+IgM− (Figure 3C). More substantial reductions were seen in B220+CD19+CD43−sIgM− small pre-B and B220+CD19+CD43−sIgM+ B cells. These results resembled previous studies that showed alterations in BM cell populations by injection of Corynebacterium parvum, LPS, adjuvant, malaria, or influenza virus.28–31 TNFα has been implicated in some of those changes, and we found increased serum levels of this cytokine 48 hours after CpG injection. Less notable IFNγ elevations were detected. FL is involved in normal DC differentiation, and CpG stimulation resulted in a 2-fold elevation in the sera of treated mice (Figure 3D).
CpG treatment preferentially depletes late-stage B lymphoid precursors in BM and simulates production of some inflammatory cytokines. (A,B) Mice were given single intraperitoneal injections of CpG, and 48 hours later lymphoid progenitors in BM were evaluated with respect to IL-7Rα expression by flow cytometry and RT-PCR. (C) Using stringent flow cytometry gating criteria, numbers of pro-B/large pre-B, small pre-B, and B cells in BM were evaluated. Data are mean plus or minus SEM (*significant difference, P < .05). (D) Levels of proinflammatory cytokines and Flt3-L (FL) in sera were tested by ELISA. Data are representative of more than 3 experiments with each having N = 3.
CpG treatment preferentially depletes late-stage B lymphoid precursors in BM and simulates production of some inflammatory cytokines. (A,B) Mice were given single intraperitoneal injections of CpG, and 48 hours later lymphoid progenitors in BM were evaluated with respect to IL-7Rα expression by flow cytometry and RT-PCR. (C) Using stringent flow cytometry gating criteria, numbers of pro-B/large pre-B, small pre-B, and B cells in BM were evaluated. Data are mean plus or minus SEM (*significant difference, P < .05). (D) Levels of proinflammatory cytokines and Flt3-L (FL) in sera were tested by ELISA. Data are representative of more than 3 experiments with each having N = 3.
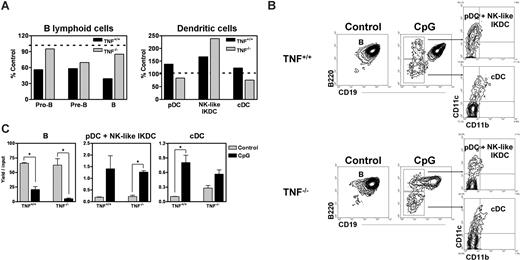
Experiments were then conducted with TNFα−/− and littermate control mice. As with normal animals, CpG treatment did not deplete CLP numbers in BM (not shown). A partial deficiency in B cells was observed in the BM and spleen of control TNFα−/− mice (not shown), possibly reflecting abnormalities in lymphoid organogenesis.32 In wild-type animals, numbers of pro-B, pre-B, and B cells declined with CpG treatment, whereas reciprocal increases were recorded in pDCs (Figure 4A). In contrast, lymphocyte numbers in the TNFα mutants were only partially diminished by CpG treatment, whereas pDC and cDCs were not significantly altered (Figure 4A). Numbers of NK-like IKDCs increased in both normal and TNFα−/− mice after treatment.
Reduction in B lineage cells in BM of CpG-treated mice is partially mediated by TNFα, but direction to dendritic fates does not require this cytokine. (A) TNFα+/+ and TNFα−/− mice were injected with LAL water (control) or CpG, and total numbers of pro-B/large pre-B, small pre-B, B, pDCs, NK-like IKDCs, and cDCs were enumerated from femurs 48 hours later. Percentage control values represent BM cellularity after CpG treatment relative to BM cellularity in controls. The results are representative of 3 independent experiments. CLPs were sorted in the same experiment and cultured under conditions designed to support B lymphopoiesis. (B) Flow cytometry was used to evaluate these cultures 8 days later. CD19− cells were further resolved on the basis of CD11b and CD11c to reveal conventional and pDC and NK-like IKDCs. (C) Yields per input progenitor were calculated as before. Data are mean plus or minus SEM (*significant difference, P < .05). Data are representative of 2 experiments with each having N = 3. Error bars represent SEM.
Reduction in B lineage cells in BM of CpG-treated mice is partially mediated by TNFα, but direction to dendritic fates does not require this cytokine. (A) TNFα+/+ and TNFα−/− mice were injected with LAL water (control) or CpG, and total numbers of pro-B/large pre-B, small pre-B, B, pDCs, NK-like IKDCs, and cDCs were enumerated from femurs 48 hours later. Percentage control values represent BM cellularity after CpG treatment relative to BM cellularity in controls. The results are representative of 3 independent experiments. CLPs were sorted in the same experiment and cultured under conditions designed to support B lymphopoiesis. (B) Flow cytometry was used to evaluate these cultures 8 days later. CD19− cells were further resolved on the basis of CD11b and CD11c to reveal conventional and pDC and NK-like IKDCs. (C) Yields per input progenitor were calculated as before. Data are mean plus or minus SEM (*significant difference, P < .05). Data are representative of 2 experiments with each having N = 3. Error bars represent SEM.
The next issue was whether lymphoid progenitors could be directly influenced by CpG in the absence of TNFα. We chose a 48-hour interval of in vivo treatment and sorted CLPs before initiating lymphoid cultures (Figure 4B). Generation of CD19+ cells was selectively reduced by at least two thirds by in vivo exposure to CpG, regardless of whether the progenitors were from normal or TNFα−/− mice. In addition, pDC + NK-like IKDC and cDC production in these cultures was dramatically stimulated (Figure 4C). A B220−CD19−CD11c−CD11b− population of unknown identity that arose from progenitors of wild-type and TNF−/− CpG-treated mice was not further investigated. Lymphoid progenitors from CD118/IFNα/β R−/− or IFNγ−/− mice were also normally redirected toward DC production by exposure to CpG (data not shown).
Thus, BM changes found in CpG-treated mice resembled those in HSV-1–infected mice. That is, late-stage B-lineage cells, but not CLPs, were depleted from the marrow, and inflammatory cytokines were not required to skew CLP toward DC fates.
Nature of DCs made from lymphoid progenitors primed by in vivo exposure to CpG
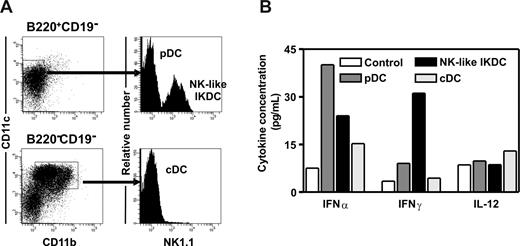
Highly purified CLPs were recovered from mice 48 hours after intraperitoneal injection of CpG and placed in stromal cell–free, serum-free lymphoid cultures. CLPs from treated mice gave rise to at least 2 major CD11c+ DC subsets (Figure 5A). To determine their nature, cells were stained with NK1.1, and it was found that the B220+CD19−CD11c+CD11b− category contained both NK1.1− (pDCs) and NK1.1+ (NK-like IKDC) cells, whereas the B220−CD19−CD11c+CD11b+ included a major NK1.1− (cDC) population (Figure 5A).
DCs generated from CLPs in response to TLR9 ligation are functional. CLPs were sorted from CpG-injected mice and cultured under conditions designed to support B lymphopoiesis. Harvested cells were then evaluated after 8 days by flow cytometry. (A) B220+CD19−CD11c+CD11b− cells were further fractionated into pDC and NK-like IKDC populations by the NK1.1 marker, whereas B220−CD19−CD11c+CD11b+ cDCs were NK1.1−. (B) The 3 categories of DCs were sorted, and their competence to respond to CpG stimulation by production of cytokines was evaluated.
DCs generated from CLPs in response to TLR9 ligation are functional. CLPs were sorted from CpG-injected mice and cultured under conditions designed to support B lymphopoiesis. Harvested cells were then evaluated after 8 days by flow cytometry. (A) B220+CD19−CD11c+CD11b− cells were further fractionated into pDC and NK-like IKDC populations by the NK1.1 marker, whereas B220−CD19−CD11c+CD11b+ cDCs were NK1.1−. (B) The 3 categories of DCs were sorted, and their competence to respond to CpG stimulation by production of cytokines was evaluated.
Consistent with previously reported properties of DC populations,33,34 pDCs were the main producers of IFNα in response to CpG stimulation, whereas NK-like IKDCs secreted more IFNγ. However, it is significant that they also produced some IFNα. All of the cells responded with modest production of IL-12 (Figure 5B). A minor B220−CD19−CD11c+CD11b−/lo population expressed NK1.1 and produced IFN-γ on CpG stimulation (not shown). These cells resemble a NKDC subset found in spleen.35 Thus, CpG treatment directed the formation of a variety of non-B lymphoid cells, including functional DCs from CLPs.
Dendritic cells with a lymphoid past are increased in CpG-treated mice
DC subsets in different tissues do not have equivalent lifespans, and their access to pathogen products may depend on proximity to infection sites. Therefore, it is difficult to quantify the contribution of TLR signals versus those elicited by cytokines, such as FL to DC numbers in any site. However, a fate mapping approach was used to determine whether any cells that had already initiated lymphopoiesis could be recruited into DC lineages. Approximately 30% of CLPs in Ig substrate transgenic mice24 are VEX fluorochrome positive and have a history of recombination. These cells were purified and shown to generate DCs on 48-hour exposure to CpG in culture (Figure 6A). Thus, lymphopoiesis can proceed beyond the Ig recombination stage and still be directed to dendritic lineages.
Transgenic mice with an Ig locus recombination substrate are used to detect redirection of lymphoid progenitors to dendritic fates. (A) CLPs with a history of Ig recombination were sorted on the basis of VEX fluorescence and placed in culture with or without CpG. Flow cytometry performed 8 days later revealed normal lymphopoiesis and continued VEX labeling for control cells (left 2 panels), whereas CpG-treated cells generated several classes of DCs and NK-like IKDCs (right 4 panels). (B) Transgenics were also injected with CpG, and BM cells were evaluated by flow cytometry 1 week later. Percentages of cells with a history of recombination (VEX+) are given for each of the indicated cell types. Data are representative of 2 independent experiments (*significant difference, P < .05). Error bars represent SEM.
Transgenic mice with an Ig locus recombination substrate are used to detect redirection of lymphoid progenitors to dendritic fates. (A) CLPs with a history of Ig recombination were sorted on the basis of VEX fluorescence and placed in culture with or without CpG. Flow cytometry performed 8 days later revealed normal lymphopoiesis and continued VEX labeling for control cells (left 2 panels), whereas CpG-treated cells generated several classes of DCs and NK-like IKDCs (right 4 panels). (B) Transgenics were also injected with CpG, and BM cells were evaluated by flow cytometry 1 week later. Percentages of cells with a history of recombination (VEX+) are given for each of the indicated cell types. Data are representative of 2 independent experiments (*significant difference, P < .05). Error bars represent SEM.
The transgenic mice were then given single intraperitoneal injections of CpG 7 days before harvest of marrow for flow cytometry analyses (Figure 6B). As with C57BL/6 mice used in all previous experiments, absolute numbers of B lineage cells declined, there were corresponding increases in pDCs, and cDC numbers were unchanged (not shown). Notably, percentages of both types of DCs that were VEX+ increased with CpG treatment, and this change reached significance for pDCs (Figure 6B). Similar results were obtained with spleen cells (not shown). Therefore, substantial numbers of pDCs in the CpG-treated mice must have originated from lymphoid progenitors.
Lymphoid progenitors express TLR9, and their differentiation potential is altered by exposure to CpG
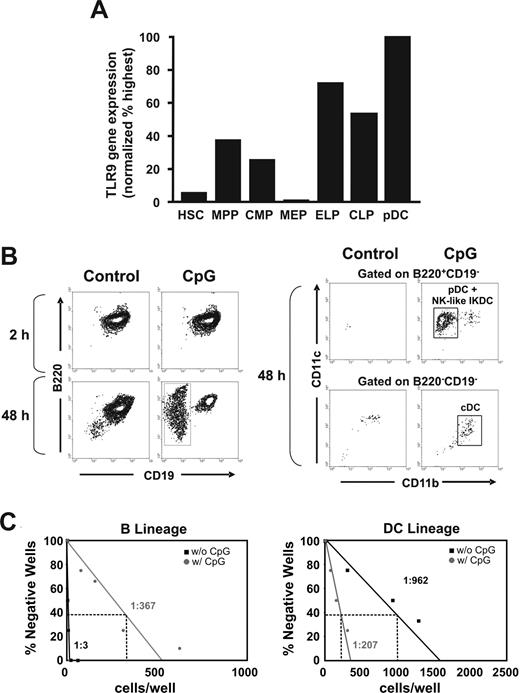
The focus of subsequent experiments was on learning whether hematopoietic progenitors were direct targets of CpG and exploring mechanisms associated with their altered differentiation. As previously determined for TLR2 and TLR4,17 RT-PCR analyses revealed that TLR9 transcripts were detectable in HSCs (Figure 7A). TLR9 expression was particularly high in subsets dedicated to lymphopoiesis, including Lin−RAG-1/GFP+Sca-1+c-Kithi early lymphoid progenitors and CLPs. This receptor was nearly absent in megakaryocyte-erythroid progenitors.
Lymphoid biased progenitors in BM express high levels of TLR9 and respond to CpG in culture. BM fractions were isolated as described in “Isolation of cell populations and flow cytometry” before analysis by quantitative RT-PCR. (A) The results are representative of 2 independent experiments and are normalized to β-actin and compared with pDC, the subset with highest expression. Normal Lin−IL-7Rα+c-kitloSca1+ CLPs were sorted and treated in serum-free, stromal-free cultures for 2 or 48 hours with 0.6 μg/mL CpG. The cultures were washed and then incubated for an additional 8 days with stem cell factor, FL, and IL-7 before flow cytometric analyses. Whereas almost pure populations of B220+CD19+ lymphocytes were present in control cultures, CD19− cells emerged as a result of extended CpG treatment (B, left panel). B220+/− CD19− cells were gated (B, right panel) and further analyzed to reveal B220+CD19−CD11c+CD11b− pDCs and/or NK-like IKDCs, and B220− CD19−CD11c+CD11b+ cDCs. Sorted CLPs were placed in limiting dilution stromal-cell free, serum-free cultures with and without 0.6 μg/mL of CpG for 48 hours. They were washed and then returned to culture for an additional 8 days before flow cytometry analysis. (C) Individual wells were scored as being positive for CD19+ B lineage and/or CD11c+ DCs.
Lymphoid biased progenitors in BM express high levels of TLR9 and respond to CpG in culture. BM fractions were isolated as described in “Isolation of cell populations and flow cytometry” before analysis by quantitative RT-PCR. (A) The results are representative of 2 independent experiments and are normalized to β-actin and compared with pDC, the subset with highest expression. Normal Lin−IL-7Rα+c-kitloSca1+ CLPs were sorted and treated in serum-free, stromal-free cultures for 2 or 48 hours with 0.6 μg/mL CpG. The cultures were washed and then incubated for an additional 8 days with stem cell factor, FL, and IL-7 before flow cytometric analyses. Whereas almost pure populations of B220+CD19+ lymphocytes were present in control cultures, CD19− cells emerged as a result of extended CpG treatment (B, left panel). B220+/− CD19− cells were gated (B, right panel) and further analyzed to reveal B220+CD19−CD11c+CD11b− pDCs and/or NK-like IKDCs, and B220− CD19−CD11c+CD11b+ cDCs. Sorted CLPs were placed in limiting dilution stromal-cell free, serum-free cultures with and without 0.6 μg/mL of CpG for 48 hours. They were washed and then returned to culture for an additional 8 days before flow cytometry analysis. (C) Individual wells were scored as being positive for CD19+ B lineage and/or CD11c+ DCs.
CLPs were sorted to high purity and exposed to the ligand CpG for either 2 or 48 hours. They were washed and placed in defined stromal cell–free cultures that are designed to support B lymphopoiesis. Untreated control progenitors, or those exposed to CpG for 2 hours, mainly gave rise to B220+CD19+ lymphocytes in the secondary cultures (Figure 7B left panel. In contrast, B lymphopoiesis was suppressed, and conspicuous CD19− populations emerged from 48-hour TLR9-ligated cells. Further gating on CD19− cells revealed that the new subsets included B220+CD19−CD11cloCD11b− pDCs and/or NK-like IKDCs, as well as B220−CD19−CD11cloCD11b+ cDCs (Figure 7B right panel). Most of the cDCs belonged to the Sirpα+ CD8α− equivalent subset36 (not shown). Limiting dilution cultures showed that the frequency of cells with B lymphoid potential dropped from 1 of 3 to 1 of 367 when CLPs were preexposed for 48 hours with CpG (Figure 7C). There was a reciprocal increase from 1 of 962 to 1 of 207 with respect to DC production. Note that progenitors normally generate small numbers of DCs in culture,7 so this may be an underestimation of the in vivo DC potential of CpG-treated CLPs. Thus, a TLR9 ligand can act as a differentiation cue, deviating highly purified progenitors under defined culture conditions to new fates.
In vitro investigation of cell population dynamics
Given the wide expression of TLR9 in BM, responses to corresponding pathogen products could be very complex. It remained unclear whether certain types of progenitors might be selectively expanded or suppressed or whether they might deviate to new fates. CLPs do not generate myeloid colonies when placed in stromal cell–free Methocel cultures containing growth and differentiation factors.8,37 It is significant that approximately 10% of CpG-treated CLPs but less than 0.2% of control CLPs produced myeloid colonies under these conditions (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
CLPs represent major intermediates in the pathway that produces most conventional NK cells.9,13 Because CpG treatment blocks production of B lineage cells, we predicted that this would also be the case for NK cells. CLPs were exposed to CpG for 48 hours before being washed and replated in B-cell cultures as described or in NK-cell cultures containing IL-15 (Figure S1B). B lymphopoiesis was reduced more than 7-fold by exposing CLPs to CpG, whereas CpG-treated CLPs produced 70% as many NK cells as control CLPs.
CMPs are normally more abundant than CLPs and are a rich source of cDCs.3,38 Furthermore, our previous studies showed that they are highly responsive to TLR2 and TLR4 ligands.17 CMPs differentiated as expected to generate B220−CD19−CD11c+CD11b+ cDCs in FL containing cultures (Figure S2). Surprisingly, CMPs were not good DC progenitors when CpG was present. Rather, we recorded increases in numbers of CD11b+GR-1+ myeloid cells.
Two recent studies identified Lin−c-KitloCD135+CD115+ pro-DC in BM and showed that they are restricted to production of DCs.6,7 Although it is known that CLPs normally have some DC potential, it was unclear whether pro-DC cells ever derive from CLPs. In preliminary experiments, CpG-stimulated CLPs did not generate pro-DCs in culture, and CLPs with CD115+ did not emerge in CpG-treated marrow (R.W., unpublished observations, 2008). Thus, B lymphoid progenitors express TLR9, respond to CpG, and generate DCs without transiting a pro-DC intermediate stage. There is also an increased tendency to generate myeloid cells, but myeloid committed CMPs were not induced to generate more DCs in response to TLR9 ligation. Furthermore, conventional NK lineage differentiation was not blocked, and production of NK-like IKDCs was enhanced.
No evidence for participation of STAT5
Survival, proliferation, and B lineage progression are critically dependent on IL-7.39 Therefore, TLR9 ligation might alter signaling events downstream of the IL-7 receptor without influencing NK-cell formation. The display of IL-7Rα was unchanged by CpG stimulation in vivo or in culture (Figures 3A,B, S3A). After 20 minutes of resting at 37°C, subgroups of cells were stimulated with IL-7 before all were fixed and stained with an antibody specific for active, phospho-STAT5. The untreated control group mounted a strong IL-7 signaling detectable by flow cytometry (Figure S3B). In contrast, signaling was reduced an average of 2-fold in cells that had been exposed to CpG. The same response was observed when CLPs were treated for either 18 or 24 but not 12 hours (not shown). Furthermore, progenitors from transgenic mice with constitutively active STAT523 were equivalent to ones from wild-type mice, and CpG treatment caused them to become DCs (Figure S3C). We conclude that STAT5 phosphorylation is diminished by TLR9 ligation but not requisite for promotion of DC production.
Autocrine stimulation does not account for the behavior of lymphoid progenitors modified by TLR9 ligation
All of these results would be consistent with a direct effect of CpG on lymphoid cells, but this point was more directly addressed with additional knockout mice. First, BM from MyD88−/− mice was transplanted into lethally irradiated, normal CD45.1 recipients. Three months later, the animals were treated with diluent or CpG for 48 hours. CLPs were then recovered by sorting and placed in lymphoid cultures. Lymphopoiesis from MyD88−/− progenitors was not substantially altered in this circumstance (not shown). This mutant not only blocks signaling from most TLR but also renders hematopoietic cells unresponsive to IL-1 and IL-18.40 Therefore, we performed culture experiments to determine whether TLR9-ligated progenitors could influence neighboring cells. As before, highly purified CLPs from wild-type mice lost 75% of their ability to generate CD19+ lymphocytes when exposed to CpG for 48 hours in culture. The same treatment had no effect on CLPs harvested from TLR9−/− mice (not shown). Progenitors from these 2 types of mice differed with respect to CD45 alleles, making it possible to discriminate them in cultures initiated with 50:50 mixtures (Figure S4). Importantly, all of the CD19− non-B lineage cells arose from wild-type progenitors, whereas the ratio of CD19+ TLR9 knockout to normal CD19+ lymphocytes increased. These findings demonstrate that the differentiation potential of lymphoid progenitors is directly influenced by TLR9 ligation, and the MyD88 adapter protein is required.
Discussion
We previously showed that highly purified stem and progenitor cells directly respond to TLR2 and TLR4 ligands under defined conditions of culture.17 The present study builds on those findings to include TLR9 and shows in vivo relevance with viral infection directing lymphopoiesis toward production of innate effector cells. TLR9 ligands interacted with progenitors, and there was no apparent cytokine requirement for altering their fates. Moreover, these newly formed CLP-derived DCs were competent to respond to CpG-ODN stimulation by production of cytokines. Therefore, health status may be a major determinant of the differentiation history of DCs.
TLRs and associated signaling molecules are important for controlling the expansion of some viruses, and one report indicates that MyD88−/− mice die more readily than normal animals to intranasal infection with HSV-1.41,42 There is more evidence that TLR mediated inflammatory responses are harmful and even fatal in HSV-1 infections.21,43 It will be interesting to learn if redirection of lymphopoiesis to generate additional innate effector cells during herpes infection participates in disease processes. The glycoprotein coats of herpes viruses ligate TLR2 on innate effector cells, stimulating cytokine production.21,43 However, our experiments did not implicate cytokines, and TLR9 was sufficient to mediate all of the changes we recorded in HSV-1–infected knockout mice. We think that cytokines and chemokines cause export of immature marrow cells, whereas TLR9 ligands set progenitors on a course to become DCs that can themselves produce factors in response to TLR9 stimulation.
Mice undergoing inflammation have immature hematopoietic cells mobilized from their BM.28–31 These responses involve reduction of the pro-adhesive CXCL12 chemokine and consequent detachment of immature cells from stromal elements.31 Homing of peripheral B cells into the marrow is also impaired.31 It was reported that pro-B, pre-B, and B cells were suppressed in BM of influenza virus infected mice and that this response was ablated by targeting TNF receptors.30 Similarly, we found that CpG depletion of these B lineage cells was greatly diminished relative to wild-type in TNFα−/− mice. However, their lymphoid progenitors were still present and directed to become DCs.
Cytokines other than TNFα might contribute to the direction of lymphoid progenitors in BM of CpG-treated or HSV-1–infected mice. The DNA of herpes viruses is rich in CpG sequences and, when internalized by marrow DCs, can function as a ligand for TLR9 and elicit large-scale interferon production.19,20 IFNγ was elevated in our CpG-treated mice, and interferons can affect lymphoid cells during early stages of viral infection.27 However, experiments conducted with IFNα/βR−/− or IFNγ−/− mice suggest that these interferons are not required for direction of lymphoid progenitors to DC production during HSV-1 infection.
The origins of the innate immune cells are not completely defined. On the one hand, apparently similar DCs can be produced from either myeloid or lymphoid progenitors.3,4,13 However, the newly identified pro-DC subset is restricted to the production of several categories of DCs, and they have not been linked developmentally to CLPs.6,7 Indeed, CLPs did not quickly express CD115, the defining marker of pro-DCs when exposed to CpG. This could mean that a unique pathway of DC production is promoted by pathogen products or that additional signals are required for formation of CD115+ intermediates. Flt3 ligand, TNF, Notch ligands, hormones, and undefined stromal cell products all encourage formation of particular DC types.44,45 Our findings suggest that microbial pathogen products also represent important differentiation cues for producing functional DCs.
IKDCs are thought to have both dendritic and NK-cell functions.11,12 Recent studies suggest they are developmentally related to NK lineage cells but different from DCs, and freshly isolated IKDCs are usually unable to produce type I interferons.13,15,16 However, these NK-like cells were conspicuous in cultures initiated with CpG stimulated CLPs, and they produced both classes of interferon in that circumstance. In addition, despite many similarities to conventional NK cells, IKDCs preferentially arose from different progenitors in transplantation experiments.13 Production of conventional NK cells in culture was not compromised by TLR stimulation. It is possible that subsets of NK-like IKDCs exist that represent different developmental origins and/or activation states.
It is difficult to know how many DCs arise through encounters with TLR ligands and how many result from cytokine stimulation. Furthermore, stem and progenitor cells in peripheral tissues might also mount local responses to pathogen products.17,18 Experiments with recombination substrate transgenic mice24 indicated that even lymphoid lineage cells with a past history of Ig locus recombination could be directed to become DCs in vivo.
The yield of DCs from CLPs is usually very low (< 0.5 per input progenitor) with stromal cell–free conditions,3,4,46 whereas primitive stem/progenitor cells are much better sources of DCs.4,7 By contrast, B lymphopoiesis from CLPs is very efficient in defined, stromal cell–free cultures,9 and there is extensive proliferation of CD19+ cells. The limiting dilution culture results suggest that there is a change in the fates of lymphopoietic cells exposed to TLR9 ligands. The culture medium and cytokines used in this study are best suited for B lineage cell production and may underestimate the DC differentiation potential of CLP after TLR9 ligation. Interestingly, myeloid-restricted CMPs expressed relatively low levels of TLR9 transcripts and produced increased numbers of myeloid but not DCs when exposed to CpG in vitro.
CLPs exposed to the TLR4 ligand LPS generated almost exclusive populations of cDCs in culture.17 We now show that additional types of functional DCs emerge when the same lymphoid progenitors are ligated with CpG. Perhaps the types of DCs that are made depend on the nature of the pathogen. Responses to both categories of TLR ligand require the MyD88 adapter protein, and it will be important to investigate downstream signaling pathways that account for such discrete responses. IL-7 stimulation normally causes marked phosphorylation of STAT5, and this response was blocked by TLR9 signaling. However, experiments with transgenic mice indicated that production of phospho-STAT5 is not the only requirement for progression in lymphoid lineages. Investigation of other signaling pathways may reveal how TLR ligation directs CLPs to DC fates.
Clinical trials are now evaluating CpG for efficacy in tumor therapy, and it has been assumed that this involves stimulation of cytotoxic effector cells.47 Our observations suggest that leukemia cells could be direct targets of TLR9 ligands. There are marked species differences with respect to Toll-like receptors, but human CD34+ TLR9+ cells do respond to CpG.48 One report showed that human cord blood CD34+ cells express TLR9- and CpG-elicited production of IL-8,48 whereas another failed to detect TLR9 in CD34+ marrow cells.49 It will be important to determine whether lymphohematopoiesis is altered in human BM as a result of viral infection or CpG therapy. These phenomena could pertain to other types of disease. For example, pDCs are major mediators of autoimmunity,50 and chronic infections might cause excessive DC production. Feedback control processes may exist to restore balance, possibly accounting for why CpG therapy rarely exacerbates autoimmune diseases in humans.47
There is much to learn about differentiation pathways responsible for generating all of the many specialized cells in the immune system, and we must now consider how hematopoietic cells integrate signals from extrinsic pathogen products with those from normal growth and differentiation factors. Patterns of blood cell differentiation under normal steady-state conditions might be quite different from those used during infections.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J. Van De Wiele for advice and reagents for the IL-7 response assay, Jacob Bass and Diana Hamilton for sorting, Tara Khamphanthala for technical assistance, Beverly Hurt for expert graphics assistance, and Shelli Wasson for professional editorial assistance.
This work was supported by the National Institutes of Health (grants AI20069, AI58162, AI053108, AI50737, AI069024, and AR054529), the US Immune Deficiency Network, a Jules and Doris Stein RPB Research Professor Award, and a Cancer Research Institute Investigator Award. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
National Institutes of Health
Authorship
Contribution: R.S.W., R.P., and K.P.G designed and performed research, analyzed data, and wrote the paper; Y.N. and T.R.W. designed and performed research and analyzed data; D.J.C., L.A.B., and M.A.F. contributed vital reagents or analytical tools and wrote the paper; and P.W.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul W. Kincade, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 Northeast 13th Street, Oklahoma City, OK 73104; e-mail: Kincade@omrf.ouhsc.edu.
References
Author notes
*R.S.W. and R.P. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal