Abstract
Dendritic cells (DCs) are the preferred targets for immunotherapy protocols focused on stimulation of cellular immune responses. However, regardless of initial promising results, ex vivo generated DCs do not always promote immune-stimulatory responses. The outcome of DC-dependent immunity is regulated by proinflammatory cytokines and neuropeptides. Proinflammatory neuropeptides of the tachykinin family, including substance P (SP) and hemokinin-1 (HK-1), bind the neurokinin 1 receptor (NK1R) and promote stimulatory immune responses. Nevertheless, the ability of pro-inflammatory tachykinins to affect the immune functions of DCs remains elusive. In the present work, we demonstrate that mouse bone marrow–derived DCs (BMDCs) generated in the presence of granulocyte macrophage–colony stimulating factor (GM-CSF) and interleukin-4 (IL-4), express functional NK1R. Signaling via NK1R with SP, HK-1, or the synthetic agonist [Sar9Met(O2)11]-SP rescues DCs from apoptosis induced by deprivation of GM-CSF and IL-4. Mechanistic analysis demonstrates that NK1R agonistic binding promotes DC survival via PI3K-Akt signaling cascade. In adoptive transfer experiments, NK1R-signaled BMDCs loaded with Ag exhibit increased longevity in draining lymph nodes, resulting in enhanced and prolonged effector cellular immunity. Our results contribute to the understanding of the interactions between the immune and nervous systems that control DC function and present a novel approach for ex vivo–generation of potent immune-stimulatory DCs.
Introduction
Because of their crucial role in initiation and control of innate and adaptive immunity, myeloid dendritic cells (DCs) are the preferred target Ag-presenting cells for positive cellular vaccination protocols.1 During the past decade, ex vivo–generated DCs have been used in immunization approaches for prevention and treatment of cancer and infectious diseases.2 Current DC-based vaccines focus on the adjuvant effect of proinflammatory mediators, conferring DCs the capability to initiate and bias T-cell immune responses.3-5 However, despite initial promising results, DC-based vaccines do not always elicit potent T-cell immunity.6
Generation of efficient T-cell immunity using ex vivo–generated DCs requires a critical number of adoptively transferred DCs capable of surviving apoptosis.7 Indeed, DCs used as cellular vaccines are exposed to proapoptotic stimuli at the injection sites and in tissue-draining lymph nodes (DLNs).7,8 Accordingly, exposure of DCs to proapoptotic stimuli triggered by lytic infections, ultraviolet B (UVB) irradiation, tumor mediators, and cytotoxic cells results in immune suppression.9 In addition, interaction of DCs with cells in early apoptosis down-regulates the T cell–stimulatory ability of DCs and induces immunological tolerance.10-13 Conversely, proinflammatory mediators and growth factors promoting DC survival, including granulocyte macrophage–colony stimulating factor (GM-CSF),14 prostaglandin E2 (PG-E2),15 lipopolysaccharide (LPS),16 and CCR717 and CD4018 ligands, correlate with enhanced T-cell immunity.
The intracellular signaling involved in DC survival is currently being elucidated. Activation through CD40 promotes DC survival by favoring a positive balance of nuclear factor kappa B (NF-κB) versus activator protein-1 (AP-1) pathway,19 whereas GM-CSF, LPS, and PG-E2 prevent DC apoptosis by signaling via phosphatidylinositol 3-kinase (PI3K) and protein kinase B (referred as Akt).14,15,20 In contrast, the immune-suppressive drug rapamycin induces DC death by antagonizing GM-CSF signaling via inhibition of the PI3K-Akt signaling cascade.14,21
Recently, it has become evident that the outcome of the immune response is highly regulated by neuropeptides. The balance between anti-inflammatory and proinflammatory neuropeptides is crucial to maintain the immune privilege of the central nervous system (CNS) and the steady-state condition in peripheral tissues, and altering this delicate balance plays a relevant role in the pathogenesis of chronic inflammatory and autoimmune diseases.22-26 Proinflammatory neuropeptides such as substance P (SP) favor CD4+ T helper (Th)1 bias and cellular immunity, whereas vasoactive intestinal peptide promotes CD4+ Th2 bias.27-34 Conversely, calcitonin gene-related peptide and the anti-inflammatory products of proopiomelanocortin cleavage, α-melanocyte-stimulating hormone and adrenocorticotropin, are potent suppressors of cellular immunity.23
The proinflammatory tachykinins SP and hemokinin-1 (HK-1) exert their immune-stimulatory functions by binding with high affinity the neurokinin 1 receptor (NK1R), a 7 transmembrane domain G protein-coupled receptor.35 SP and HK-1 induce interferon-γ secretion and proliferation of T cells.25,26,36-38 Our laboratory has recently described that skin DCs express functional NK1R and elicit in vivo CD4+ Th1 and CD8+ cytotoxic T lymphocyte (CTL) immunity in response to NK1R agonists.30 Nevertheless, because the NK1R is expressed by several skin cells, the potential of NK1R agonists to modulate directly the immune-stimulatory function of DCs remains unknown.
In the present study, we examined the expression of functional NK1R by bone marrow–derived DCs (BMDCs) and the ability of NK1R agonists SP, HK-1, and the synthetic NK1R ligand [Sar9Met(O2)11]-SP (SarSP) to rescue BMDCs from apoptosis induced by deprivation of GM-CSF and IL-4. We also investigated in vivo the immunological relevance of signaling BMDCs with these proinflammatory neuropeptides.
Methods
Mice
Eight- to 12-week-old wild-type C57BL/6 mice (B6) (The Jackson Laboratory, Bar Harbor, ME) and B6 NK1R−/−KO mice (kindly provided by Dr Christopher Paige, University of Toronto, Toronto, ON) were housed in the pathogen-free animal facility of the University of Pittsburgh and used according to institutional guidelines with approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
Generation of BMDCs
BMDCs were generated by culturing mouse BM-precursors for 6 days in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini, West Sacramento, CA; complete medium) and GM-CSF and IL-4 cytokines (both at 1000 units/mL; R&D Systems, Minneapolis, MN), as described previously.39,40 Total CD11c+ BMDCs were purified by histodenz gradient (16% [wt/vol], purity of CD11c+ cells ≥ 85% as determined by fluorescence-activated cell sorting [FACS] analysis). The DC morphology was confirmed in cytospins of purified CD11c+ cells obtained, as described previously,41 and stained with Hema-3 (Thermo Fisher Scientific, Waltham, MA).
Quantitative RT-PCR
The expression of NK1R mRNA was detected by SYBR green-based quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using reverse-transcribed cDNA isolated from CD11c+ BMDCs cultured for 24 hours in complete medium with or without LPS (500 ng/ml; Sigma-Aldrich, St Louis, MO). Total RNA was isolated using the RNeasy isolation kit (QIAGEN, Valencia, CA) and was reverse-transcribed using the QuantiTect Reverse Transcription Kit (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. cDNA template amplification was performed with the use of the Quantitect SYBR Green PCR kit with specifically designed NK1R and phosphoglycerate kinase 1 (PGK1) primers (QIAGEN). qRT-PCR was conducted using the ABI Prism 7900HT system (Applied Biosystems, Foster City, CA) in the Genomics and Proteomics Core Laboratories, University of Pittsburgh (Pittsburgh, PA) with cycling conditions consisting of 15 minutes Taq activation at 95°C followed by denature, anneal, and extension phases for 15 seconds at 94°C, 30 seconds at 54°C, and 30 seconds at 72°C, respectively, for 40 cycles. Analysis was performed using Sequence Detection System software 2.2.2 (Applied Biosystems, Foster City, CA), and the quantification of PGK1-normalized NK1R was expressed as mRNA fold increase.
Immunofluorescence microscopy and FACS analysis
The expression of NK1R protein on the surface of BMDCs was analyzed in cytospins. BMDC suspensions were FcR-blocked with anti-CD16/32 monoclonal antibody (mAb) and stained with a purified goat anti–mouse IgG specific for the N-terminal domain of the NK1R (Santa Cruz Biotechnology, Santa Cruz, CA), followed by donkey anti–goat fluorescein isothiocyanate (FITC)–IgG F(ab′)2 (Jackson ImmunoResearch Laboratories, West Grove, PA) fixed in 2% paraformaldehyde (PFA). After labeling, BMDCs were cytospun as described previously39 and analyzed. Microscopy analyses was performed using an AxioStar Plus microscope equipped with epi-fluorescence, an AxioCamera, and the image analyzing software AxioVision (Carl Zeiss Vision Imaging Systems, Thornwood, NY).
Quantification of NK1R in BMDCs was performed by FACS analysis (FACSCalibur; BD Biosciences, San Jose, CA). BMDCs were (1) stained with anti–mouse allophycocyanin (APC)–CD11c and phycoerythrin (PE)–CD86 or PE-CD83 mAbs (BD Biosciences Pharmingen, San Diego, CA); (2) fixed in 2% PFA; (3) permeabilized in 0.1% saponin (Sigma-Aldrich); and (4) incubated with goat anti-NK1R IgG (recognizing the N-terminal domain) or rabbit anti-NK1R IgG (specific for the C-terminal domain; Advanced Targeting Systems, San Diego, CA), followed by anti–goat or anti–rabbit FITC-IgG F(ab′)2 (Jackson ImmunoResearch Laboratories). Negative controls included species-matched isotype IgG and secondary antibodies (Abs).
Quantification of anti- and proapoptotic proteins in BMDCs was assessed by intracellular staining and FACS analysis. BMDCs were cultured in complete medium or supplemented with [Sar9Met(O2)11]-SP (10−9 mol/L; Sigma-Aldrich) or GM-CSF and IL-4 (both at 1000 units/ml). After 24 hours, cells were (1) labeled with FITC-CD11c mAb (BD Pharmingen); (2) fixed and permeabilized as described above; and (3) incubated with one of the following anti–mouse mAbs: Alexa Fluor 488-pAktSer473 (Abcam, Cambridge, MA), PE-active caspase 3 (BD Pharmingen) or PE-Bcl-2 (BD Pharmingen). For detection of pBad, BMDCs were incubated with goat anti–mouse pBadSer136 mAb (Santa Cruz Biotechnology) followed by anti-goat FITC-IgG F(ab′)2.
Induction and quantification of apoptosis
BMDCs were cultured for 24 to 72 hours in complete medium with or without the NK1R antagonist 17-hydroxy-17-ethynyl-5α-androstano(3,2-b)pyrimido(1,2-a)benzimidazole (WIN-51708; 10−8 mol/L; Sigma-Aldrich) and/or one of the following NK1R agonists: (1) [Sar9Met(O2)11]-SP; (2) SP (Bachem, King of Prussia, PA); or (3) HK-1 (Bachem; all at 10−9 or 10−5 mol/L). Positive controls included BMDC cultures supplemented with GM-CSF and IL-4 or agonistic CD40 mAb (10 μg/mL; BD Pharmingen).41 At the indicated time points 24, 48, or 72 hours, BMDCs were harvested, and cell viability was assessed by trypan blue exclusion. The morphology of BMDCs undergoing apoptosis was examined in cytospins stained with Hema-3 (Thermo Fisher) or 4,6-diamidino-2-phenylindole (DAPI; Invitrogen). DNA fragmentation was further assessed by FACS analysis in BMDCs immunostained using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) kit (Roche, Indianapolis, IN), according to the manufacturer's protocol. For quantification of apoptosis, FACS analysis was performed on BMDCs that were immunostained with APC-CD11c, FITC–annexin V (BD Pharmingen), and propidium iodide (PI; Sigma-Aldrich). Percentage of apoptotic cell rescue was calculated by the formula: [1 − (% apoptotic NK1R-signaled BMDCs)/(% apoptotic nontreated BMDCs)] × 100.
Experiments addressing specificity of signaling via NK1R used BMDCs generated from B6 NK1R−/−KO and B6 NK1R+/+ mice, the latter treated for 30 minutes with WIN-51708 (10−8 or 10−5 mol/L) before stimulation with [Sar9Met(O2)11]-SP (10−9 mol/L).
Blockade of intracellular signaling
BMDCs were cultured in complete medium with one of the following cell-permeable inhibitors: (1) 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002, a PI3K inhibitor, 5 μmol/L; BIOMOL Research Laboratories, Plymouth Meeting, PA); (2) Akt inhibitor X (5 μmol/L; Calbiochem, San Diego, CA); (3) 2′-amino-3′-methoxyflavone (PD98059, an extracellular signal-regulated kinase [ERK] inhibitor; 20 μmol/L; BIOMOL); (4) SN-50 (NFκB inhibitor; 18 μmol/L; Calbiochem); or (5) c-Jun NH2-terminal kinase (JNK) inhibitor II (10 μmol/L; Calbiochem). After a 30-minute blockade, BMDC cultures were treated or not with [Sar9Met(O2)11]-SP (10−9 mol/L) for 24 hours. Quantification of apoptotic BMDCs was analyzed by FACS as described above.
Adoptive transference of BMDCs
BMDCs were cultured in complete medium with or without [Sar9Met(O2)11]-SP (10−9 mol/L). After 24 hours, BMDCs were left untreated (DCs, control) or haptenized with trinitrobenzene sulfonic acid (TNBS, 1 mM, 25 minutes at 37°C; Sigma-Aldrich; TN-DCs). DCs and TN-DCs were then labeled with carboxyfluorescein succinimidyl ester (CFSE, 1 μmol/L, 15 minutes at 37°C; Invitrogen). After thorough rinsing, CFSE-labeled DCs and TN-DCs were adoptively transferred (2 × 106 cells/50 μL PBS, footpad, subcutaneously) into B6 mice (n = 3 animals per group). For in vivo studies analyzing the survival of DCs in the presence or absence of CD40 ligation, mice were treated (or not) with 2 doses of anti-CD154 blocking MR1 mAb (Bio Express, West Lebanon, NH) injected simultaneously and 2 days after BMDC transfer (250 μg per dose, flank, intraperitoneally). At indicated time points, mice were euthanized and local (popliteal) and distant (cervical and axillary) DLNs were dissected. The number of viable CFSE-labeled CD11c+ BMDCs in DLNs was analyzed by FACS, as described previously.30 An equal number of CD11c+ DCs (106) per experimental situation was acquired by FACS, and the percentage of CFSE-labeled CD11c+ BMDCs was calculated with the following formula: [(number of CFSE+CD11c+ BMDCs)/(total number of CD11c+ DCs) × 100].
Delayed-type hypersensitivity assays
B6 mice (n = 6 animals/group) were sensitized with 1 dose of BMDCs (2 × 106 cells/50 μL PBS, footpad, subcutaneously) pretreated as follows: (1) TNBS haptenized (TN-DCs); (2) TN-DCs cultured with SarSP (10−9 mol/L) (SarSP-TN-DCs); (3) TN-DCs cultured with CD40 mAb (CD40-TN-DCs); (4) nonhaptenized DCs (DCs); or (5) nonhaptenized DCs cultured with SarSP (SarSP-DCs). Elicitation of delayed-type hypersensitivity (DTH) was performed 6 days after sensitization by applying trinitrochlorobenzene (TNCB, 1% in acetone/olive oil, 4:1; Sigma-Aldrich) on the dorsal surface of the ears. The severity of the DTH responses was assessed by comparing the thickness of ears measured before and after elicitation for up to 6 days, using an electronic caliper (Mitutoyo, Aurora, IL). The DTH responses were expressed as the percentage of ear thickness increase using the formula: [(thickness of challenged ear − thickness of control ear)/(thickness of control ear) × 100]. For histological analysis, mice were euthanized 6 days after elicitation, and samples from ear skin were fixed in 4% formaldehyde, embedded in paraffin, stained with hematoxylin and eosin (H&E), and analyzed by microscopy or embedded in Tissue-Tek OCT (Miles Laboratories, Elkhart, IN) and snap-frozen in methyl-butane (Sigma-Aldrich). The composition of the cellular infiltrate 6days after elicitation was assessed by immunofluorescence microscopy of cryostat sections of the ears, as described previously.30 Cryostat sections (8 μm) were mounted on slides pretreated with Vectabond (Vector Laboratories, Burlingame, CA), fixed in rechilled 96% ethanol, and incubated with Alexa Fluor 488–CD4 mAb (Invitrogen) and biotin-CD8 (eBioscience, San Diego, CA) or biotin-F4/80 mAbs (Invitrogen) followed by Cy3-streptavidin (Jackson ImmunoResearch Laboratories). Cell nuclei were stained with DAPI.
Statistical analysis
Statistical analysis was performed using the Prism 4 software (GraphPad Software, San Diego, CA). Differences between more than 2 means plus or minus 1 SD were analyzed by 1-way analysis of variance followed by a Student-Newman-Keuls test. Comparisons between 2 different means plus or minus 1 SD were performed by a Student t test. A P value less than .05 was considered significant.
Results
Expression of NK1R by BMDCs
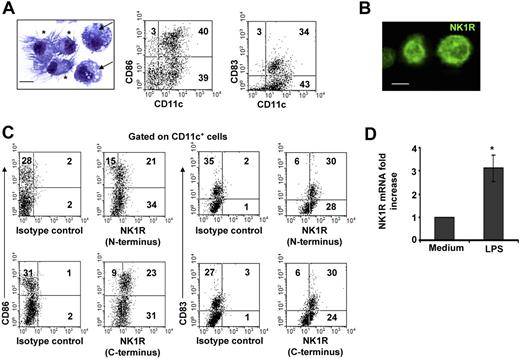
The population of day –6 BMDCs was composed of immature and mature CD11c+ DCs. Similar percentages of CD11c+ DCs exhibited short dendrites and a round nucleus, features of immature DCs, or well-developed dendrites and a bean-shaped nucleus, indicative of mature DCs (Figure 1A). Likewise, FACS analysis demonstrated that BMDC cultures contained CD11c+CD86−/lowCD83− immature DCs and CD11c+CD86highCD83+ mature DCs (Figure 1A).
BMDCs express NK1R. (A) Microscopic and phenotypic characteristics of day–6 BMDCs. Cytospin showing immature DCs (→) identified by round nuclei and short membrane prolongations and mature DCs (*) with long membrane processes and bean-shaped nuclei. Hema-3 stain, ×500; scale bar = 5 μm. Dot plots of BMDCs illustrating the percentages (numbers in dot plots) of mature (CD11c+CD86high or CD11c+CD83+) and immature (CD11c+CD86−/low or CD11c+CD83−) DCs. (B,C) Expression of NK1R by BMDCs. (B) Cytospin of purified BMDCs showing the expression of NK1R on their cell surface. Fluorescence microscopy, ×500; scale bar equals 5 μm. (C) NK1R expression by CD11c+CD86+CD83+ mature and CD11c+CD86−CD83− immature DCs analyzed by FACS. Mature and immature BMDCs express the N-terminal (top panels) and the C-terminal (bottom panels) motifs of the NK1R, indicating expression of the full-length functional NK1R. (A-C) Data are representative of 5 independent experiments. (D) qRT-PCR analysis of NK1R mRNA transcripts in BMDCs cultured with or without LPS. *P < .05. Means (± SD) of 3 independent experiments are shown.
BMDCs express NK1R. (A) Microscopic and phenotypic characteristics of day–6 BMDCs. Cytospin showing immature DCs (→) identified by round nuclei and short membrane prolongations and mature DCs (*) with long membrane processes and bean-shaped nuclei. Hema-3 stain, ×500; scale bar = 5 μm. Dot plots of BMDCs illustrating the percentages (numbers in dot plots) of mature (CD11c+CD86high or CD11c+CD83+) and immature (CD11c+CD86−/low or CD11c+CD83−) DCs. (B,C) Expression of NK1R by BMDCs. (B) Cytospin of purified BMDCs showing the expression of NK1R on their cell surface. Fluorescence microscopy, ×500; scale bar equals 5 μm. (C) NK1R expression by CD11c+CD86+CD83+ mature and CD11c+CD86−CD83− immature DCs analyzed by FACS. Mature and immature BMDCs express the N-terminal (top panels) and the C-terminal (bottom panels) motifs of the NK1R, indicating expression of the full-length functional NK1R. (A-C) Data are representative of 5 independent experiments. (D) qRT-PCR analysis of NK1R mRNA transcripts in BMDCs cultured with or without LPS. *P < .05. Means (± SD) of 3 independent experiments are shown.
To determine whether BMDCs might be targets of pro-inflammatory neuropeptides that signal via NK1R, we investigated NK1R protein and mRNA transcript expression in BMDCs. Presence of NK1R was analyzed by fluorescence microscopy (Figure 1B) and FACS (Figure 1C) using an Ab recognizing the N-terminal extracellular domain of the receptor. Both immature and mature BMDCs expressed surface NK1R (Figure 1B,C top panels).
Because NK1R exists as a full-length (functional) or a C-terminally truncated variant (deficient in signaling),42 we investigated whether BMDCs express functional NK1R, using an Ab recognizing the C-terminal motif of the receptor. As determined by FACS analysis, immature and mature BMDCs expressed the functional variant of NK1R (Figure 1C bottom panels).
Next, we analyzed whether BMDCs up-regulate NK1R mRNA after exposure to the proinflammatory stimulus LPS by quantifying NK1R transcripts via qRT-PCR. The levels of NK1R mRNA increased 3-fold (P < .05) in BMDCs cultured with LPS (Figure 1D). These results demonstrate that mature and immature BMDCs express surface functional NK1R, and transcription of NK1R mRNA was up-regulated by the DC-activating mediator LPS.
