Abstract
Clinical trials have indicated that immunoablation followed by autologous hematopoietic stem cell transplantation (ASCT) has the potential to induce clinical remission in patients with refractory systemic lupus erythematosus (SLE), but the mechanisms have remained unclear. We now report the results of a single-center prospective study of long-term immune reconstitution after ASCT in 7 patients with SLE. The clinical remissions observed in these patients are accompanied by the depletion of autoreactive immunologic memory, reflected by the disappearance of pathogenic anti–double-stranded DNA (dsDNA) antibodies and protective antibodies in serum and a fundamental resetting of the adaptive immune system. The latter comprises recurrence of CD31+CD45RA+CD4+ T cells (recent thymic emigrants) with a doubling in absolute numbers compared with age-matched healthy controls at the 3-year follow-up (P = .016), the regeneration of thymic-derived FoxP3+ regulatory T cells, and normalization of peripheral T-cell receptor (TCR) repertoire usage. Likewise, responders exhibited normalization of the previously disturbed B-cell homeostasis with numeric recovery of the naive B-cell compartment within 1 year after ASCT. These data are the first to demonstrate that both depletion of the autoreactive immunologic memory and a profound resetting of the adaptive immune system are required to reestablish self-tolerance in SLE. This trial was registered at www.clinicaltrials.gov as #NCT00742300.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with heterogeneous clinical manifestations. It is characterized by the generation of pathogenic antibodies directed against a variety of autoantigens, including nuclear and cytoplasmic antigens, such as double-stranded DNA (dsDNA), nucleosomes, and by complement activation.1 It is thought that, in genetically susceptible persons, an initial breakdown of peripheral tolerance permits the activation of autoreactive lymphocytes, which then propagate autoimmune responses in a self-perpetuating process.2,3 We recently demonstrated that autoimmune reactions in lupus-prone mice (NZB/W) result in the generation of long-lived plasma cells, which secrete pathogenic autoantibodies and are resistant to conventional immunosuppression and B-cell depletion therapy.4,5
Whereas glucocorticoids and immunosuppressants ameliorate manifestations of SLE in many patients, current therapies are insufficient to control the disease in a subset of patients, and their clinical prognosis remains poor because of the development of vital organ failure, cumulative drug toxicity, and the increased risk of cardiovascular disease and malignancy.6 Immunoablative chemotherapy followed by autologous hematopoietic stem cell transplantation (ASCT) has recently emerged as a promising experimental therapy for severely affected patients, providing them the potential to achieve treatment-free, long-term remission.7,8 The rationale for applying ASCT to autoimmune diseases has been the hope that immunoablation could eliminate inflammation-driving pathogenic cells from the immune system and that regeneration of the patients' immune system from hematopoietic precursors could reestablish immunologic tolerance.9,10 So far, direct evidence for that is lacking, and no study has determined whether immunoablation and ASCT can actually “reset the immunologic clock” in SLE.
Here we describe the long-term reconstitution of T- and B-cell subsets and serologic changes in 7 patients with SLE for up to 8 years after receiving immunoablation and ASCT, and show that immunoablation with high-dose chemotherapy, methylprednisolone, and antithymocyte globulin (ATG) efficiently depletes naive and memory T and B cells and long-lived plasma cells, including those that are autoreactive. In addition, ASCT reactivated the thymus, leading to the development of a tolerant, “juvenile” adaptive immune system, which is reflected by long-term, treatment-free, clinical remissions.
Methods
Clinical trial protocol
Enrollment in the monocentric phase 1/2 clinical trial included a diagnosis of SLE according to American College of Rheumatology classification criteria11 and failure of remission despite treatment with 2 different standard immunosuppressive therapies, including at least 6 cycles of intravenous cyclophosphamide at doses of 500 to 1000 mg/m2. A detailed description of the patients and trial design has been published previously.8 Peripheral blood stem cells were collected by leukapheresis after infusion of 2.0 g/m2 cyclophosphamide followed by daily granulocyte colony-stimulating factor (10 μg/kg; Amgen, Thousand Oaks, CA) beginning 72 hours after cyclophosphamide infusion. The graft was enriched for CD34+ cells by CliniMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). Data on the purity of CD34+-enriched hematopoietic stem cells are reported in Table 1. Immune ablation was achieved by 50 mg/kg per day of cyclophosphamide intravenously for 4 days (days −5 to −2) and 30 mg/kg per day of rabbit ATG (Fresenius, Bad Homburg, Germany) intravenously for 3 days (days −4 to −2); 1 g of methylprednisolone was administered intravenously on each day of ATG infusion. The study was approved by the responsible ethics committee and was conducted in conformity with European League Against Rheumatism and European Group for Blood and Marrow Transplantation guidelines for blood and bone marrow stem cell transplantation. Seven patients with SLE (Table 2) gave informed consent and were consecutively enrolled in the study. This study was performed in accordance with the Declaration of Helsinki.
Purity of the CD34+-enriched hematopoietic stem cell grafts
| Patient no. . | Percentage of CD34+ . | No. of infused CD34+/kg . | Percentage of CD3+ . | No. of infused CD3+/kg . |
|---|---|---|---|---|
| 1 | 92.2 | 3.0 × 106 | 0.48 | 1.6 × 104 |
| 2 | 89.3 | 2.4 × 106 | 0.52 | 1.4 × 104 |
| 3 | 99.1 | 6.1 × 106 | 0.05 | 1.0 × 104 |
| 4 | 98.0 | 4.2 × 106 | 0.05 | 1.0 × 104 |
| 5 | 97.5 | 2.6 × 106 | 0.02 | 0.5 × 104 |
| 6 | 95.3 | 2.0 × 106 | 0.03 | 0.6 × 104 |
| 7 | 78.6 | 2.4 × 106 | 0.03 | 0.4 × 104 |
| Patient no. . | Percentage of CD34+ . | No. of infused CD34+/kg . | Percentage of CD3+ . | No. of infused CD3+/kg . |
|---|---|---|---|---|
| 1 | 92.2 | 3.0 × 106 | 0.48 | 1.6 × 104 |
| 2 | 89.3 | 2.4 × 106 | 0.52 | 1.4 × 104 |
| 3 | 99.1 | 6.1 × 106 | 0.05 | 1.0 × 104 |
| 4 | 98.0 | 4.2 × 106 | 0.05 | 1.0 × 104 |
| 5 | 97.5 | 2.6 × 106 | 0.02 | 0.5 × 104 |
| 6 | 95.3 | 2.0 × 106 | 0.03 | 0.6 × 104 |
| 7 | 78.6 | 2.4 × 106 | 0.03 | 0.4 × 104 |
Demographic data and clinical features of patients
| Patient no. . | Sex/age, y . | Clinical manifestation . | SLEDAI pretransplantation . | Therapy pretransplantation . | Follow-up, mo . | Clinical outcome . | Current therapy . |
|---|---|---|---|---|---|---|---|
| 1 | F/27 | Nephritis, abdominal vasculitis, polyserositis, APS, cytopenia | 25 | CY, AZA, HCQ, CSA, MMF | 96 | Clinical remission | 2 mg prednisolone |
| 2 | F/48 | Nephritis, peripheral neuropathy, polyserositis, ventricular arrhythmia | 23 | CY, AZA, HCQ, MTX, MMF | 96 | Clinical remission | None |
| 3 | M/37 | Nephritis WHO IV, ventricular arrhythmia, polyserositis, APS, cytopenia | 30 | CY, AZA, HCQ, CSA, MTX, MMF | 38 | Relapse 18+ mo, exitus letalis 38+ mo | — |
| 4 | F/24 | Nephritis WHO V, seizures, psychosis, polyserositis, APS, cytopenia | 28 | CY, AZA, CSA | 72 | Clinical remission | None |
| 5 | F/31 | Nephritis WHO II, seizures, psychosis, APS, cytopenia | 26 | CY, AZA, MTX | 3 | Exitus letalis 3+ mo | — |
| 6 | F/30 | Nephritis WHO IIa, APS, cytopenia | 23 | CY, AZA, MTX, HQC | 48 | Clinical remission | 4 mg prednisolone |
| 7 | M/19 | Nephritis, cerebritis, APS cytopenia | 19 | CY, AZA, MTX, HCQ | 24 | Clinical remission | 4 mg prednisolone |
| Patient no. . | Sex/age, y . | Clinical manifestation . | SLEDAI pretransplantation . | Therapy pretransplantation . | Follow-up, mo . | Clinical outcome . | Current therapy . |
|---|---|---|---|---|---|---|---|
| 1 | F/27 | Nephritis, abdominal vasculitis, polyserositis, APS, cytopenia | 25 | CY, AZA, HCQ, CSA, MMF | 96 | Clinical remission | 2 mg prednisolone |
| 2 | F/48 | Nephritis, peripheral neuropathy, polyserositis, ventricular arrhythmia | 23 | CY, AZA, HCQ, MTX, MMF | 96 | Clinical remission | None |
| 3 | M/37 | Nephritis WHO IV, ventricular arrhythmia, polyserositis, APS, cytopenia | 30 | CY, AZA, HCQ, CSA, MTX, MMF | 38 | Relapse 18+ mo, exitus letalis 38+ mo | — |
| 4 | F/24 | Nephritis WHO V, seizures, psychosis, polyserositis, APS, cytopenia | 28 | CY, AZA, CSA | 72 | Clinical remission | None |
| 5 | F/31 | Nephritis WHO II, seizures, psychosis, APS, cytopenia | 26 | CY, AZA, MTX | 3 | Exitus letalis 3+ mo | — |
| 6 | F/30 | Nephritis WHO IIa, APS, cytopenia | 23 | CY, AZA, MTX, HQC | 48 | Clinical remission | 4 mg prednisolone |
| 7 | M/19 | Nephritis, cerebritis, APS cytopenia | 19 | CY, AZA, MTX, HCQ | 24 | Clinical remission | 4 mg prednisolone |
APS indicates antiphospholipid syndrome; CY, cyclophosphamide; AZA, azathioprine; HCQ, hydroxychloroquine; CSA, cyclosporine; MTX, methotrexate; MMF, mycophenolate mofetil; and —, not applicable.
Clinical responses to ASCT
All 7 patients successfully completed the protocol treatment. Clinical remission, defined as a Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) of less than 3 without immunosuppressive treatment or use of antimalarials and with less than 7.5 mg of prednisolone daily, was achieved in all 7 patients. One patient relapsed after being free of clinical symptoms for 18 months after transplantation, as described earlier12 ; he died of SLE-related pulmonary embolism 38 months after ASCT. Another patient died because of uncontrolled invasive central nervous system aspergillosis 3 months after ASCT. The remaining 5 patients showed no clinical or serologic evidence of SLE activity during a median follow-up of 60 months (range, 24-96 months).
Blood samples and cell preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom).
Flow cytometry
Absolute CD4+ and CD19+ lymphocyte numbers were calculated based on the total lymphocyte count and the percentage of CD4+ and CD19+ cells, as identified by flow cytometry using the BD Multitest panel (BD Biosciences, San Jose, CA). The following monoclonal antibodies (mAbs) were used for phenotypic analyses: anti-CD19-peridinin chlorophyll protein Cy5.5 (SJ25C1), anti–CD20-phycoerythrin (PE; 2H7), and anti–IgD–fluorescein isothiocyanate (FITC; IA6-2), anti–CD4–peridinin chlorophyll protein Cy5.5 (SK3), anti–CD31-PE (MEC13.3), anti–CD45RA-FITC (L48), and anti–CD45RO-allophycocyanin (APC; UCHL-1), all obtained from BD Biosciences. Anti–CD27-Cy5 (2E4) was conjugated to Cy5 (GE Healthcare) according to the manufacturer's instructions. Immunofluorescence staining was performed by incubating PBMCs in the presence of mAbs in 1% bovine serum albumin in phosphate-buffered saline on ice for 10 minutes after blocking with 10 μg of human IgG for 10 minutes. Cells were washed before analysis on a FACSCalibur flow cytometer (BD Biosciences).
FoxP3 expression analysis was performed using freshly isolated PBMCs stained with anti–CD4-FITC (TT1), anti–CD25-APC (2A3; BD), and anti–Foxp3-PE (PCH101) using the anti-human FoxP3 Staining Set (eBioscience, San Diego, CA) according to the manufacturer's instructions. At least 2.5 × 104 CD4+ T cells were acquired.
We performed analysis of T-cell receptor (TCR) Vβ expression on freshly isolated peripheral blood CD4+ T cells by 4-color flow cytometry using 22 TCR Vβ-specific mononuclear antibodies (IOTest Beta Mark; Beckman Coulter, Fullerton, CA) as described recently.13 TCR designations are according to Arden's nomenclature.14 At least 2.5 × 104 CD4+ T cells were acquired. Normal ranges were established for each Vβ member based on confidence intervals (CIs) of 97.5% determined in 20 healthy persons. Perturbations of Vβ families were considered to be significant in patients when they were outside of these normal intervals.
Bone marrow mononuclear cells (BM-MNCs) were collected by bone marrow aspiration in 1 patient after ASCT and in 1 healthy volunteer from the femur after joint surgery. BM-MNCs were isolated by Ficoll-Hypaque density gradient centrifugation (GE Healthcare) and stained with anti–CD38-APC (HIT2; BD PharMingen, San Diego, CA) and anti–CD138-PE (B-B4; Chemicon International, Temecula, CA). Cells were washed before acquisition (LSR II flow cytometer; BD Biosciences) and analysis (FlowJo Software; TreeStar, San Carlos, CA).
Stimulation assays
For in vitro lymphocyte stimulation assays, 1 mL freshly collected heparinized peripheral blood was stimulated in the presence of 1 μg/mL αCD28 (clone 28.2; BD PharMingen) for 6 hours at 37°C with the following antigens: 1 μg/mL Staphylococcus aureus enterotoxin B as the positive control (Sigma Chemie, Deisenhofen, Germany), 20 μg/mL nucleosomes, as described previously,15 10 μg/mL SmD1 peptide, as described previously,16 varicella zoster virus lysate and cytomegaly virus (CMV) lysate (both Biodesign International, Kennebunk, ME). Brefeldin A (Sigma Chemie) was added for the last 4 hours of stimulation. Erythrocytes were lysed with FACS lysing solution and permeabilized with FACS-Perm2 (both from BD PharMingen) according to the manufacturer's instructions. Fixed cells were stained for 30 minutes at room temperature with the following antibodies: anti–CD154-PE (TRAP1) or anti–CD69-PE (FN-50), anti–CD4-PerCp Cy5.5 (RPA-T4), anti–CD14-FITC (M5E2) and anti–interferon gamma (IFN-γ)–APC (B27) (all purchased from BD PharMingen). At least 2 × 105 CD4+ T cells were analyzed.
Serologic analysis
Antinuclear antibodies (ANAs) were assessed by indirect immunofluorescence on HEp-2 cells. Anti-dsDNA antibodies were detected by Crithidia luciliae immunofluorescence and commercial enzyme-linked immunosorbant assay (ELISA).
Statistical analysis
T- and B-lymphocyte subpopulation frequencies were calculated using CellQuest software (BD Biosciences). A paired t test was used to compare (per patient and immune parameter) pretransplantation and posttransplantation data using Graph Pad Prism 4 software (version 4.03; Graph Pad Software, San Diego, CA). Based on distributional assumptions, the Mann-Whitney U test was used to compare data from patients treated by ASCT with those from healthy controls and conventionally treated SLE patients. All P values were 2-sided; statistical significance was set at α = 0.05.
Results
Engraftment and leukocyte recovery
The median time for recovery to 0.5 × 109/L neutrophils and 109/L leukocytes in peripheral blood was 14 days. Platelets recovered to more than 20 × 109/L by a median of 12 days. No patient received unselected backup stem cell support. All patients had significantly reduced baseline lymphocyte counts (mean, 0.33 ± 0.14 × 103 cells/μL), reflecting lupus activity or side effects of immunosuppressive therapy. As in patients with hematologic diseases treated with a similar regimen,17 peripheral lymphocyte counts reconstituted slowly after ASCT and were still slightly reduced at the 6-month follow-up (0.94 ± 0.32 × 103 cells/μL). Mean absolute lymphocyte counts were back to normal at the 1-year follow-up (1.11 ± 0.29 × 103 cells/μL) and remained stable thereafter in responding patients.
Increased naive T cells after posttransplantation immune reconstitution
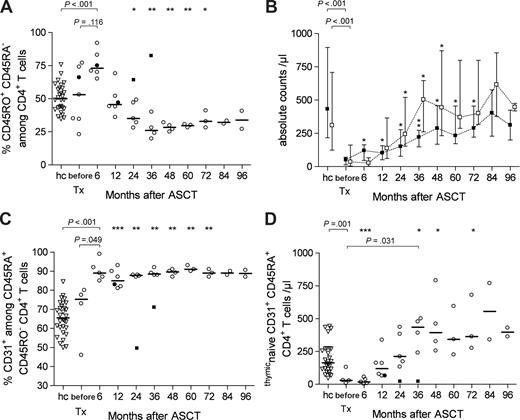
In SLE, pathogenic T-cell functions are thought to be mediated by autoreactive memory or memory effector CD4+ T cells. Elimination of such cells in vivo by immunoablation is therefore presumed to ameliorate autoimmune inflammation. Conversely, thymic reactivation is presumably required to reestablish central tolerance and to generate natural FoxP3+ regulatory T cells. To assess the effect of immunoablation and ASCT on the CD4+ T-cell compartment, we first used the phenotypic markers CD45RA and CD45RO to discriminate between (CD45RA+ CD45RO−) naive and (CD45RO+ CD45RA−) memory CD4+ T cells.
The longitudinal analysis of reconstituting naive and memory CD4+ T cells is shown in Figure 1. At baseline, patients displayed a significant CD4+ T-cell lymphopenia compared with age-matched healthy controls, which was attributable to both CD45RA+ naive (median, 35/μL vs 288/μL, P < .001) and CD45RO+ memory CD4+ T cells (median, 51/μL vs 433/μL, P < .001), reflecting the disturbed T-cell homeostasis of active SLE (Figure 1B). In the regenerative phase, the memory phenotype (CD45RO+CD45RA−) was the predominant CD4+ T-cell subset; there was a significant increase in this subpopulation at 6 months after treatment compared with baseline (median, 73.4% vs 53.3%, P = .116; Figure 1A) with a doubling of absolute counts (median, 121/μL vs 51/mL, P = .027; Figure 1B). Naive CD4+ CD45RA+ T-cell counts were low or undeterminable at that time but later increased continuously, reaching complete recovery 24 months after ASCT with significant higher values than before ASCT (median, 244/μL vs 35/μL, P = .014; Figure 1B). CD45RO+ Th counts (Figure 1B) remained significantly diminished until the 4-year follow-up.
Recovery of CD4+ T-cell subsets over time in SLE patients treated by immunoablation and ASCT versus levels in age-matched healthy controls. (A) CD45RO+ CD45RA− CD4+ T-cell frequencies (median values) in patients (○, •) versus controls (hc, ▿, n = 28). The patient represented by closed symbols had a complete relapse 18 months after ASCT (patient 3); data after the flare (■) were excluded from statistical considerations. A Mann-Whitney U test was used for group comparison; a paired t test was performed to compare pre-ASCT data and corresponding post-ASCT data (*P < .05, **P < .005, ***P < .001). (B) Absolute counts of CD45RA+ CD45RO− naive (□) and CD45RO+ CD45RA− memory (■) CD4+ T cells (median values and ranges) in patients versus controls (hc, n = 28). (C) CD31 expression on CD45RA+ CD45RO− CD4+ T cells (median values) in patients versus controls (hc, n = 28). (D) Absolute counts of CD45RA+ CD31+thymicnaive CD4+ T cells (median values) in patients versus controls (hc, n = 28).
Recovery of CD4+ T-cell subsets over time in SLE patients treated by immunoablation and ASCT versus levels in age-matched healthy controls. (A) CD45RO+ CD45RA− CD4+ T-cell frequencies (median values) in patients (○, •) versus controls (hc, ▿, n = 28). The patient represented by closed symbols had a complete relapse 18 months after ASCT (patient 3); data after the flare (■) were excluded from statistical considerations. A Mann-Whitney U test was used for group comparison; a paired t test was performed to compare pre-ASCT data and corresponding post-ASCT data (*P < .05, **P < .005, ***P < .001). (B) Absolute counts of CD45RA+ CD45RO− naive (□) and CD45RO+ CD45RA− memory (■) CD4+ T cells (median values and ranges) in patients versus controls (hc, n = 28). (C) CD31 expression on CD45RA+ CD45RO− CD4+ T cells (median values) in patients versus controls (hc, n = 28). (D) Absolute counts of CD45RA+ CD31+thymicnaive CD4+ T cells (median values) in patients versus controls (hc, n = 28).
Increased output of RTEs
To determine whether CD45RA+CD45RO−CD4+ T cells in the regenerated immune system were homeostatically expanded peripheral T cells or naive T cells newly generated in the thymus, we analyzed their expression of CD31, a surrogate marker of recent thymic emigrants (RTEs).18 Six months after ASCT, 85.2% to 98.8% (median, 89.7%) of the CD45RA+CD4+ T cells in responding patients coexpressed CD31; this was significantly more than before ASCT in the same patients (median, 75.3%, P = .049) and in age-matched healthy controls (median, 64.2%, P < .001; Figure 1C). CD31 expression in CD45RA+ CD4+ T cells of the responding patients remained at these high frequencies during the entire observation period. Recovery of this T-cell subset to numbers comparable with healthy controls was completed between 12 and 24 months after ASCT (Figure 1D), reaching on average 5.2 to 12.1 times the baseline levels. Remarkably, the number of recent thymic emigrants continued to increase in responding patients; not before 36 months after ASCT, they reached a plateau at twice the level observed in age-matched healthy controls (median, 435/μL vs 164/μL, P = .016), and at 15 times the baseline levels (median, 435/μL vs 29/μL, P = .031). The number of RTEs decreased only in the patient who suffered a relapse 18 months after transplantation.
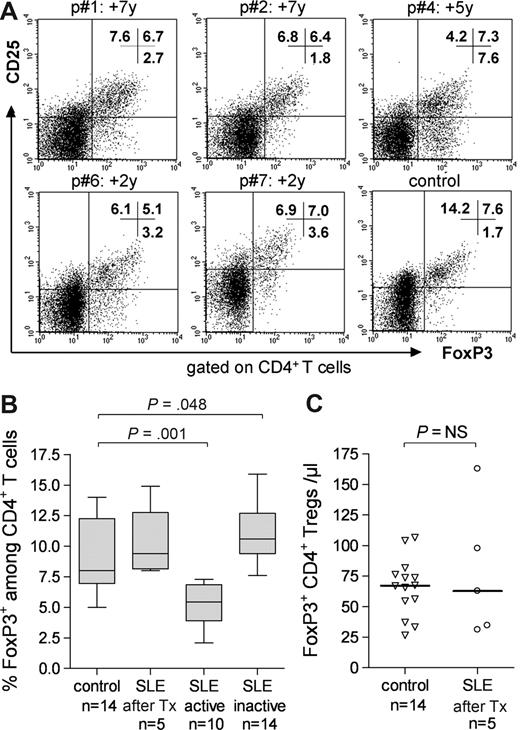
The thymus contributes to the regeneration of FoxP3+ regulatory T cells
Regeneration of the regulatory CD4+ T cell (Treg) compartment was evaluated after ASCT by identifying peripheral blood CD4+ T cells costaining brightly for CD25 and expressing intracellular FoxP3 (Figure 2A). Conventionally treated patients with active SLE had significantly lower frequencies of peripheral FoxP3+ Tregs than normal controls (median, 5.5% vs 8.0%, P = .001), as illustrated in Figure 2B. Those with inactive SLE had comparable, if not higher, frequencies of FoxP3+ CD4+ T cells than the controls (median, 10.5% vs 8.0%, P = .048). FoxP3+ CD4+ T-cell frequencies in regenerated immunoablated ASCT patients at time points from 2 to 7 years after ASCT (as depicted in Figure 2A) were as high as those in normal controls (median, 9.4%, vs 8.0%, P = .229; Figure 2B). Overall, the absolute numbers of FoxP3+ CD4+ T cells were similar in both groups (median, 62.8/μL vs 67.2/μL, P = .963; Figure 2C). However, the patients were heterogeneous with respect to numbers of FoxP3+ CD4+ T cells. The later the follow-up date, the higher the patients' peripheral FoxP3+ CD4+ T-cell count (161.8/μL in patient 1 at 7 years) and the lower the count in patients analyzed at earlier time points after ASCT (31.1/μL in patient 7 at 2 years).
Phenotypic analysis of FoxP3+ Treg levels in 5 patients after ASCT compared with those in healthy controls and conventionally treated SLE patients. (A) CD25 and FoxP3 expression on CD4+ T cells at indicated times after ASCT in 5 patients and 1 control. (B) Median FoxP3 expression levels in CD4+ T cells (as determined by flow cytometry in panel A) in 5 patients versus 14 healthy controls, 10 conventionally treated patients with active SLE (SLEDAI ≥ 6), and 14 conventionally treated with inactive SLE (SLEDAI < 6). Group comparisons were performed using the Mann-Whitney U test. (C) FoxP3+ CD4+ Tregs (median absolute counts, as determined by flow cytometry in panel A) in 5 patients versus 14 normal controls. A Mann-Whitney U test was used for group comparison.
Phenotypic analysis of FoxP3+ Treg levels in 5 patients after ASCT compared with those in healthy controls and conventionally treated SLE patients. (A) CD25 and FoxP3 expression on CD4+ T cells at indicated times after ASCT in 5 patients and 1 control. (B) Median FoxP3 expression levels in CD4+ T cells (as determined by flow cytometry in panel A) in 5 patients versus 14 healthy controls, 10 conventionally treated patients with active SLE (SLEDAI ≥ 6), and 14 conventionally treated with inactive SLE (SLEDAI < 6). Group comparisons were performed using the Mann-Whitney U test. (C) FoxP3+ CD4+ Tregs (median absolute counts, as determined by flow cytometry in panel A) in 5 patients versus 14 normal controls. A Mann-Whitney U test was used for group comparison.
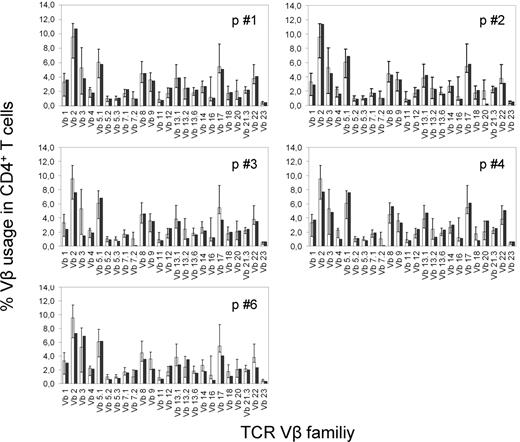
Thymic output generates a new and diverse TCR repertoire
CD4+ T-cell diversity in the patients' regenerating immune systems was analyzed with a panel of TCR Vβ-specific monoclonal antibodies by flow cytometry, as recently described.19 At baseline, all patients analyzed (n = 4) showed significantly expanded TCR Vβ-expressing CD4+ T cells (Table 3) in line with previous findings on restricted TCR repertoires in active SLE.20 Early after ASCT (+ 3 months), CD4+ T cells in these patients still exhibited a highly restricted TCR repertoire, however, with a different TCR Vβ family usage profile (Table 3). At the time point of assessment, patients showed no clinical signs of active infection or lupus flare except for 1 patient with an acute systemic herpes infection (HHV-6, patient 4). However, all patients contracted frequent infections during the period of neutropenia shortly after ASCT. Along with the emergence of thymic naive CD31+ T cells, the CD4+ TCR repertoire gradually normalized within 1 year after ASCT (Table 3). Except for 2 patients showing transient TCR Vβ expansion (patient 4, Vβ5.1 at 4 years and patient 6, Vβ12 at 2 years) after ASCT, the TCR Vβ profiles of CD4+ T cells remained stable and heterogeneous throughout follow-up. At the 3-year follow-up, the regenerated CD4+ T-cell TCR Vβ family usage was normal in all patients (Figure 3).
Significantly expanded TCR Vβ-expressing CD4+ T cells at baseline and during follow-up after ASCT in patients 4 to 7
| Patient no.TCR . | Beforetransplantation . | 3 months after transplantation . | 6 months after transplantation . | 1 year after transplantation . | 2 years after transplantation . | 3 years after transplantation . | 4 years after transplantation . |
|---|---|---|---|---|---|---|---|
| 4 | |||||||
| BV5.1 | 5.5 | 8.1* | 11.8* | 8.9* | 7.9 | 7.6 | 8.3* |
| BV5.2 | 1.7 | 2.4* | 1.8 | 1.5 | 1.2 | 1.1 | 1.1 |
| BV7.1 | 1.9 | 3.2* | 1.9 | 2.7* | 2.2 | 2.1 | 2.2 |
| BV9 | 4.5 | 6.4* | 4.3 | 3.5 | 3.0 | 3.3 | 2.9 |
| BV13.6 | 1.5 | 6.7* | 1.9 | 2.2 | 0.6 | 2.3 | 2.0 |
| BV16 | 5.7* | 0.5 | 0.3 | 0.6 | 0.2 | 1.1 | 0.7 |
| 5 | |||||||
| BV14 | 8.4* | — | — | — | — | — | — |
| 6 | |||||||
| BV7.2 | 2.7* | 1.7 | 1.4 | 1.9 | 1.6 | 1.4 | 2.0 |
| BV12 | 2.1 | 2.3 | 2.5 | 2.2 | 2.7* | 2.5 | 2.6 |
| BV13.2 | 5.0* | 3.5 | 2.9 | 3.4 | 3.4 | 3.1 | 3.5 |
| BV20 | 3.7* | 4.7* | 1.1 | 1.5 | 2.1 | 1.4 | 2.1 |
| 7 | |||||||
| BV4 | 2.6* | 1.9 | 2.6* | 2.2 | 2.3 | — | — |
| BV12 | 2.1 | 3.4* | 3.2* | 2.5 | 1.7 | — | — |
| BV13.1 | 5.2 | 12.9* | 4.7 | 4.8 | 5.1 | — | — |
| Patient no.TCR . | Beforetransplantation . | 3 months after transplantation . | 6 months after transplantation . | 1 year after transplantation . | 2 years after transplantation . | 3 years after transplantation . | 4 years after transplantation . |
|---|---|---|---|---|---|---|---|
| 4 | |||||||
| BV5.1 | 5.5 | 8.1* | 11.8* | 8.9* | 7.9 | 7.6 | 8.3* |
| BV5.2 | 1.7 | 2.4* | 1.8 | 1.5 | 1.2 | 1.1 | 1.1 |
| BV7.1 | 1.9 | 3.2* | 1.9 | 2.7* | 2.2 | 2.1 | 2.2 |
| BV9 | 4.5 | 6.4* | 4.3 | 3.5 | 3.0 | 3.3 | 2.9 |
| BV13.6 | 1.5 | 6.7* | 1.9 | 2.2 | 0.6 | 2.3 | 2.0 |
| BV16 | 5.7* | 0.5 | 0.3 | 0.6 | 0.2 | 1.1 | 0.7 |
| 5 | |||||||
| BV14 | 8.4* | — | — | — | — | — | — |
| 6 | |||||||
| BV7.2 | 2.7* | 1.7 | 1.4 | 1.9 | 1.6 | 1.4 | 2.0 |
| BV12 | 2.1 | 2.3 | 2.5 | 2.2 | 2.7* | 2.5 | 2.6 |
| BV13.2 | 5.0* | 3.5 | 2.9 | 3.4 | 3.4 | 3.1 | 3.5 |
| BV20 | 3.7* | 4.7* | 1.1 | 1.5 | 2.1 | 1.4 | 2.1 |
| 7 | |||||||
| BV4 | 2.6* | 1.9 | 2.6* | 2.2 | 2.3 | — | — |
| BV12 | 2.1 | 3.4* | 3.2* | 2.5 | 1.7 | — | — |
| BV13.1 | 5.2 | 12.9* | 4.7 | 4.8 | 5.1 | — | — |
— indicates not applicable.
Significant expansions according to criteria described in “Methods.”
CD4 TCR Vβ repertoires in 5 patients after ASCT. TCR Vβ family usage in peripheral blood CD4+ T cells (■) in 5 patients 3 years after treatment. □ represent median values in 20 healthy donors; boundaries indicate the 2.5th and 97.5th percentiles.
CD4 TCR Vβ repertoires in 5 patients after ASCT. TCR Vβ family usage in peripheral blood CD4+ T cells (■) in 5 patients 3 years after treatment. □ represent median values in 20 healthy donors; boundaries indicate the 2.5th and 97.5th percentiles.
Early expansion of memory CD4+ T cells is not driven by autoantigens
The specificity of CD4+ memory T cells was analyzed by ex vivo short-term restimulation of whole blood with viral antigens and autoantigens, and subsequent enumeration of reactivated T cells expressing CD69 and IFN-γ (Th1 memory cells) or CD154 (all memory Th cells) during the early phase of immune reconstitution.15,16,21 Notably, these ex vivo restimulation assays were performed in patients with identified viral infections based on clinical symptoms and corresponding serologic findings (patient 1: varicella-zoster virus, patient 4: human herpes virus 6, patient 6: CMV reactivation, patient 7: herpes simplex virus 1). During viral infections, Th effector memory cells specific for VZV (patient 1) and CMV (patient 6) were readily detectable as CD69+ or CD154+ CD4+ T cells coexpressing IFN-γ. Inversely, T cells reacting to nucleosomes or SmD1 were not detectable early after ASCT (Figure 4).
In vitro whole-blood stimulation assays in 4 patients early after ASCT. Levels of reactivated CD4+ T cells coexpressing CD69 or CD154 and intracellular IFN-γ after ex vivo short-term restimulation of whole blood for 6 hours with viral antigens, autoantigens, and without antigens as control (without [w/o]) in 4 patients at indicated time points after ASCT.
In vitro whole-blood stimulation assays in 4 patients early after ASCT. Levels of reactivated CD4+ T cells coexpressing CD69 or CD154 and intracellular IFN-γ after ex vivo short-term restimulation of whole blood for 6 hours with viral antigens, autoantigens, and without antigens as control (without [w/o]) in 4 patients at indicated time points after ASCT.
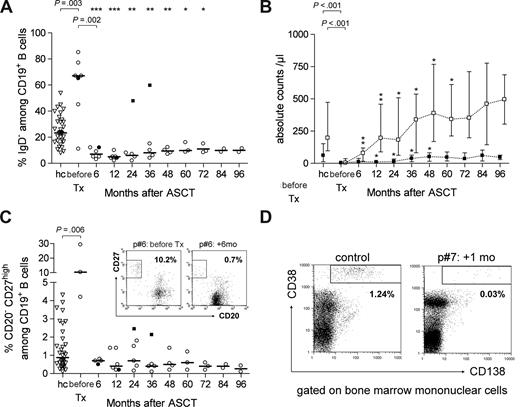
Normalization of disturbed B-cell homeostasis after ASCT
Active SLE is characterized by marked B lymphocytopenia, which reportedly affects CD27− naive B cells more than CD27+ memory B cells.22 A prominent population of peripheral plasma blasts has also been observed in active disease.22,23 To evaluate the effect of immunoablation and ASCT on these B-cell disturbances, we analyzed peripheral blood B lymphocytes from regenerating immune systems for IgD, CD27, and CD20 expression.22,24,25
Before treatment, patients had significantly lower numbers of IgD+ naive B cells than normal controls (median, 4/μL vs 202/μL, P < .001; Figure 5) as well as a predominance of IgD− memory B lymphocytes (median, 67.2% vs 23.5%, P = .003) and a prominent population of CD27high CD20− plasma blasts (median, 10.3% vs 0.9%, P = .006) in peripheral blood. After ASCT, B lymphocytes predominantly displayed a naive IgD+ phenotype. Complete numeric recovery of this subset was observed by 12 months after ASCT, with counts 50 times higher than at baseline (median, 196/μL vs 4/μL, P = .024). Absolute naive B-cell counts in responders were well maintained throughout follow-up.
Recovery of CD19+ B-cell subsets over time in SLE patients treated by immunoablation and ASCT versus levels in healthy controls. (A) Levels of IgD− memory B cells among CD19+ B cells (median values) in patients (○, •) versus controls (hc, ▿, n = 32). The patient represented by closed symbols had a complete relapse 18 months after ASCT (patient 3); data after the flare (■) were excluded from statistical considerations. A Mann-Whitney U test was used for group comparison; a paired t test was performed to compare pre-ASCT data and corresponding post-ASCT data (*P < .05, **P < .005, ***P < .001). (B) Absolute numbers of IgD+ naive (□) and IgD− memory (■) CD19+ B cells (median and range) in patients versus controls (hc, n = 32). A Mann-Whitney U test was used for group comparison; a paired t test was used to compare pre-ASCT data and corresponding post-ASCT data. (C) Levels of CD27highCD20− plasma blasts among circulating CD19+ B cells (median values) in patients versus controls (hc, n = 32). Dot plots show representative examples in 1 patient (patient 6) at baseline and 6 months after ASCT. (D) Surface expression of CD38 and CD138 on bone marrow mononuclear cells in 1 patient (patient 7) early after ASCT (+1 month) and in 1 healthy control.
Recovery of CD19+ B-cell subsets over time in SLE patients treated by immunoablation and ASCT versus levels in healthy controls. (A) Levels of IgD− memory B cells among CD19+ B cells (median values) in patients (○, •) versus controls (hc, ▿, n = 32). The patient represented by closed symbols had a complete relapse 18 months after ASCT (patient 3); data after the flare (■) were excluded from statistical considerations. A Mann-Whitney U test was used for group comparison; a paired t test was performed to compare pre-ASCT data and corresponding post-ASCT data (*P < .05, **P < .005, ***P < .001). (B) Absolute numbers of IgD+ naive (□) and IgD− memory (■) CD19+ B cells (median and range) in patients versus controls (hc, n = 32). A Mann-Whitney U test was used for group comparison; a paired t test was used to compare pre-ASCT data and corresponding post-ASCT data. (C) Levels of CD27highCD20− plasma blasts among circulating CD19+ B cells (median values) in patients versus controls (hc, n = 32). Dot plots show representative examples in 1 patient (patient 6) at baseline and 6 months after ASCT. (D) Surface expression of CD38 and CD138 on bone marrow mononuclear cells in 1 patient (patient 7) early after ASCT (+1 month) and in 1 healthy control.
IgD− memory-phenotype B-cell frequencies drastically declined from a median of 67.2% at baseline to 7.0% within 6 months after ASCT (P = .002). During immune regeneration, IgD− B-cell frequencies remained lower than in healthy controls over the entire follow-up period of up to 8 years (Figure 5A). IgD− memory B-cell counts did not recover until 3 years after ASCT (Figure 5B). IgD− memory B lymphocytes did not detectably expand before that time, except in the patient with the lupus flare (Figure 5A). CD20− CD27high plasma blast frequencies among CD19+ B cells normalized within 6 months after ASCT in all patients analyzed, and normal levels persisted during the entire follow-up period (Figure 5C).
Autoreactive and protective antibodies in serum are largely extinguished after ASCT
All patients had ANAs and persistently high anti–double-stranded (anti-ds) DNA serum antibody titers before enrollment. After immunoablation and ASCT, anti-dsDNA antibodies disappeared in all patients within 1 month (Table 4) and recurred only in the patient with reactivated disease (patient 3). Four of 6 patients with a follow-up of at least 6 months after transplantation showed a decrease in ANA titers to negative or 1:80, which is regarded as clinically not significant (Table 4). From these 4 patients, only 1 (patient 4) showed relevant ANA recurrence, which persisted from the 3-year follow-up onward without clinical symptoms of SLE. In 2 patients, ANA persisted, albeit in significantly reduced titers.
Titer of ANAs and anti–dsDNA antibodies before and after ASCT
| Patient no./antibodies . | Beforetransplantation . | After ASCT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 month . | 6 months . | 12 months . | 24 months . | 36 months . | 48 months . | 60 months . | 72 months . | 84 months . | 96 months . | ||
| 1 | |||||||||||
| ANA | 5120 | 320 | Negative | Negative | 320 | 80 | 160 | 320 | 80 | 80 | 80 |
| dsDNA | 8 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 2 | |||||||||||
| ANA | 5120 | 320 | 80 | 80 | 80 | 80 | 80 | 160 | 80 | 80 | 80 |
| dsDNA | 64 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 3 | |||||||||||
| ANA | 2560 | 160 | 80 | 80 | 5120 | 2560 | — | — | — | — | — |
| dsDNA | 64 | Negative | Negative | Negative | Negative | Negative | — | — | — | — | — |
| 4 | |||||||||||
| ANA | 20 480 | 640 | Negative | Negative | Negative | 320 | 1280 | 1280 | 1280 | — | — |
| dsDNA | 64 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | — | — |
| 5 | |||||||||||
| ANA | 2560 | 320 | — | — | — | — | — | — | — | — | — |
| dsDNA | 32 | Negative | — | — | — | — | — | — | — | — | — |
| 6 | |||||||||||
| ANA | 10 240 | 2560 | 2560 | 2560 | 2560 | 5120 | 5120 | — | — | — | — |
| dsDNA | 80 | Negative | Negative | Negative | Negative | Negative | Negative | — | — | — | — |
| 7 | |||||||||||
| ANA | 10 240 | 1280 | 1280 | 640 | 640 | — | — | — | — | — | — |
| dsDNA | 80 | Negative | Negative | Negative | Negative | — | — | — | — | — | — |
| Patient no./antibodies . | Beforetransplantation . | After ASCT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 month . | 6 months . | 12 months . | 24 months . | 36 months . | 48 months . | 60 months . | 72 months . | 84 months . | 96 months . | ||
| 1 | |||||||||||
| ANA | 5120 | 320 | Negative | Negative | 320 | 80 | 160 | 320 | 80 | 80 | 80 |
| dsDNA | 8 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 2 | |||||||||||
| ANA | 5120 | 320 | 80 | 80 | 80 | 80 | 80 | 160 | 80 | 80 | 80 |
| dsDNA | 64 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 3 | |||||||||||
| ANA | 2560 | 160 | 80 | 80 | 5120 | 2560 | — | — | — | — | — |
| dsDNA | 64 | Negative | Negative | Negative | Negative | Negative | — | — | — | — | — |
| 4 | |||||||||||
| ANA | 20 480 | 640 | Negative | Negative | Negative | 320 | 1280 | 1280 | 1280 | — | — |
| dsDNA | 64 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | — | — |
| 5 | |||||||||||
| ANA | 2560 | 320 | — | — | — | — | — | — | — | — | — |
| dsDNA | 32 | Negative | — | — | — | — | — | — | — | — | — |
| 6 | |||||||||||
| ANA | 10 240 | 2560 | 2560 | 2560 | 2560 | 5120 | 5120 | — | — | — | — |
| dsDNA | 80 | Negative | Negative | Negative | Negative | Negative | Negative | — | — | — | — |
| 7 | |||||||||||
| ANA | 10 240 | 1280 | 1280 | 640 | 640 | — | — | — | — | — | — |
| dsDNA | 80 | Negative | Negative | Negative | Negative | — | — | — | — | — | — |
ANA indicates antinuclear antibodies, inverse titer; dsDNA, anti–double-stranded DNA antibodies (C luciliae assay), inverse titer; and —, not applicable.
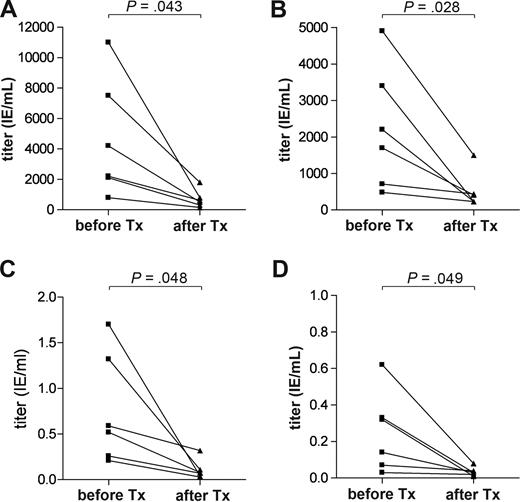
Not only autoantibodies but also protective serum antibodies specific for measles, mumps, tetanus, and diphtheria disappeared in the immunoablated patients. All patients had received the World Health Organization-recommended vaccination before enrollment. Even though not all patients had reached protective levels of vaccine-specific antibodies before immunoablation, significant decreases in serum antibody levels for measles (P = .043, Figure 6A), mumps (P = .028, Figure 6B), tetanus toxoid (P = .048, Figure 6C), and diphtheria (P = .049, Figure 6D) were observed when tested 1 or 2 years after ASCT (Figure 6). The serologic data point to an effective depletion of long-lived plasma cells from the bone marrow.5,26 A bone marrow aspiration sample from 1 patient (patient 7), obtained early after ASCT (+1 month), exhibited almost complete depletion of CD38+ CD138+ plasma cells with only 0.03% among BM-MNC compared with a normal control bone marrow with 1.24% of such cells (Figure 5D).
Changes in protective antibody titers over time after ASCT. Protective serum antibody titers for (A) measles, (B) mumps, (C) tetanus, and (D) diphtheria declined significantly within 1 to 2 years after transplantation in all 6 patients with a follow-up of at least 6 months after transplantation. A paired t test was used to compare pretransplantation and posttransplantation data.
Changes in protective antibody titers over time after ASCT. Protective serum antibody titers for (A) measles, (B) mumps, (C) tetanus, and (D) diphtheria declined significantly within 1 to 2 years after transplantation in all 6 patients with a follow-up of at least 6 months after transplantation. A paired t test was used to compare pretransplantation and posttransplantation data.
Discussion
Immunoablation followed by ASCT is an emerging treatment option for patients with severe autoimmune diseases refractory to conventional therapies, including SLE.7,10,27,28 In accordance with previous reports,7,28 this regimen achieved long-term (up to 8 years) clinical and serologic treatment-free remissions in our lupus patients. Notably, these patients originally had high disease activity and a poor prognosis, reflected by high SLEDAI scores and persistent anti-dsDNA antibody titers. Although the clinical efficacy of this experimental therapy is rapidly becoming evident, it remains obscure how it works. Our detailed analysis of the long-term reconstitution of the patients' immune systems with respect to the recurrence of T- and B-lymphocyte subsets and the course of serologic changes over time demonstrated successful depletion of autoreactive immunologic memory and the regeneration of a tolerant immune system from hematopoietic stem cells. Regeneration involved reactivation of the thymus and extensive renewal of antigen-receptors, in other words, a “resetting of the immunologic clock.”
Depletion of autoreactive immunologic memory by immunoablation was most drastically reflected in the complete disappearance of autoantibodies, particularly dsDNA-specific antibodies. Depletion of immunologic memory was not restricted to autoreactive memory. In addition, pathogen-specific serum antibodies for mumps, measles, tetanus, and diphtheria were largely extinguished. This drastic ablation of humoral memory suggests that the ATG used for immunoablation directly targets the plasma cells secreting these serum antibodies. It was recently shown that the plasma cells providing humoral memory are long-lived cells that reside mostly in the bone marrow, where they dwell in specialized niches providing essential survival signals.5 Recent in vitro experiments indicated that polyclonal rabbit ATG directly targets plasma cells and B cells via complement-mediated lysis and apoptosis.29,30 In line with this hypothesis, we were able to stain plasma cells ex vivo with the polyclonal rabbit ATG used for immunoablation (data not shown); moreover, plasma cells disappeared from bone marrow 1 month after immunoablation in 1 case. The depletion of long-lived plasma cells might be of particular relevance for the success of immunoablative therapy. It has been demonstrated that these plasma cells are resistant to immunosuppression by cyclophosphamide, irradiation, and CD20-mediated B-cell depletion.4,5,31 Hence, autoreactive long-lived plasma cells represent a key component of autoreactive immunologic memory. Persistent autoantibodies secreted by long-lived plasma cells could maintain chronic inflammation and accelerate autoimmunity.32 A retrospective survey by the European Blood and Marrow Transplant and European League Against Rheumatism Registry revealed that patients without complete loss of autoantibody responses after immunoablation and ASCT had higher rates of relapse.28 In our cohort, the only patient who relapsed became anti-dsDNA-negative after immunoablation, but anti-Ro/SSA and anti-La/SSB antibodies persisted until the relapse. From the other patients, only 1 had anti-Ro/SSA antibodies before enrollment (patient 6). Similar to the patient with the relapse, anti-Ro/SSA antibodies persisted in this patient after ASCT, albeit without evidence for SLE reactivation. So far, it is not clear why plasma cells secreting these autoantibodies seem to be more resistant to the immunoablative regimen and if their persistence characterizes patients with a higher risk for relapse.
T-cell reconstitution after immunoablation was characterized by continued generation of new naive CD4+ T cells for up to 8 years after ASCT. In particular, naive CD45RA+CD4+ T cells expressing CD31 with high overall clonal diversity of the CD4+ TCR repertoire were generated. These cells have been shown to be recent thymic emigrants.18 In the regenerated patients, absolute CD45RA+CD31+ naive CD4+ T-cell counts continuously increased to levels twice as high as those in age-matched controls, resembling those in young children. This observation supports the notion that, after immunoablation and ASCT, the naive CD4+ T-cell compartment is regenerated by thymic reactivation rather than by lymphopenic expansion of surviving naive T cells, emphasized earlier for patients undergoing immunoablation and ASCT for treatment of hematologic malignancies33 and multiple sclerosis.34 In our SLE patients, the finding is even more relevant in light of the disease- and treatment-related impairment of the naive T-cell compartment, which has been attributed to intrinsic impairment of thymic export.35 Immunoablation and ASCT obviously can correct this deficiency, rejuvenate the CD4+ T-cell compartment, and normalize naive T-cell homeostasis.
After monitoring the TCR Vβ family repertoire of the recurring CD4+ T-cell compartment, we observed a drastic change in clonal diversity of the TCR repertoire. The originally observed clonal expansions and deletions disappeared, suggesting that treatment had led to the ablation of expanded clones and to the generation of a complete repertoire of recent thymic emigrants.36
Among CD4+ T cells, FoxP3+ regulatory T cells regenerated to frequencies and absolute numbers comparable with those in normal controls. The fact that regeneration of the Treg compartment was accompanied by the reappearance of naive T cells and recent thymic emigrants suggests that these regulatory T cells were generated in the thymus. Similar observations have been made in patients undergoing immunoablation and ASCT for juvenile idiopathic arthritis, suggesting a common mechanism of action of stem cell transplantation in different autoimmune diseases.37
Whereas regeneration of thymic naive Th cells was delayed for up to 1 year after ASCT, mature CD45RO+ memory CD4+ T cells reappeared faster with on average a doubling of absolute counts at 6 months after transplantation compared with baseline values. However, their TCR Vβ repertoires were highly restricted, reflecting responses to a limited array of available antigens during lymphopenia.38,39 If peripheral T-cell expansion had involved lymphopenia-driven proliferation of memory T cells in response to low-affinity self-antigens, expansion of autoreactive T-cell clones should have been observed.40 However, we found no evidence of clonal expansion of autoreactive T cells specific for SLE-associated autoantigens, such as nucleosomes or SmD1. This implies that the early expansion of memory CD4+ T cells is not driven by autoantigens and, in particular, not by those involved in SLE. Rather, we showed that clonally expanded memory T cells reacted to virus-specific antigens in patients infected with specific viruses. This implicates protective pathogen-specific immune responses as a cause of clonal expansion of memory-phenotype T cells. The expansion of protective pathogen-specific T cells in response to treatment may contribute to the control of autoimmunity by restricting the space available in the effector-memory compartment for autoreactive T cells, the expansion of which is driven by (weak) reactions to autoantigens.
Regeneration of the B lymphocyte compartment in the treated SLE patients resembled that of patients receiving ASCT for treatment of hematologic malignancies.17,41 The majority of repopulating B cells initially showed a naive (IgD+) phenotype. Memory (IgD−) B cells did not reappear until later. In the present study, this regeneration of the B-cell compartment was remarkable in view of the significant disturbances observed in our active SLE cohort before ASCT. These patients had shown naive B-cell lymphopenia, relative predominance of phenotypically memory B cells, and expansion of CD27high CD20− plasma cell precursors. The complete normalization of these preexisting disturbances indicates that immunoablation had removed all autoreactive B cells. Apparently, the B-cell compartment also regenerates from stem cells after immunoablation and ASCT, and it is tolerant to self-antigens, including those that had been relevant in the patients before treatment.
In conclusion, this study provides direct evidence for a profound regeneration of the adaptive immune system in SLE patients after immunoablation and ASCT. All patients except 1 achieved long-lasting clinical and serologic remissions and are no longer reliant on immunosuppressive therapy. The 1 exception relapsed after having been in clinical remission for more than a year. The relapse might be the result of insufficient ablation of autoreactive immunologic memory, presentation of tolerance-breaking autoantigen forms to the regenerated immune system, or genetic predispositions that restart the disease in the regenerated immune system. Our findings would propose that chronic autoimmunity is not an endpoint depending on continuous treatment with specific anti-inflammatory agents but may be cured by combining specific targeting of autoreactive memory and effector cells with a reactivation of thymic activity.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Bundesministerium fur Bildung und Forschung (01GI9944/DRFZ C4.1) and the Sonderforschungsbereiche (SFB) 650 TP12.
Authorship
Contribution: T.A., A.S., S.K., and H.M. did most of the experiments; F.H., R.A., A.T., and A.R. developed the concept and designed the clinical trial; T.A., O.R., G.M., H.R., E.G.-I., G.-R.B., R.A., and F.H. conducted the clinical trial; and T.A., A.T., F.H., and A.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tobias Alexander, Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Charitéplatz 1, 10117 Berlin, Germany; e-mail: tobias.alexander@charite.de.
References
Author notes
*T.A. and A.T. contributed equally to this work.
†R.A., A.R., and F.H. (all senior authors) contributed equally to this work.

![Figure 4. In vitro whole-blood stimulation assays in 4 patients early after ASCT. Levels of reactivated CD4+ T cells coexpressing CD69 or CD154 and intracellular IFN-γ after ex vivo short-term restimulation of whole blood for 6 hours with viral antigens, autoantigens, and without antigens as control (without [w/o]) in 4 patients at indicated time points after ASCT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/1/10.1182_blood-2008-07-168286/5/m_zh80240828220004.jpeg?Expires=1769097900&Signature=s2K5n2q08TvI5zeEEnEKcEc6UiwoKUQiowJDK~GJvgBNIQ9pyAgjcOrtKDOmpM4wQ2ycefUCjGXNvVg28jLepY9Nksok5QsZd49Mk1~8pJp1bj1uAfX-Cg-HuZb4rsTpWvCS8AHvupGxYvGGpsXNU8M~u0XdxiqlD6SiBTu0W6o1QSKeJxQEraQfs1ubTT8uwMO3lUIJ07DtnUHQEkyzYxAfNabaA8W2QgoInplqQQ19i-mVVwNqvkLiGWnV5kSW~NWSRk3sz~LiKtI8X~t1zDG0yEC1QRVVoGb5OZrRgRoJNleO3~98ID6JqC6bSKI8fWbfhsbUSqCs8-ib84DZKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal