Abstract
Activation of dendritic cells (DCs) leads to cell maturation, which is accompanied by a regulated pattern of gene expression changes. Two significant and contradictory consequences of DC activation are that, although activation is necessary for maximal T-cell stimulation, it also leads to the initiation of gene expression that results ultimately in cell death. We have identified a gene, MINOR (mitogen-inducible nuclear orphan receptor), that becomes highly up-regulated on activation and whose expression leads to apoptosis in mature DCs. MINOR is a member of the Nur77 family of nuclear orphan receptors, which includes Nur77 and Nurr1. Although Nur77 and Nurr1 are expressed in macrophages and DCs, their expression levels do not change on DC activation. We thus tested the hypothesis that induction of MINOR would lead to an activation-induced cell death in DCs and that its inhibition would increase the lifespan of DCs and improve their vaccine efficacy. To block natural expression of MINOR by DCs, we generated a lentiviral vector that expresses a small interfering RNA. Our results indicate that blockade of MINOR expression dramatically decreases apoptosis in DCs and suggest that this approach may be a novel means to improve the potency of ex vivo–generated DC vaccines.
Introduction
Although dendritic cells (DCs) are critical for initiating immune responses, they are relatively short lived in vitro and in vivo, and their transience probably affects their potential for therapeutic use.1 Activation of DCs leads to a specific pattern of gene expression that allows for maximal immune responses but also signals the initiation of cell death. Although many advances have been made in understanding the nature of antigen processing and presentation, the genes that are important for regulating DC lifespan have not been well understood. Studies using labeled DCs suggest that they are replaced every 3 to 4 days,2 implying that there is only a limited window for T cells to encounter antigen-presenting DCs. Although prone to apoptosis, DCs are more resistant to certain apoptotic pathways than other cell types as a result of the expression of molecules, such as FLICE inhibitory protein (cFLIP), which blocks caspase 8 activation and the subsequent apoptotic cascade. Mature DCs derived from bone marrow (BM) or spleen express high levels of Fas but are insensitive to Fas-mediated killing.3,4 In addition, signaling by TRANCE or CD154 has been shown to prevent apoptosis in DCs5,6 ; nevertheless, these cells generally have a very short lifespan in vivo. Indeed, animal models and clinical trials suggest that one major issue with ex vivo expansion and loading of DCs followed by reinjection is that relatively few DCs successfully traffic to spleen or lymph nodes,7 and those that do are rapidly cleared by host cytotoxic T lymphocytes (CTLs)8 and increased CD154 by tumors decreases their cytotoxicity to DCs,9 indicating the physiologic relevance of this finding. Thus, there is a coordinated regulation of proapoptotic and antiapoptotic factors during DC activation.
Another gene family that regulates apoptosis is the Nur77 family. The Nur77 family consists of 3 orphan nuclear receptors: Nur77, Nurr1, and MINOR.10,11 Nur77 was originally identified as an immediate-early gene that was rapidly induced in fibroblasts by serum stimulation and in PC12 cells by nerve growth factor.12 Nur77 is a transcription factor that binds to NGFI-B/Nur77 response element (NBRE) motifs as a monomer and to palindromic NurRE sites as a dimer.13 Both Nur77 and MINOR have been implicated in mediating the cell fate and differentiation of immune cells, including macrophages, thymocytes, and T and B cells.14,15 Although much of the work on the Nur77 superfamily members has been focused on T-cell apoptosis, their role in DC apoptosis has not been examined. In the present studies, we have identified a Nur77 family member that is highly up-regulated in mature DCs, and whose expression leads to the induction of apoptosis in DCs. MINOR was originally identified by a subtractive hybridization analysis in which the gene expression profile of activated DCs was compared with that of activated macrophages.16 Nur77 is involved in activation-induced cell death of T cells10,11 and also caspase-independent cell death of macrophages.15 Here we show that, although DCs express Nur77 constitutively, its expression does not change on DC activation, whereas the MINOR gene becomes highly up-regulated, which suggests the possibility that MINOR might provide an analogous role in creating an activation-induced cell death signal in DCs. In support of this hypothesis, our results show that forced expression of MINOR induces apoptosis in DC2.4 cells, which are similar to immature DCs, and blockade of MINOR expression suppresses natural apoptosis in DCs, indicating that MINOR is a pivotal gene in regulating DC lifespan. In addition, its inhibition leads to an enhancement in DC vaccine potency.
Methods
Mice
BALB/c mice (6-8 weeks old) were purchased from the National Cancer Institute (Frederick, MD) and Harlan (Indianapolis, IN). The 6.5 T-cell receptor (TCR)–transgenic mice that express a TCR recognizing a I-Ek–restricted hemagglutinin (HA) epitope were bred within the animal facility at Johns Hopkins University. All animal studies were approved by the Johns Hopkins University Animal Care and Use Committee.
Antibodies and flow cytometry staining
All antibodies, annexin V–phycoerythrin (PE), and 7-amino-actinomycin D (7-AAD) were purchased from BD Biosciences PharMingen (San Diego, CA). Flow cytometry analyses were performed on a FACSCalibur instrument (BD Biosciences PharMingen) and analyzed using CellQuest (BD Biosciences PharMingen) and FlowJo (TreeStar, Ashland, OR).
Preparations of DCs
Bone marrow–derived DCs (BMDCs) were generated by standard methods. On day 8, DCs were collected for fluorescence-activated cell sorting (FACS) based on CD86 and major histocompatibility complex class II (MHC II) expression. Immature and mature DCs were sorted as CD11c+CD86lowMHCIIlow and CD11c+CD86highMHCIIhigh cells, respectively. For transduction with lentiviral vectors, DCs were infected for 12 to 24 hours with self-inactivating LV-MINOR-small interfering RNA (siRNA) or LV-control-siRNA at a multiplicity of infection of 5 on days 3 and 4 with polybrene (8 μg/mL). The efficiency of transduction was analyzed on day 6 by flow cytometry; and, in some cases, positively transduced DCs as identified by their green fluorescent protein (GFP) fluorescence were FACS-sorted for further experiments. DC subpopulations were prepared from spleens and lymph nodes of BALB/c mice using methods as previously described.17,18
Bone marrow transplant and transduction of HSCs
BALB/c BM was harvested and enriched for hematopoietic stem cells (HSCs) using the StemSep separation kit (StemCell Technologies, Vancouver, BC). HSCs were transduced for 3 days with either LV-siRNA-control/GFP or LV-siRNA-MINOR/GFP followed by transplantation into myeloablatively (850 cGy radiated)–treated Balb/c mice. Mice were killed after 8 to 10 weeks of engraftment time, and their spleens and lymph nodes were harvested for DC isolation.
Adoptive transfer of T cells, DCs, and A20-HA cells
A total of 6.5 CD4+ HA-specific TCR-transgenic T cells were prepared from pooled spleen and lymph nodes of 6.5 TCR-transgenic mice. CD4+ T cells were prepared using the CD4 isolation kit (Miltenyi Biotec, Auburn, CA). Clonotypic percentage was determined by flow cytometry analysis. The purified carboxyfluorescein succinimidyl ester-labeled T cells (2.5 × 106) were injected intravenously. On day 3, HA peptide-loaded DCs (100 μg class II HA peptide/1 × 106 cells) were resusupended in phosphate-buffered saline for subcutaneous injection. The A20-HA is a B-cell lymphoma, previously described.19
Quantification of transcripts
Quantitative polymerase chain reaction (PCR) analysis was conducted using the Bio-Rad iCycler system (Bio-Rad, Hercules, CA). The MINOR primer sequences are: forward: 5′-AGCAGCTTAAAGGACCACCA-3′ and reverse: 5′-GGGTGTCAAGGAAGAGCTTG-3′. Values have been normalized to β-actin.
Induction of apoptosis by transient expression of MINOR
DC 2.4 cells were transfected with either the rat homolog MINOR cDNA (pCI MINOR, which was obtained from Astar Winoto [Berkeley, CA] and inserted into HindIII-BamHI digested peGFPN2; Clontech, Mountain View, CA) or pEGFP-N2. Expression of MINOR was achieved by plasmid DNA transfection using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). Two days later, cells were stained with annexin V–7-AAD and analyzed by flow cytometry.
siRNA design and generation of lentivirus-based siRNA
siRNAs were designed corresponding to the mouse MINOR gene (GenBank accession no. NP_056558).20 Sequences were chosen using the Oligoengine software (Oligoengine, Seattle, WA). siRNAs with no sequence homology to any known mouse gene were used as negative controls. All siRNA sequences were BLAST searched in the National Center for Biotechnology Information's (NCBI)21 “search for short nearly exact matches” mode against all mouse sequences deposited in the GenBank and were not found to have significant homology to genes other than MINOR. To generate a vector-based suppression of MINOR expression, the construct pSUPER-retro (Oligoengine) was used as a template. The siRNA oligonucleotides designed contained a sense strand of 19-nucleotide sequence followed by a short spacer (TTCAAGAGA), the reverse complement of the sense strand, and 5 thymidines as a RNA polymerase III transcriptional stop signal. Briefly, the pSUPER-retro vector was digested with BglII and HindIII and the annealed oligos (5′-GAT CCC CTG CCC TTG TCC GAG CTT TAT TCA AGA GAT AAA GCT CGG ACA AGG GCA TTT TTG GAA A-3′; forward and 5′-AGC TTT TCC AAA AAT GCC CTT GTC CGA GCT TTA TCT CTT GAA TAA AGC TCG GAC AAG GGC AGG G-3′; reverse or for some experiments a different vector with the following sequences: forward 5′-GATCCGATCAGACGTTACTTATAGTTCAAGAGACTATAAGTAACGTCTGATCTTTTTTGGAAA-3′ and reverse 5′-AGCTTTTCCAAAA AAGATCAGACGTTACTTATAGTCTCTTGAACTATAAGTAACGTCTGATCG-3′) were ligated into the vector. For generating lentivectors encoding siRNA construct (LV-siRNA), the complete human H1-RNA promoter and the siRNA cassette as well as the PGK promoter were subcloned at XhoI and NheI sites before the reporter eGFP gene of the third generation self-inactivating lentiviral vector, Sin-18 provided by D. Trono (University of Geneva, Geneva, Switzerland). All inserts were confirmed by sequencing.
Western blotting
BALB/c day-8 BMDCs were generated as previously described. Lysates were prepared in RIPA buffer; protein concentration was quantified and then run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. After transfer, the blot was probed with the anti-NOR1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistics
All statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA). Either ANOVA or t tests were conducted, depending on the experiments. Survival analysis was conducted using both t tests to determine significance at a given time point and Kaplan-Meier to determine significance at completion of experiments.
Results
MINOR expression is induced upon DC maturation and forced expression of MINOR induces apoptosis
To assess patterns of expression of MINOR, we FACS-sorted populations of BMDCs into immature (CD11c+MHCIIlowCD86low) and mature (CD11c+MHCIIhighCD86high) groups by FACS and then determined relative expression using real-time quantitative reverse-transcription PCR (RT-PCR) analysis. Figure 1A shows that mature-activated BMDCs expressed significantly higher levels of MINOR transcript compared with immature BMDCs or macrophages. Although Nur77 and Nurr1 transcripts were detected in all DC samples tested, their expression levels did not increase on DC maturation and were much lower than those of activated T cells (data not shown). We also assessed whether in vivo activation of DCs would lead to a similar pattern of up-regulation. For these studies, mice were injected with vaccinia virus to stimulate DC activation, and splenic DCs were sorted into activated (based on CD86 expression) conventional and plasmacytoid DCs. Both of these populations also demonstrated an up-regulation of MINOR after in vivo activation (Figure 1B). Because Nur77 family members induce T lymphocyte apoptosis, we hypothesized that MINOR expression plays an analogous role in mediating apoptosis in activated DCs. To test this hypothesis, we transiently expressed a MINOR construct whose N terminus has been fused with GFP in a DC-like cell line, DC2.4, and examined induction of apoptosis. MINOR-expressing DC2.4 cells (GFP-MINOR fusion) displayed a significantly higher level of annexin V– and 7-AAD–positive (dead) cells relative to control (GFP vector only) cells, indicating that MINOR overexpression led to DC cell death (Figure 1C).
MINOR expression is highly up-regulated in activated DC populations in vitro and in vivo, and its forced expression induces apoptosis in DC2.4 cells. (A) Day-4 BM-derived CD11c+ cells (D4 CD11c+), immature (D8 CD11c+MHClowCD86low), and activated (D8 CD11c+MHC IIhighCD86high) BMDCs were FACS-sorted and examined for their expression of MINOR, Nur77, and Nurr1 using real-time quantitative RT-PCR as described in “Quantification of transcripts.” Transcript levels were normalized to β-actin. Expression levels are expressed as fold increase compared with those of activated macrophages. (B) The results of experiments in which mice were injected with vaccinia virus to induce activation in vivo, and splenic DCs were then sorted based on their expression of CD86, CD11c, and B220 into naive and activated populations of conventional or plasmacytoid DCs, which were then analyzed for MINOR expression by quantitative PCR. Transient expression of MINOR induced DC2.4 apoptosis. GFP-control (left panel) and GFP-MINOR-expressing (right panel) DC2.4 cells were analyzed for apoptotic markers using FACS analysis, shown in panel C. A representative FACS plot of annexin V versus 7-AAD staining is shown along with a statistical analysis of results of 3 different experiments. *P < .05, analysis of variance or t test.
MINOR expression is highly up-regulated in activated DC populations in vitro and in vivo, and its forced expression induces apoptosis in DC2.4 cells. (A) Day-4 BM-derived CD11c+ cells (D4 CD11c+), immature (D8 CD11c+MHClowCD86low), and activated (D8 CD11c+MHC IIhighCD86high) BMDCs were FACS-sorted and examined for their expression of MINOR, Nur77, and Nurr1 using real-time quantitative RT-PCR as described in “Quantification of transcripts.” Transcript levels were normalized to β-actin. Expression levels are expressed as fold increase compared with those of activated macrophages. (B) The results of experiments in which mice were injected with vaccinia virus to induce activation in vivo, and splenic DCs were then sorted based on their expression of CD86, CD11c, and B220 into naive and activated populations of conventional or plasmacytoid DCs, which were then analyzed for MINOR expression by quantitative PCR. Transient expression of MINOR induced DC2.4 apoptosis. GFP-control (left panel) and GFP-MINOR-expressing (right panel) DC2.4 cells were analyzed for apoptotic markers using FACS analysis, shown in panel C. A representative FACS plot of annexin V versus 7-AAD staining is shown along with a statistical analysis of results of 3 different experiments. *P < .05, analysis of variance or t test.
Blockade of MINOR expression inhibits cell death in BMDCs
We next tested whether inhibition of MINOR expression would decrease cell death because its overexpression resulted in apoptosis. To this end, we generated a lentivirus vector expressing a siRNA against MINOR, coupled with GFP as a reporter (LV-MINOR-siRNA). A control vector expressing siRNA with identical nucleic acid composition whose sequence does not target any murine genes (LV-control-siRNA) was also prepared as a negative control. LV-MINOR-siRNA–transduced BMDCs exhibited a significant decrease in MINOR expression (by ∼90%) as indicated by real-time quantitative RT-PCR (Figure 2A) and Western blotting (Figure 2B), compared with the LV-control-siRNA–transduced BMDCs. No change in MINOR family members, Nur77, or Nurr1 was observed by quantitative PCR (Figure 2A). A protective effect on DC apoptosis was observed in cells transduced with LV-MINOR-siRNA but not the control lentivirus. Transient transfection of MINOR into LV-MINOR-siRNA–transduced BMDCs led to a reduction in cell death, as measured by annexin V and 7-AAD staining (Figure 2C). In contrast, such an apoptosis-protective effect was not observed in LV-control-siRNA–transduced DC2.4 cells, indicating that suppression of MINOR expression could prolong the lifespan of DCs.
LV-MINOR-siRNA suppressed MINOR expression in DCs and dampened DC apoptosis. BMDCs were transduced with either LV-MINOR-siRNA or LV-control-siRNA. Expression of MINOR, Nur77, and Nurr1 was assessed by real-time quantitative RT-PCR as shown in Figure 2A. Western blot analysis of MINOR was also conducted; relative expression of MINOR and GFP (as a control for equal loading, expressed by the vector) is shown in Figure 2B. Representative FACS plots of annexin V versus 7-AAD staining of LV-MINOR-siRNA– or LV-control-siRNA–transduced BMDCs are shown in Figure 2C. (Top panel) LV-control-siRNA–transduced cells. (Bottom panel) LV-MINOR-siRNA–transduced cells. (Left panel) GFP-negative (untransduced control) cells. (Right panel) GFP-positive cells. Results of a statistical analysis using a t test are shown in the graph for 3 separate experiments.
LV-MINOR-siRNA suppressed MINOR expression in DCs and dampened DC apoptosis. BMDCs were transduced with either LV-MINOR-siRNA or LV-control-siRNA. Expression of MINOR, Nur77, and Nurr1 was assessed by real-time quantitative RT-PCR as shown in Figure 2A. Western blot analysis of MINOR was also conducted; relative expression of MINOR and GFP (as a control for equal loading, expressed by the vector) is shown in Figure 2B. Representative FACS plots of annexin V versus 7-AAD staining of LV-MINOR-siRNA– or LV-control-siRNA–transduced BMDCs are shown in Figure 2C. (Top panel) LV-control-siRNA–transduced cells. (Bottom panel) LV-MINOR-siRNA–transduced cells. (Left panel) GFP-negative (untransduced control) cells. (Right panel) GFP-positive cells. Results of a statistical analysis using a t test are shown in the graph for 3 separate experiments.
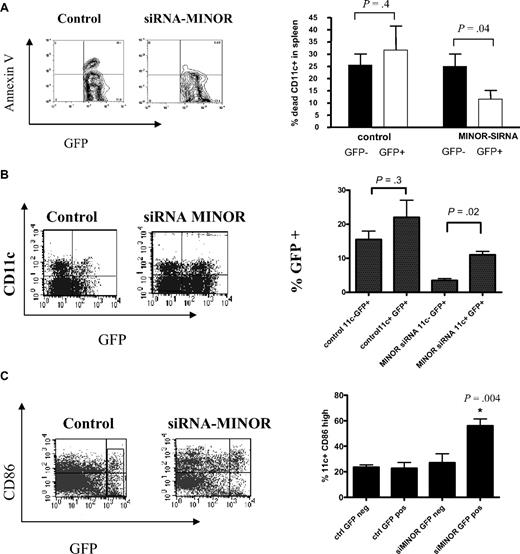
In vivo silencing of MINOR promotes DC survival
We next investigated whether inhibiting MINOR would impact on the development of DCs from progenitors in vivo, using transduced HSCs in a BM-transplantation model. HSCs transduced with LV-MINOR siRNA or LV-control-siRNA were transplanted into lethally irradiated recipients, which were then allowed to reconstitute, and the mice were then analyzed for GFP expression on DCs in lymphoid compartments. Our results show that MINOR siRNA-transduced splenic DCs exhibited a decrease in apoptosis (Figure 3A). In addition, there was a higher percentage of GFP-positive DCs in the mice that received transplants with the LV-MINOR siRNA (Figure 3B), suggesting a selective survival advantage of DCs (or their progenitors) by MINOR suppression. Further, DCs derived from HSCs transduced with LV-MINOR-siRNA expressed a higher level of CD86, suggesting that suppression of MINOR expression promoted an enhanced survival of the most mature DC populations (Figure 3C). These results suggest that inhibiting MINOR in progenitors allows for an increased number of DCs to survive, particularly those in their most activated states.
In vivo silencing of MINOR promoted DC survival. Mice received BM transplants with either LV-MINOR-siRNA– or LV-control-siRNA–transduced HSCs as described in “Bone marrow transplant and transduction of HSCs.” (A) Representative plots and an analysis of percentages of apoptotic DCs collected ex vivo during purification from spleens of those mice. (B) Representative plots and percentages of GFP-positive cells in total DC population in mice that received transplants showing that there was a bias toward retention of MINOR-inhibited CD11c+ cells. (C) Representative FACS plots of CD86 surface expression on DCs derived from the mice that received transplants indicating a higher level of CD86 expression in MINOR-inhibited CD11c+ cells. Statistical analyses were conducted on results of 2 separate experiments. *P < .05, analysis of variance or t test.
In vivo silencing of MINOR promoted DC survival. Mice received BM transplants with either LV-MINOR-siRNA– or LV-control-siRNA–transduced HSCs as described in “Bone marrow transplant and transduction of HSCs.” (A) Representative plots and an analysis of percentages of apoptotic DCs collected ex vivo during purification from spleens of those mice. (B) Representative plots and percentages of GFP-positive cells in total DC population in mice that received transplants showing that there was a bias toward retention of MINOR-inhibited CD11c+ cells. (C) Representative FACS plots of CD86 surface expression on DCs derived from the mice that received transplants indicating a higher level of CD86 expression in MINOR-inhibited CD11c+ cells. Statistical analyses were conducted on results of 2 separate experiments. *P < .05, analysis of variance or t test.
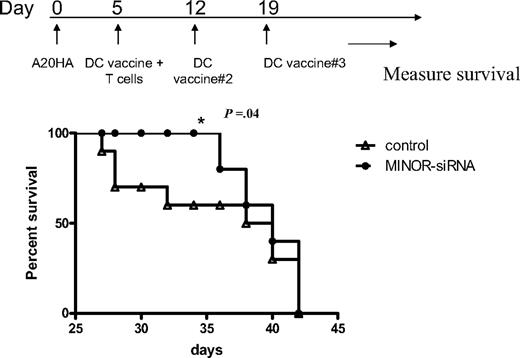
Inhibition of MINOR expression in a DC vaccine delays tumor progression
To test whether prolonging DC survival would result in an enhanced antitumor effect of an ex vivo DC vaccine, we tested the efficacy of the vaccine on an established tumor (the A20-HA B-cell lymphoma). For these experiments, we injected A20-HA cells into mice and allowed the tumor to progress for 5 days. We then vaccinated the mice with either LV-control-siRNA– or LV-MINOR-siRNA–transduced DCs (subcutaneously with 1-2 × 106 DCs that were pulsed with class II HA peptide) and then measured survival. Although ultimately all mice died of this rapidly growing tumor and overall there was no significant difference in long-term survival, inhibition of MINOR expression in the DC vaccines led to a significant delay in tumor progression, as determined by percentage surviving at intermediate time points (Figure 4).
Silencing of MINOR in DCs improves short-term antitumor efficacy. Mice were injected with 5 × 105 A20-HA cells 5 days before treatment with weekly DC vaccines, which were transduced with either LV-control-siRNA or LV-MINOR-siRNA and pulsed with MHC II–restricted HA peptide. HA-reactive T cells (6.5) were administered at the time of the first vaccine. Mice were followed for survival (killed as appropriate according to Institutional Animal Care and Use Committee guidelines). Shown is 1 of 3 similar experiments; tumor progression was significantly delayed in mice receiving the DC vaccine transduced with the MINOR-siRNA (t test conducted on results at day 38).
Silencing of MINOR in DCs improves short-term antitumor efficacy. Mice were injected with 5 × 105 A20-HA cells 5 days before treatment with weekly DC vaccines, which were transduced with either LV-control-siRNA or LV-MINOR-siRNA and pulsed with MHC II–restricted HA peptide. HA-reactive T cells (6.5) were administered at the time of the first vaccine. Mice were followed for survival (killed as appropriate according to Institutional Animal Care and Use Committee guidelines). Shown is 1 of 3 similar experiments; tumor progression was significantly delayed in mice receiving the DC vaccine transduced with the MINOR-siRNA (t test conducted on results at day 38).
Inhibition of MINOR improved T-cell responses
To investigate the mechanism of the observed improvement during the early phase of vaccination, we evaluated the effects of MINOR blockade on secretion of interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α) by DCs and on their capacity to activate T cells in vivo. BMDCs were generated as before, modified with either the LV-control-siRNA or the LV-MINOR-siRNA and then tested by quantitative PCR for IL-12 expression. As shown, suppression of MINOR led to an increase in IL-12 production (Figure 5A). To test whether MINOR inhibition in DCs improved their ability to activate tumor-responsive T cells, we adoptively transferred HA-specific TCR-transgenic CD4+ (6.5) T cells into mice, then 1 day later immunized with transduced DCs pulsed with HA class II peptide. MINOR-inhibited DCs stimulated a robust in vivo expansion of clonotypic 6.5 CD4+ T cells in lymph nodes relative to control-transduced DCs (Figure 5B). Further, an enhanced TNF-α production was seen when the expanded T cells from mice that were injected with antigen-pulsed LV-MINOR-siRNA–transduced DCs were rechallenged with HA-specific peptides compared with control-transduced samples (Figure 5C).
MINOR inhibition in DCs improved cytokine expression and T-cell expansion. BMDCs were transduced with either LV-control-siRNA or LV-MINOR-siRNA, pulsed with MHC II–restricted HA peptide and analyzed for their ability to secrete cytokines in vitro and stimulate CD4+ T cells in vivo. RNA was isolated from BMDCs and analyzed for IL-12 expression by quantitative PCR (A). For in vivo analysis, anti-HA T cells (6.5 cells) were injected 1 day before DC vaccination with transduced DCs; T-cell expansion was measured by harvesting spleens and measuring percentage of clonotypic 6.5 cells by FACS. (B) Percentages of clonotypic cells, from mice vaccinated with LV-control-siRNA– or LV-MINOR-siRNA–transduced DCs (or unvaccinated control, phosphate-buffered saline) pulsed with MHC II–restricted HA peptide. (C) Statistical analysis of percentages of cells expressing TNF-α by 6.5 CD4+ T cells rechallenged with HA-specific peptides. Results from 2 separate experiments are shown; statistical analysis is by t test.
MINOR inhibition in DCs improved cytokine expression and T-cell expansion. BMDCs were transduced with either LV-control-siRNA or LV-MINOR-siRNA, pulsed with MHC II–restricted HA peptide and analyzed for their ability to secrete cytokines in vitro and stimulate CD4+ T cells in vivo. RNA was isolated from BMDCs and analyzed for IL-12 expression by quantitative PCR (A). For in vivo analysis, anti-HA T cells (6.5 cells) were injected 1 day before DC vaccination with transduced DCs; T-cell expansion was measured by harvesting spleens and measuring percentage of clonotypic 6.5 cells by FACS. (B) Percentages of clonotypic cells, from mice vaccinated with LV-control-siRNA– or LV-MINOR-siRNA–transduced DCs (or unvaccinated control, phosphate-buffered saline) pulsed with MHC II–restricted HA peptide. (C) Statistical analysis of percentages of cells expressing TNF-α by 6.5 CD4+ T cells rechallenged with HA-specific peptides. Results from 2 separate experiments are shown; statistical analysis is by t test.
Discussion
DCs have a short lifespan after activation in vitro and in vivo. Although much emphasis has been placed on studying antigen uptake, processing and presentation, as well as costimulatory signal delivery by DCs, little is known about regulation of DC lifespan. Modifying DC longevity by targeting MINOR expression may provide a new strategy to promote T-cell immunity. Emerging technology has allowed the identification of genes that may be important to DC function, based on their relative expression levels, and MINOR was identified as the one member of the Nur77 family that is up-regulated on maturation of DCs. The increase in expression was observed both in vitro and in vivo and in plasmacytoid as well as conventional DCs, indicating that its up-regulation was a function of activation and independent of the type of DC. To test the hypothesis that the increase in MINOR expression led to cell death in DCs, we investigated 2 separate strategies: (1) we determined whether forced overexpression of MINOR would lead to apoptosis; and (2) we blocked the natural up-regulation of expression to assess effects of induction of apoptosis. Transduction of an immature DC-like cell line with a vector expressing the MINOR gene led to an increase in apoptosis in these cells; similarly, blockade of MINOR expression with RNA interference led to a decrease in DC apoptosis. These findings support a role for MINOR in the activation-induced cell death of DCs and also the possibility of targeting this gene to extend the lifespan of DCs.
Because up-regulation of MINOR was observed in vivo as well as in vitro after activation of DCs, we evaluated the effects of MINOR blockade on the de novo generation of DCs. We assessed both steady-state apoptosis and maturation state of transduced DCs to determine the impact of MINOR blockade as DCs were being generated from progenitors. Our results revealed several interesting findings. Consistent with the in vitro results, we observed a decrease in the numbers of apoptotic DCs after MINOR blockade. We also noted that there was a relatively higher proportion of gene-modified DCs in the MINOR-siRNA–transduced cells, which indicated a selective maintenance of expression of vector in the DC population. This suggests that there is a survival advantage to DCs associated with inhibition of MINOR expression. In addition, there was a skewing toward expression in the mostly highly activated populations, which is consistent with the fact that mature DCs exhibit maximal levels of MINOR and thus suppressing MINOR expression should have its greatest effect on the survival of the most mature DCs.
In vivo, the interaction between T cells and DCs is extremely brief and often limited by a low abundance of antigen-specific T cells. Thus, increasing the lifespan of antigen-pulsed DCs may increase the probability for antigen-presenting DCs to encounter rare antigen-specific T cells; promoting DC survival increases the likelihood of TCR engagement and clonal T-cell expansion. In vitro loading of human DCs with tumor antigens, followed by induction of maturation and subcutaneous injection, has been exploited as a means to vaccinate against cancer.22,23 Although effective for generating an antitumor immune response, a limitation in this technology has been the low number of DCs migrating to the draining lymph nodes.24 One possible explanation is the result of the limited lifespan of DCs after injection in vivo, and previous studies have indicated that blocking apoptotic pathways can improve the efficacy of vaccines.25,26 To test whether our observed antiapoptotic effects of MINOR inhibition would improve the therapeutic efficacy of a DC vaccine for preexisting tumors, we compared survival of mice treated with DCs that were modified with either the LV-MINOR-siRNA vector or control. Mice were inoculated with the B-cell lymphoma A20-HA, which was allowed to progress for 5 days before the institution of a DC vaccine. Mice then received 3 weekly injections of DCs that were modified with either the control vector or the siRNA-MINOR vector and pulsed with HA peptide. Mice that received the siRNA-MINOR DC vaccines had a significant delay in the onset of disease, although approximately 3 weeks after vaccines were stopped, the tumors began to grow and ultimately the mice died of this aggressive tumor. It is possible that, although activated DCs are present, they are sufficient to generate an effective T-cell response, but after they disappear, the balance is shifted toward a more tolerizing environment, allowing the tumors to begin to grow rapidly. A previous study demonstrated that prolonging DC lifespan via inhibition of Bak and Bax resulted in an increase in CD8+ T cells and subsequent improvement in vaccination strategies. Results of those studies suggested that, although DC lifespan was prolonged during both priming and boosting, it was probable that a large part of the effect was the result of better DC survival during the boosting phase as that was the time in which DCs were most probably killed by activated T cells.27 This is particularly relevant when taken in the context of our results showing that the most mature DCs have a preferential survival advantage if MINOR is inhibited. It is possible that either additional regimens of boosting with MINOR-inhibited DCs or combinations with other antiapoptotic molecules or inhibition of regulatory T cells would improve long-term survival. Our ongoing studies are elucidating mechanisms of inhibition of cell death, and this information will allow us to investigate combination strategies that may result in enhancement of antitumor effects by delaying cell death of DCs even further. In addition, inhibition of tolerance or enhancement of activation may be 2 means by which the therapy could be improved in the future. Our present results indicated that our current approach would probably be the most effective in a situation in which the tumor burden was low, such as in a minimal residual disease. To assess the mechanism behind the delay, we evaluated the characteristics of the DC vaccine and the antitumor T-cell responses. DCs that were transduced with the siRNA-MINOR vector had a significantly higher level of expression of IL-12, which might be expected because higher levels of IL-12 are secreted by activated DCs. We hypothesized that the inhibition of apoptosis in the vaccine would allow for an increase in expansion of a higher level of tumor reactive T cells, which might in part explain the improvement in vaccine efficacy. Our results showed that MINOR inhibition led not only to a higher level of tumor reactive T cells but also an increase in secretion of TNF-α by these cells, both of which may contribute to the delay in tumor progression.
Of note, it is interesting that suppression of MINOR expression delays DC apoptosis without causing undesirable oncogenic effects. No sign of lymphoproliferative syndrome or autoimmunity has been observed with mice transplanted with MINOR-siRNA–transduced HSCs for more than 6 months. Taken together, the results of our studies provide evidence of a novel role of MINOR in triggering DC apoptosis and suggest a new method of improving the efficacy of DC vaccines through inhibition of MINOR expression.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (Bethesda, MD; R01-CA111989).
National Institutes of Health
Authorship
Contribution: T.W., Q.J., C.C., K.S.G., E.M., and D.K. performed research, designed experiments, and analyzed data; D.P. designed experiments and analyzed data; and K.A.W. performed research, conducted data analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katharine A. Whartenby, Johns Hopkins School of Medicine, 1650 Orleans St, CRB-Rm 2M46, Baltimore, MD 21231; e-mail: whartenby@jhmi.edu.