Agonisitc signaling via NK1R rescues BMDCs from apoptosis
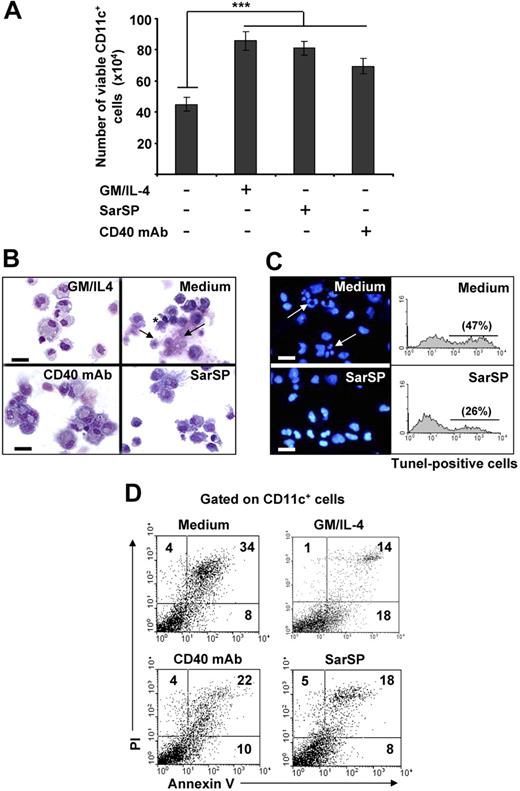
Withdrawal of GM-CSF and IL-4 from BMDC cultures triggers DC death.14,43,44 Therefore, we investigated the effects of signaling through NK1R on the viability of BMDCs after cytokine withdrawal. BMDCs (106) were cultured for 24 hours in complete medium with or without GM-CSF and IL-4, the synthetic NK1R agonist SarSP (10−9 mol/L),45 or agonistic CD40 mAb (DC survival signal, positive control). Withdrawal of GM-CSF and IL-4 significantly decreased the number of viable BMDCs (P < .001), whereas addition of SarSP, CD40 mAb, or GM-CSF and IL-4 yielded similar high numbers of viable BMDCs (Figure 2A). GM-CSF and IL-4 withdrawal induced apoptosis of BMDCs characterized by plasma membrane blebs, nuclear condensation, and DNA fragmentation (Figure 2B,C). Conversely, DCs cultured in medium with SarSP or agonistic CD40 mAb exhibited the morphology of viable cells (Figure 2B). Accordingly, SarSP reduced DNA fragmentation in BMDC cultures deprived of GM-CSF and IL-4, as determined by fluorescent microscopy and FACS analysis of nuclear staining with DAPI and TUNEL, respectively (Figure 2C).
Agonistic signaling through NK1R in BMDCs prevents apoptosis. (A) Number of viable CD11c+ BMDCs recovered 24 hours after culture in different conditions. ***P < .001. Means (± SD) of 6 independent experiments are shown. (B,C) Morphological and nuclear characteristics of apoptosis by BMDCs harvested 24 hours after culture in different conditions. (B) Morphological analysis of apoptosis as evaluated by cytospins of CD11c+ BMDCs stained with Hema-3. (C) Nuclear analysis of apoptosis as determined by cytospins of CD11c+ BMDCs stained with the nuclear dye DAPI (left panels) or suspensions of CD11c+ BMDCs stained with TUNEL (right panels). Withdrawal of GM-CSF (GM) and IL-4 (Medium) induces apoptosis of BMDCs characterized by surface membrane blebs (B top right panel, *), nuclear breakage (B top panel, →), chromatin condensation, and DNA fragmentation (C top panels, arrows), ×500; scale bar equals 20 μm. (D) Quantification of apoptosis in CD11c+ BMDCs by FACS analysis of annexin V and PI labeling. Numbers in dot-plots represent percentage of cells. Signaling via NK1R with SarSP (10−9 mol/L) prevents apoptosis of BMDCs to a similar extent as signaling via CD40 or addition of GM and IL-4 (positive controls). Data are representative of 10 independent experiments.
Agonistic signaling through NK1R in BMDCs prevents apoptosis. (A) Number of viable CD11c+ BMDCs recovered 24 hours after culture in different conditions. ***P < .001. Means (± SD) of 6 independent experiments are shown. (B,C) Morphological and nuclear characteristics of apoptosis by BMDCs harvested 24 hours after culture in different conditions. (B) Morphological analysis of apoptosis as evaluated by cytospins of CD11c+ BMDCs stained with Hema-3. (C) Nuclear analysis of apoptosis as determined by cytospins of CD11c+ BMDCs stained with the nuclear dye DAPI (left panels) or suspensions of CD11c+ BMDCs stained with TUNEL (right panels). Withdrawal of GM-CSF (GM) and IL-4 (Medium) induces apoptosis of BMDCs characterized by surface membrane blebs (B top right panel, *), nuclear breakage (B top panel, →), chromatin condensation, and DNA fragmentation (C top panels, arrows), ×500; scale bar equals 20 μm. (D) Quantification of apoptosis in CD11c+ BMDCs by FACS analysis of annexin V and PI labeling. Numbers in dot-plots represent percentage of cells. Signaling via NK1R with SarSP (10−9 mol/L) prevents apoptosis of BMDCs to a similar extent as signaling via CD40 or addition of GM and IL-4 (positive controls). Data are representative of 10 independent experiments.
The ability of SarSP to rescue BMDCs from apoptosis was quantified by FACS in BMDCs stained with annexin V and PI. Withdrawal of GM-CSF and IL-4 increased significantly the percentage of apoptotic BMDCs, whereas addition of SarSP, at physiological concentration, reduced the level of apoptosis by 47% (P < .001; Figure 2D). The antiapoptotic effect of SarSP was sustained up to 72 hours, and addition of higher concentrations of SarSP (from 10−7 to 10−5M) did not result in further apoptotic cell rescue (not shown). Cultures of BMDCs supplemented with agonistic CD40 mAb showed a slightly less antiapoptotic effect than BMDCs treated with SarSP or GM-CSF and IL-4, a result that could be explained by the low percentage of immature BMDCs that do not express CD40.39 These results indicate that NK1R agonistic signaling rescues BMDCs from apoptosis induced by GM-CSF and IL-4 withdrawal.
Proinflammatory tachykinins prevent apoptosis of BMDCs by binding NK1R
The naturally occurring NK1R agonists SP and HK-1 also ligate NK2R and NK3R,46 which decreases their proinflammatory function. In addition, at very high concentrations, SP and HK-1 bind other neuropeptide receptors47 or exert effects independently of receptor ligation.48 Therefore, we investigated whether SP and HK-1, similar to SarSP, induce antiapoptotic effects on BMDCs and whether the apoptotic cell rescue induced by SarSP is mediated through NK1R signaling.
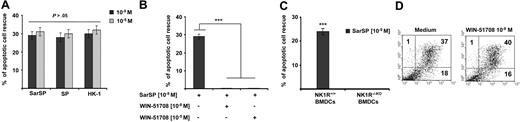
SP, HK-1, and SarSP exerted similar antiapoptotic effects on BMDCs at their physiological concentration (10−9 mol/L; P < .001), and a higher concentration (10−5 mol/L) did not enhance this effect (P > .05; Figure 3A). The antiapoptotic effect of SarSP was mediated by NK1R binding, as demonstrated by the lack of apoptotic rescue by SarSP, in NK1R blockade assays of BMDCs pretreated with the selective NK1R antagonist WIN-51708 (P < .001) or in BMDCs generated from B6 NK1R−/−KO mice (P < .001; Figure 3B,C, respectively). Blockade of NK1R in BMDC cultures with WIN-51708 alone did not significantly increase the level of apoptosis induced by GM-CSF and IL-4 withdrawal (Figure 3D). Together, these results demonstrate that the synthetic NK1R agonist SarSP, SP, and HK-1 exert similar antiapoptotic effects on BMDCs, which requires signaling via NK1R.
Proinflammatory tachykinins prevent apoptosis of BMDCs by NK1R signaling. (A) Comparison of the antiapoptotic effects of SarSP, SP, and HK-1 at physiological (10−9 mol/L) and nonphysiological (10−5 mol/L) concentrations on CD11c+ BMDCs as determined by FACS analysis of annexin V and PI labeling. (B,C) Bar-diagrams representing the antiapoptotic effect of SarSP on CD11c+ BMDCs is abrogated by (B) preincubation with the highly selective NK1R antagonist WIN-51708 or (C) in NK1R−/−KO BMDCs. ***P < .001. (A-C) Means (± SD) of 3 independent experiments are illustrated. (D) Dot-plots illustrating the percentage of apoptosis (numbers in dot-plots) of CD11c+ BMDCs signaled with NK1R antagonist WIN-51708 alone. Data are representative of 4 independent experiments.
Proinflammatory tachykinins prevent apoptosis of BMDCs by NK1R signaling. (A) Comparison of the antiapoptotic effects of SarSP, SP, and HK-1 at physiological (10−9 mol/L) and nonphysiological (10−5 mol/L) concentrations on CD11c+ BMDCs as determined by FACS analysis of annexin V and PI labeling. (B,C) Bar-diagrams representing the antiapoptotic effect of SarSP on CD11c+ BMDCs is abrogated by (B) preincubation with the highly selective NK1R antagonist WIN-51708 or (C) in NK1R−/−KO BMDCs. ***P < .001. (A-C) Means (± SD) of 3 independent experiments are illustrated. (D) Dot-plots illustrating the percentage of apoptosis (numbers in dot-plots) of CD11c+ BMDCs signaled with NK1R antagonist WIN-51708 alone. Data are representative of 4 independent experiments.
Agonistic binding of NK1R activates PI3K-Akt to prevent apoptosis of BMDCs
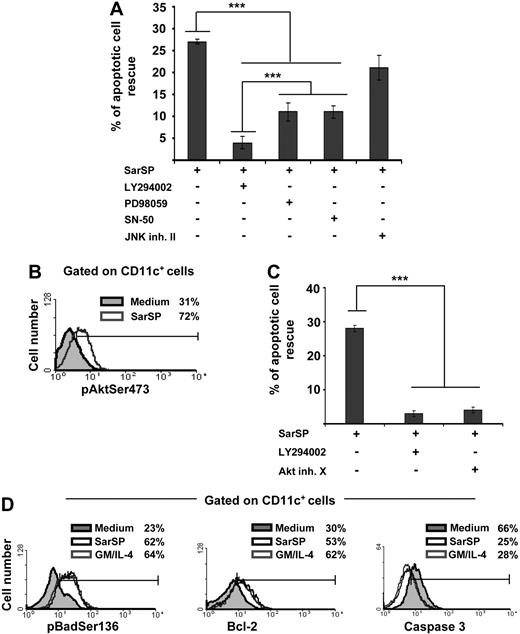
The mechanisms by which NK1R agonists exert their antiapoptotic effect on BMDCs were analyzed by investigating the role of intracellular pathways involved in DC survival in blockade assays using cell-permeable inhibitors specific for PI3K (LY294002),7,20,49 NF-κB (SN-50),50 ERK (PD98059),16 and JNK-AP-1 (JNK inhibitor II).19 Blockade of PI3K decreased significantly the ability of SarSP to rescue BMDCs from apoptosis (P < .001), whereas inhibition of ERK or NF-κB exerted lower but still significant decreases of BMDC apoptosis (Figure 4A). Conversely, inhibition of JNK-AP-1 did not interfere with the antiapoptotic effect of SarSP (P > .05; Figure 4A).
Signaling BMDCs via NK1R prevents apoptosis through activation of the PI3K-Akt cascade. (A) Bar diagram representing the effect of intracellular pathway blockade on the antiapoptotic effect exerted by SarSP on CD11c+ BMDCs. Before culture with or without SarSP, BMDCs were left untreated (control) or incubated with the cell-permeable inhibitors LY294002 (PI3K), PD98059 (ERK), SN-50 (NFκB), or JNK inhibitor II (JNK-AP1). ***P < .001. (B) FACS analysis showing the level of pAktSer473 expression by CD11c+ BMDCs cultured in the presence of SarSP (gray empty histogram) or medium alone (gray filled histogram). (C) Comparative analysis of the effects of LY294002 and Akt inhibitor X (pAktSer473 inhibitor) on the antiapoptotic effect of SarSP on BMDCs. ***P < .001. (D) FACS analysis illustrating the level of Bcl-2, pBadSer136, and active caspase 3 expression by CD11c+ BMDCs cultured in the presence of SarSP (black empty histogram), GM and IL-4 (gray empty histogram), or medium alone (gray filled histogram). (A, C) Means (± SD) of 4 independent experiments are shown. (B, D) Data are representative of 4 independent experiments. Numbers in histograms indicate percentage of CD11c+ BMDCs.
Signaling BMDCs via NK1R prevents apoptosis through activation of the PI3K-Akt cascade. (A) Bar diagram representing the effect of intracellular pathway blockade on the antiapoptotic effect exerted by SarSP on CD11c+ BMDCs. Before culture with or without SarSP, BMDCs were left untreated (control) or incubated with the cell-permeable inhibitors LY294002 (PI3K), PD98059 (ERK), SN-50 (NFκB), or JNK inhibitor II (JNK-AP1). ***P < .001. (B) FACS analysis showing the level of pAktSer473 expression by CD11c+ BMDCs cultured in the presence of SarSP (gray empty histogram) or medium alone (gray filled histogram). (C) Comparative analysis of the effects of LY294002 and Akt inhibitor X (pAktSer473 inhibitor) on the antiapoptotic effect of SarSP on BMDCs. ***P < .001. (D) FACS analysis illustrating the level of Bcl-2, pBadSer136, and active caspase 3 expression by CD11c+ BMDCs cultured in the presence of SarSP (black empty histogram), GM and IL-4 (gray empty histogram), or medium alone (gray filled histogram). (A, C) Means (± SD) of 4 independent experiments are shown. (B, D) Data are representative of 4 independent experiments. Numbers in histograms indicate percentage of CD11c+ BMDCs.
Because the previous experiment demonstrated that DC survival promoted by the NK1R agonist was abrogated by PI3K blockade, we further investigated the role of effector molecules downstream of PI3K that could be involved in the antiapoptotic effect of SarSP on BMDCs.51 We analyzed whether signaling via NK1R in BMDCs activates Akt by intracellular labeling with an Ab recognizing phosphorylated-Akt at the Ser473 residue (pAKTSer473), an event that occurs downstream of PI3K and is necessary for full-activation of Akt. Signaling BMDCs with SarSP increased the percentage of BMDCs expressing pAktSer473 from 31% to 72%, as determined by FACS analysis (Figure 4B). Moreover, blockade of pAktSer473 with the Akt Inhibitor X decreased the antiapoptotic effect of SarSP (P < .001) to an extent similar to that observed with PI3K blockade (Figure 4C).
Next, we compared the abilities of SarSP and GM-CSF and IL-4 to (1) induce phosphorylation of Bad, reducing its proapoptotic activity; (2) increase antiapoptotic Bcl-2 expression; and (3) decrease proapoptotic caspase 3 activity, which are the final effectors of the PI3K-Akt cascade.52,53 Signaling BMDCs with SarSP or GM-CSF and IL-4 induced similar significant increases of intracellular levels of the Akt-dependent phosphorylated-Bad at the Ser136 residue (pBadSer136) and Bcl-2 (Figure 4D). It is noteworthy that we observed a significant decrease of active caspase 3 levels, a protease that executes apoptosis by activation of nuclear-DNAses.54,55 Collectively, these results demonstrate that SarSP promotes survival of BMDCs through activation of the PI3K-Akt pathway.
NK1R signaled-BMDCs show enhanced survival in vivo
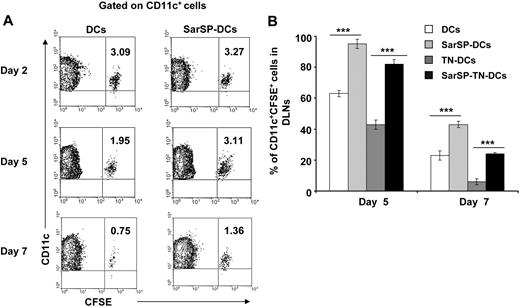
The relevance of our previous results was analyzed in vivo by studying the effect of signaling via NK1R on the survival of adoptively transferred BMDCs. CFSE-labeled BMDCs, preincubated or not with SarSP (SarSP-DCs and DCs, respectively) were injected (footpad, subcutaneously) into B6 mice, and the number of viable CFSE-labeled CD11c+ BMDCs was quantified in local DLNs for a period of 7 days by FACS. Two days after adoptive transference, similar numbers of SarSP-DCs and control DCs were detected in DLNs, indicating that both DC subsets displayed equivalent DLN-homing capacities (Figure 5A). Five days after injection, 95% of SarSP-DCs remained in DLNs compared with 63% of control DCs and by day 7, 42% of SarSP-DCs were present versus 24% of control DCs (Figure 5A). No CFSE dilution was detected in CD11c+ BMDCs homed to local DLNs, indicating that the higher percentage of SarSP-DCs observed in the LNs was due to prolonged DC survival and not DC proliferation (Figure 5A).56 Conversely, CFSE+ cells were not detected in nondraining homolateral or collateral LNs (not shown).
Agonistic signaling via NK1R prolongs BMDC survival in vivo. (A) Contour-plots illustrating the percentage of SarSP-DCs or control DCs (CD11c+CFSE+) in DLNs after their adoptive transference into B6 mice and quantification by FACS analysis at different time points. Data are representative of 3 independent experiments. (B) Comparative analysis of the percentages of CD11c+CFSE+ BMDCs in DLNs after adoptive transference of TN-DCs, SarSP-TN-DCs, SarSP-DCs, or untreated DCs as quantified by FACS analysis at different time points. ***P < .001. Means (± SD) of 3 independent experiments are displayed.
Agonistic signaling via NK1R prolongs BMDC survival in vivo. (A) Contour-plots illustrating the percentage of SarSP-DCs or control DCs (CD11c+CFSE+) in DLNs after their adoptive transference into B6 mice and quantification by FACS analysis at different time points. Data are representative of 3 independent experiments. (B) Comparative analysis of the percentages of CD11c+CFSE+ BMDCs in DLNs after adoptive transference of TN-DCs, SarSP-TN-DCs, SarSP-DCs, or untreated DCs as quantified by FACS analysis at different time points. ***P < .001. Means (± SD) of 3 independent experiments are displayed.
These findings confirm that signaling via NK1R enhances survival of BMDCs in vivo. Next, we addressed whether SarSP prolongs survival of Ag-loaded BMDCs in DLNs. CFSE-labeled SarSP-DCs or control DCs were left untreated or haptenized with TNBS (SarSP-TN-DCs and TN-DCs, respectively), injected (footpad, subcutaneously) into B6 mice, and quantified by FACS analysis of DLNs at days 2, 5, and 7 after transfer. Regardless of the treatment, BMDCs showed similar DLN-homing capacities (not shown). However, the percentage of TN-DCs detected in DLNs 5 and 7 days after DC administration was significantly lower than the number of nonhaptenized DCs (P < .001; Figure 5B). It is noteworthy that the percentage of SarSP-TN-DCs homed in DLNs was significantly higher than that of TN-DCs, indicating that signaling via NK1R prolongs the survival of Ag-loaded BMDCs in DLNs.
NK1R and CD40 signaling act synergistically to prolong DC survival
The finding that SarSP-TN-DCs exhibit prolonged survival once they are homed in DLNs could be ascribed to sustained expression of antiapoptotic molecules induced by SarSP or a combined effect of NK1R and CD40 signaling, the latter provided by DC-activated CD4+ T cells in DLNs.8,57-61 Thus, we analyzed the individual and collective contribution of NK1R and CD40 signaling in the expression of Bcl-2 and on the viability of BMDCs in vitro and in vivo, respectively.
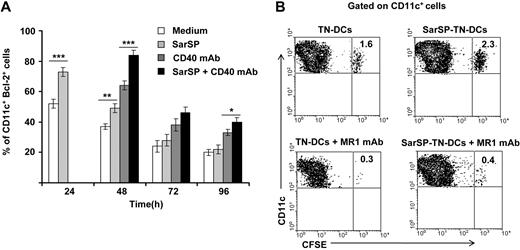
For in vitro experiments, BMDCs were pretreated (or not) with SarSP during the first 24 hours of culture and signaled with agonistic CD40 mAb added at 24 hours of culture. Expression of Bcl-2 by CD11c+ BMDCs was quantified up to 96 hours by FACS. As expected, NK1R signaling significantly increased the percentage of BMDCs expressing Bcl-2 after 24 hours of culture (P < .001), and signaling via CD40 further enhanced Bcl-2 expression in both SarSP-DCs and control DCs 48 hours after culture (P < .001; Figure 6A). By 96 hours, the percentage of BMDCs expressing Bcl-2 diminished regardless of the treatment used; however, CD40 ligation of SarSP-DCs significantly enhanced the number of cells expressing Bcl-2 versus DCs (P < .001), SarSP-DCs (P < .01) or DCs signaled only via CD40 (P < .05) (Figure 6A).
NK1R and CD40 signaling act synergistically to promote BMDC survival. (A) Bar-diagram representing the percentage of CD11c+ BMDCs expressing Bcl-2 after culture in medium alone or with SarSP for different time points. Agonistic CD40 mAb was added at 24 hours to cultures, and the percentage of CD11c+ BMDCs expressing Bcl-2 was analyzed at each time point. *P < .05, **P < .01, and ***P < .001. Means (± SD) of 3 independent experiments are shown. (B) Contour-plots illustrating the presence of SarSP-TN-DCs or TN-DCs (CD11c+CFSE+) in DLNs 5d after adoptive transference of DCs in B6 mice treated with or without the CD154 (CD40L) neutralizing MR1 mAb. Data are representative of 3 independent experiments.
NK1R and CD40 signaling act synergistically to promote BMDC survival. (A) Bar-diagram representing the percentage of CD11c+ BMDCs expressing Bcl-2 after culture in medium alone or with SarSP for different time points. Agonistic CD40 mAb was added at 24 hours to cultures, and the percentage of CD11c+ BMDCs expressing Bcl-2 was analyzed at each time point. *P < .05, **P < .01, and ***P < .001. Means (± SD) of 3 independent experiments are shown. (B) Contour-plots illustrating the presence of SarSP-TN-DCs or TN-DCs (CD11c+CFSE+) in DLNs 5d after adoptive transference of DCs in B6 mice treated with or without the CD154 (CD40L) neutralizing MR1 mAb. Data are representative of 3 independent experiments.
Next, we compared in vivo the survival of adoptively transferred BMDCs homed in DLNs in the presence or absence of CD40-CD40L (CD154) interaction blockade. CFSE-labeled TN-DCs or SarSP-TN-DCs were injected (footpad, subcutaneously) into B6 mice treated or not with CD154 (CD40L) blocking MR1 mAb (250 μg, intraperitoneally) simultaneously and 2 days after DC administration. In vivo blockade of the CD40-CD154 interaction decreased the percentage of transferred TN-DCs and SarSP-TN-DCs in DLNs 5 days after injection. Nevertheless, mice that were nontreated with CD154 blocking mAb had a significantly higher number of SarSP-TN-DCs remaining in DLNs compared with TN-DCs (Figure 6B). Together, these results suggest that the prolonged viability of BMDCs homed in DLNs was caused by a synergistic effect of NK1R and CD40 signaling.
BMDCs signaled via NK1R promote enhanced cellular immunity
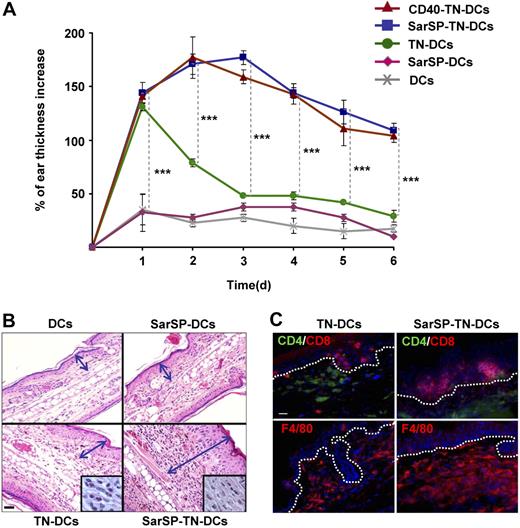
The previous results indicate that combination of NK1R and CD40 signaling enhances the interaction of BMDCs with T cells in DLNs, which might result in robust immune responses. To evaluate the immunological relevance of our findings, we compared the capability of TN-DCs and SarSP-TN-DCs to induce DTH responses. B6 mice were sensitized by administration of TN-DCs or SarSP-TN-DCs (footpad, subcutaneously) and DTH was elicited 5 days after by TNCB application to the dorsal skin of the ears. Mice were injected with haptenized CD40-TN-DCs or nonhaptenized (untreated or SarSP-signaled) DCs to be included as positive and negative controls, respectively. The effector cellular immune response was analyzed by measuring the increase of ear thickness up to 6 days after elicitation. As expected, mice sensitized with TN-DCs, SarSP-TN-DCs, and CD40-TN-DCs exhibited a significant ear thickness increase versus control mice 1 day after elicitation (P < .001) (Figure 7A). In mice sensitized with TN-DCs, the ear thickness diminished significantly 2 days after elicitation, and showed minimal differences versus control mice 3 days after elicitation (Figure 7A). Conversely, mice sensitized with SarSP-TN-DCs and CD40-TN-DCs exhibited a further increase 2 days after elicitation, and showed a significant prolonged increase in ear thickness in comparison to TN-DCs and control mice for up to 6 days (Figure 7A). We also characterized the cellular infiltrate in the ears of mice obtained 6 days after elicitation by microscopic analysis. Mice sensitized with TN-DCs presented scarce cellular infiltrate, whereas mice sensitized with SarSP-TN-DCs exhibited severe inflammatory infiltrate composed of mononuclear cells (Figure 7B,C). Immunofluorescence-microscopy analysis revealed that the enhanced cellular inflammatory infiltrate consisted of epidermal CD8+ T cells and dermal CD4+ T cells and F4/80+ macrophages (Figure 7C), demonstrating that the immune response generated was a type-1 DTH response.
Signaling BMDCs via NK1R prolongs effector cellular immune responses. (A) Ear thickness increase of mice sensitized with adoptively transferred TN-DCs, SarSP-TN-DCs, CD40-TN-DCs, or control (nonhaptenized) SarSP-DCs or untreated DCs, as analyzed at different time points after DTH elicitation. Means (± SD) of 6 mice per experimental group are illustrated. ***P < .001. (B) Histological analysis of ear skin sections dissected 6 days after DTH elicitation shows severe mononuclear cell infiltrate in samples obtained from mice sensitized with SarSP-TN-DCs compared with mice sensitized with TN-DCs or control SarSP-DCs or untreated DCs. The mononuclear cell infiltrate is shown at higher magnification in the insets. Arrows indicate the thickness of the skin (excluding the hypodermis). H&E, ×200 (insets, ×1000); scale bar equals 20 μm. (C) Characterization of the cellular infiltrate in ear skin of mice sensitized with TN-DCs or SarSP-TN-DCs. Dotted-lines indicate the epidermal-dermal junction. Immunofluorescence, ×500; scale bar equals 20 μm. (B,C) One representative skin section of 5 analyzed per experimental group is shown.
Signaling BMDCs via NK1R prolongs effector cellular immune responses. (A) Ear thickness increase of mice sensitized with adoptively transferred TN-DCs, SarSP-TN-DCs, CD40-TN-DCs, or control (nonhaptenized) SarSP-DCs or untreated DCs, as analyzed at different time points after DTH elicitation. Means (± SD) of 6 mice per experimental group are illustrated. ***P < .001. (B) Histological analysis of ear skin sections dissected 6 days after DTH elicitation shows severe mononuclear cell infiltrate in samples obtained from mice sensitized with SarSP-TN-DCs compared with mice sensitized with TN-DCs or control SarSP-DCs or untreated DCs. The mononuclear cell infiltrate is shown at higher magnification in the insets. Arrows indicate the thickness of the skin (excluding the hypodermis). H&E, ×200 (insets, ×1000); scale bar equals 20 μm. (C) Characterization of the cellular infiltrate in ear skin of mice sensitized with TN-DCs or SarSP-TN-DCs. Dotted-lines indicate the epidermal-dermal junction. Immunofluorescence, ×500; scale bar equals 20 μm. (B,C) One representative skin section of 5 analyzed per experimental group is shown.
Discussion
The survival of DCs transporting Ag from peripheral tissues to DLNs is crucial for initiation of cellular immunity, and administration of DCs unable to survive in the periphery or DLNs represents a major drawback for current DC-based immune-stimulatory therapies.7,62,63 Despite the relevance of DC survival for the purpose of positive vaccination, the mechanisms that control viability of adoptively transferred DCs have not been fully elucidated.
The nervous and immune systems have been demonstrated to interact to modulate the immune response via secretion of neuropeptides. The balance of proinflammatory and anti-inflammatory neuropeptides in the CNS and peripheral tissues is crucial in maintaining self-tolerance under the steady state. Indeed, a predominant secretion of the proinflammatory SP versus anti-inflammatory neuropeptides favors chronic inflammatory and autoimmune disorders in the CNS and peripheral tissues.28,64,65
We hypothesized that agonistic binding of NK1R promotes DC viability during their migration from peripheral tissues and in DLNs, which will result in a more efficient DC-T-cell interaction. Likewise, it has been demonstrated that proinflammatory tachykinins that signal via NK1R promote survival of neutrophils,66 macrophages,67 and B- and T-cell precursors.37,38 Recent publications have demonstrated that SP recruits immature DCs to inflammation sites,68 and that signaling skin DCs via NK1R participates in Th1 polarization and induction of CTL/Tc1 bias.30 However, the mechanisms involved in the relevant DC-stimulatory activities of proinflammatory tachykinins remain greatly unknown. Moreover, the ability of proinflammatory tachykinins to promote the survival of ex vivo–generated DCs relevant for DC-based immunotherapies has not been investigated.
In the present work, using a model of ex vivo–generated BMDCs, we demonstrated that, regardless of their maturation stage, BMDCs constitutively expressed the functional variant of NK1R. It is noteworthy that BMDCs increased the levels of NK1R mRNA after LPS stimulation, indicating that DCs are capable of up-regulating NK1R and are direct targets of increased levels of proinflammatory neuropeptides that are secreted during inflammation.28 Current approaches for adoptive transference of mouse or human DCs use BM precursor- or monocyte-derived DCs generated ex vivo in the presence of GM-CSF and IL-4, and deprivation of these cytokines results in DC apoptosis.7,14,43,44 Here, we confirmed that deprivation of GM-CSF and IL-4 results in significant apoptosis of BMDCs, and this effect is prevented by signaling BMDCs via NK1R with the natural agonists SP or HK-1 or the synthetic NK1R ligand SarSP.
At high concentrations, SP and SP analogs bind other neuropeptide receptors, including bombesin and serpin-enzyme complex receptors47,69 or, alternatively, exert their functions independently from receptor-ligation as described for mast cells.48 Under our conditions, the proinflammatory tachykinins induced their antiapoptotic effects on BMDCs at physiological concentrations, and this function was abrogated completely by the presence of NK1R antagonists or in BMDCs generated from NK1R−/−KO mice, demonstrating that the effect of the tachykinins on DC survival was mediated by NK1R binding. Moreover, our data suggest that antagonistic blockade of the NK1R does not exert apoptosis per se, but prevents from NK1R agonistic apoptotic rescue by competing for NK1R binding. In addition, BMDCs might not secrete significant levels of endogenous NK1R agonists to account for apoptotic rescue via an autocrine feedback loop.
Our results showed that signaling BMDCs through NK1R with proinflammatory neuropeptides activates the PI3K-Akt cascade to prolong DC survival. In addition, we observed that NF-κB or ERK signaling was less significant in the antiapoptotic effect mediated by NK1R agonists. These findings agree with recent studies, which emphasized the relevance of the PI3K-Akt cascade in promoting DC survival, with less prominent roles of MAPK and NF-κB pathways.7,20 Under our conditions, the relative lower contribution of ERK and NF-κB activation could be attributed to their interaction with the PI3K-Akt cascade, because cross-activation of these pathways have been described.49
Downstream effectors of the PI3K-Akt cascade include the antiapoptotic proteins from the Bcl-2 family.61 The Bcl-2 family includes anti- and proapoptotic proteins with characteristic homology sequences (BH domains) that are crucial for regulating mitochondrial outer membrane permeability, leading to inhibition or activation, respectively, of terminal effector caspases.53 Accordingly, Bad, a proapoptotic Bcl-2 family protein, exerts its effects by sequestering the antiapoptotic Bcl-2 through dimerization.70 Phosphorylation of Bad at the Ser136 residue by Akt disrupts its affinity for Bcl-2, allowing the free Bcl-2 to block the release of cytochrome c from the mitochondria and prevent the activation of caspases.52 Our results demonstrate that NK1R signaling of BMDCs, promotes Akt-dependent pBadSer136 and enhances Bcl-2 expression, with a consequent decrease of caspase 3.
DCs homing in secondary lymphoid organs become targets of CD8+ CTLs71,72 and NK cells73 that eliminate DCs in Ag-dependent or -independent fashions, respectively. Conversely, cognate activation via CD40 provided by CD4+ Th cells presents DCs with antiapoptotic signals necessary to survive CTL killing.74,75 Our data demonstrate that signaling via NK1R prolongs survival of BMDCs adoptively transferred into mice and their subsequent homing in DLNs, in Ag-independent and, importantly, Ag-dependent manners. Mechanistic analysis of the latter phenomenon revealed that signaling BMDCs via NK1R followed by CD40 stimulation exerted a synergistic effect to sustain in vitro Bcl-2 expression and prevent in vivo, the clearance of BMDCs homed in DLNs. The synergistic effect of signaling BMDCs via NK1R and CD40 was further confirmed in vivo by those results showing a significant increase of viable SarSP-TN DCs in the absence of the CD40L inhibition, compared with the number of TN-DCs that received CD40 signaling in the absence of exogenous NK1R agonist. The fact that inhibition of CD40-CD40L interaction diminished greatly the antiapoptotic effects exerted by both DC survival signals demonstrates the relevance of DC-CD4+ Th cell interaction in DLNs for prolonging survival of NK1R-signaled BMDCs.
It is noteworthy that enhanced longevity of Ag-loaded BMDCs signaled via NK1R and CD40 resulted in elicitation of a potent and sustained cellular immunity mediated mainly by CD8+ T cells and macrophages. Moreover, the observation that elicitation of DTH responses promoted by BMDCs signaled via NK1R persisted up to 1 week implies that the effector cells overcame the mechanisms of resolution of inflammation and remained active for the period that the Ag was present in the skin.76
In conclusion, we have demonstrated that signaling BMDCs via NK1R with proinflammatory tachykinins enhances DC survival in vitro and in vivo, resulting in induction of potent immune-stimulatory DCs that promote robust cellular immunity. Our data provide insight into the mechanisms by which the nervous system regulates the function of DCs and the subsequent cellular immune responses and helps to clarify the requirements for the development of more effective immune-stimulatory DCs for cell-based vaccination.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Christopher Paige (Department of Immunology, University of Toronto) for providing the B6 NK1R−/−KO mice.
This work was supported by National Institutes of Health (Bethesda, MD) grants R01-CA100893 to A.T.L., R01-HL075512 and R01-HL077545 to A.E.M., and T32-CA82084 fellowship to A.R.M.
National Institutes of Health
Authorship
Contribution: B.M.J. collaborated with experimental design, performed most of the experimental work, and wrote the manuscript; A.R.M. and G.E. contributed with qRT-PCR design and assistance; A.R.M. helped write the manuscript; O.A.T. performed BMDC cultures and other experimental techniques; W.J.S. performed immunofluorescence staining and analysis; A.E.M. contributed with experimental design and flow cytometric analysis; and A.T.L. coordinated the experimental approach and helped with data analysis and interpretation as well as with manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adriana T. Larregina, W1051 Biomedical Science Tower, 200 Lothrop St, Pittsburgh, PA 15213; e-mail: adrianal@pitt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal