Abstract
CD8+ T cells play an important role in controlling viral infections. Defective CD8+ T-cell responses during HIV infection could contribute to viral persistence. Early initiation of highly active antiretroviral therapy during acute primary HIV infection helps to preserve HIV-specific immune responses. Here, we describe a particular CD27+ CD45RO−/RA+ HIV-specific CD8+ T cell in participants treated early during the primary infection. These cells, which were present at a very low frequency during primary HIV infection, increased markedly after early treatment, whereas their frequency remained unchanged in untreated participants and in participants treated later. These nonnaive antigen-experienced cells are in a resting state and have characteristics of long-lived memory cells. They also possess direct effector capabilities, such as cytokine production, and are able to proliferate and to acquire cytotoxic functions on reactivation. Our results suggest that these HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells, observed when early viral replication is inhibited, form a pool of resting cells with memory characteristics.
Introduction
Virus-specific CD8+ T cells play a central role in controlling viral infections. During acute viral infections, naive specific CD8+ T cells expand, differentiate, and acquire effector functions.1,2 After the acute phase, the CD8+ T-cell response generally subsides because of massive apoptosis of antigen-specific effector CD8+ T cells. However, some of these cells persist to form a pool of memory cells. Memory cells can be divided into 2 subpopulations. The first, the central memory (CM) population, resides in lymph nodes. These cells are resting and long-lived. On reexposure to their cognate antigen, they expand, leave the lymph nodes, and acquire effector capabilities. The second, the effector memory (EM) population, is composed of circulating cells that can exert their effector functions immediately, for rapid control of reinfection.3,4
Various surface markers have been proposed to distinguish among naive, memory, and effector cells. One is the domiciliation marker CCR7, which allows cells expressing it to home to lymph nodes. Naive cells express CCR7 and the CD45RA isoform of the CD45 molecule. CM cells also express CCR7 and the reciprocal CD45 isoform, CD45RO. EM and effector cells do not express CCR7 and variably express the costimulatory molecule CD27 and the CD45RA/RO isoforms. In their initial description, van Lier et al defined EM cells in terms of their CD27 expression, as effectors do not express this marker (they are CCR7− CD27− CD45RO+ and become CD45RA+ when terminally differentiated).5-7 Another classification is based on CD45RA/RO isoform expression3,8 : EM cells express the CD45RO isoform (regardless of CD27 expression), whereas effectors express the CD45RA isoform.
During primary HIV infection (PHI), HIV-specific CD8+ T cells generally only partially inhibit viral replication, thus allowing the virus to persist.9-11 Several quantitative, qualitative, and homing defects of HIV-specific CD8+ T-cell responses have been described during PHI.12-14 In particular, HIV-specific CD8+ T cells appear to be blocked at an intermediate stage of differentiation, in the form of EM cells with limited antiviral capacities.8,15,16 However, such virus-specific CD8+ T cells with intermediate differentiation may be found during the acute phase of most viral infections, including acute Epstein-Barr virus (EBV) and hepatitis C virus infections.17 It is their persistence after the initial stage of infection that has been thought to be responsible for the lack of control of HIV infection.18-20 Indeed, several studies have shown that HIV-infected patients with fully differentiated HIV-specific CD8+ T cells tend to have better outcomes.18-20 However, other authors consider that the pattern of differentiation may vary among different virus specific CD8+ T cells, is not directly related to effector capacity,21,22 and has little effect on the control of viral replication.17
Here we tested the hypothesis that rapid and early control of viremia during the primary acute infection might modify the differentiation status of HIV-specific CD8+ T cells. We observed a significant increase in a particular subset of HIV-specific CCR7− CD27+ CD45RO−/RA+ CD8+ T cells during therapy, and went on to characterize them in terms of their activation, survival, and functions.
Methods
Study participants
We studied 35 persons with primary HIV-1 infection enrolled in the French PRIMO multicenter cohort (Agence Nationale de Recherche sur le SIDA, CO6-PRIMO Cohort). Primary infection was defined by HIV RNA positivity with a negative or emerging antibody response shortly after a high-risk exposure. Patients' informed consent was obtained as required for the study in accordance with the Declaration of Helsinki, with the approval of the Comité Consultatif pour la Protection des Personnes dans la Recherche Biomédicale of Université Paris-Sud. After the diagnosis of acute HIV infection, participants were given the choice to begin highly active antiretroviral therapy (HAART) immediately or to postpone it, after discussing it with their physician.
At inclusion, 22 participants were on HAART and the other 13 were untreated. The median baseline plasma HIV RNA levels were, respectively, 5.13 and 4.86 log10/mL (interquartile range [IQR], 4.69-5.51 and 4.51-5.58, respectively). The median CD4+ T-cell counts were, respectively, 539/μL and 554/μL (IQR, 302-651 and 412-884, respectively) and the median CD8+ T-cell counts were 846/μL and 1340/μL (IQR, 581-1205 and 952-1955, respectively). Clinical and biologic characteristics of the 35 participants at the time of primary HIV infection are shown in the Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). As controls, 12 healthy persons were studied.
Cells and peptides
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood by Ficoll density gradient centrifugation and were preserved in liquid nitrogen. Human leukocyte antigen (HLA) typing was performed with the complement-dependent microlymphocytotoxic technique (One Lambda, Montpellier, France). Specific responses to peptides corresponding to optimal HIV–cytotoxic T lymphocyte (CTL) epitopes (National Institutes of Health HIV Molecular Immunology Database, http://www.hiv.lanl.gov/content/immunology/tables/optimal_ctl_summary.html) were measured by IFNγ–enzyme-linked immunosorbent spot assay.
Peptide-HLA class 1 multimers
Virus-specific CD8+ T cells were identified with soluble phycoerythrin (PE)- or allophycocyanin (APC)-labeled peptide-HLA class 1 multimers (Proimmune, Oxford, United Kingdom) derived from the HIV proteins Gag, Nef, Pol, and Env and from the EBV proteins BMLF-1 and BZLF-1. The HIV-epitopes used were the HLA-A*0201-restricted peptide ligands SLYNTVATL (Gag 77-85) and ILKEPVHGV (Pol 476-484), the A*0301-restricted peptide ligands RLRPGGKKK (Gag 20-28) and QVPLRPMTYK (Nef 73-82), the A*2402-restricted peptide ligand RYPLTFGWCY (Nef 134-143), the B*0702-restricted peptide ligand IPRRIRQGL (Env 848-856), the B*0801-restricted peptide ligands GEIYKRWII (Gag 259-267) and FLKEKGGL (Nef 90-97), the B*2705-restricted peptide ligand KRWIILGLNK (Gag 263-272), and B*5701 restricted ligands KAFSPEVIPMF (Gag 162-172) and TSTLQEQIGW (Gag 240-249). The EBV epitopes used were the HLA-A*0201-restricted peptide ligand GLCTLVAML (BMLF-1 259-267) and the B*0801-restricted peptide ligand RAKFKQLL (BZLF-1 190-197).
Flow cytometry
PBMCs were analyzed by 4-color or 6-color flow cytometry. Samples were stained first with PE- or APC-conjugated HLA class 1 peptide multimers for 20 minutes at room temperature, then with labeled antibodies against surface markers for 15 minutes at 4°C. Antibodies against the following antigens were used to characterize HIV-specific CD8+ T cells: fluorescein isothiocyanate (FITC)–coupled: CCR7 (R&D Systems Europe, Lille, France), CD27, CD28, CD62L, CD38, CD45RA, and HLA-DR (BD Biosciences, San Jose, CA); PE-coupled: CD127 (R&D Systems Europe); PE-Texas Red (ECD)–coupled: CD45RO and CD8 (Beckman Coulter, Fullerton, CA); PE-cyanin 5 (PC5)–coupled: CD3 and CD8 (Beckman Coulter); peridin chlorophyll protein-cyanin 5.5 (PerCP-Cy5.5)–coupled: CD8 (BD Biosciences). To detect intracellular proteins, samples were incubated with anti–Bcl-2–, anti–Ki-67–, and anti–perforin-FITC antibodies (BD Biosciences) for 30 minutes at room temperature after incubation with FACS permeabilizing solution (BD Biosciences). Samples were acquired on an Epics XL flow cytometer (Beckman Coulter) or a BD FACSCanto flow cytometer (BD Biosciences) and analyzed with RXP software (Beckman Coulter).
Intracellular cytokine production
PBMCs were stimulated for 15 hours in medium containing the relevant optimal EBV or HIV peptide (2 μg/mL), anti-CD28, and anti-CD49d (1 μg/mL each, BD Biosciences). After 1 hour of stimulation, cytokine secretion was blocked by adding brefeldin A (10 μg/mL, Sigma-Aldrich Chemie, Saint-Quentin Fallavier, France). After further incubation, samples were stained as described in “Flow cytometry” for intracellular detection using anti–IFN-γ–FITC or -PE, anti–TNF-α–FITC or anti–IL-2–PE antibodies (BD Biosciences). A negative control (medium) and a positive control staphylococcal enterotoxin B (SEB) were included in each experiment.
HIV-specific CD8+ T-cell viability
The CD45RA+ and CD45RA− T-cell subsets were separated from PBMCs using CD45RA microbeads and LS columns (Miltenyi Biotec, Paris, France). Purity was greater than 95% and 85%, respectively.
CD45RA+ and CD45RA− T cells were cultured for 6 days at 37°C with 5% CO2 in RPMI 1640 medium (Invitrogen, Cergy Pontoise France), supplemented with 10% fetal calf serum (FCS; Dominique Dutscher, Brumath, France) and antibiotics. The percentage of viable HIV-specific CD8+ T cells was determined on day 0 and day 6.
Perforin content in HIV-specific CD8+ T cells
Isolated CD45RA+ and CD45RA− cells were cultured in RPMI medium with 10% FCS and antibiotics plus 2 μg/mL of the appropriate optimal HIV peptide for 6 days. Perforin expression was determined by flow cytometry before and after culture.
CFSE proliferation assay
PBMCs were stained with 0.35 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) for 10 minutes at 37°C. After washes, they were cultured for 6 days in RPMI medium with 10% FCS and antibiotics plus the relevant optimal EBV or HIV peptide (2 μg/mL). After incubation, the cells were washed and stained as described in “Flow cytometry.” A negative control (medium) and a positive control (SEB) were included in each experiment.
Statistical analysis
Data were analyzed with GraphPad Prism 5 software (GraphPad Software, San Diego, CA). Variables were compared with nonparametric tests (the Wilcoxon test for paired values and the Mann-Whitney U test for unpaired values). P values less than or equal to .05 were considered to denote significant differences.
Results
Effects of early treatment on differentiation status of HIV-specific CD8+ T cells
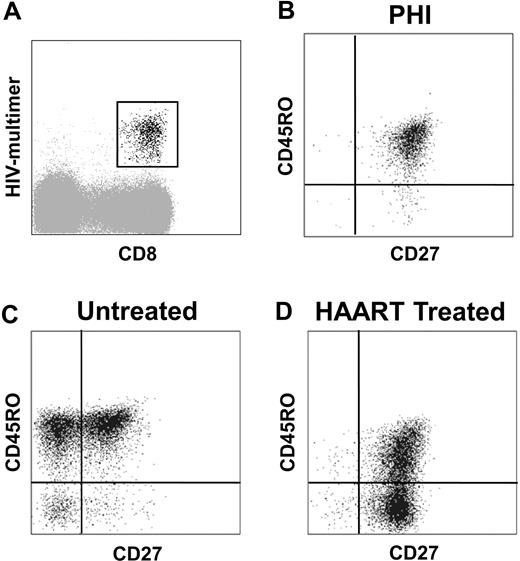
It is generally reported that sequential loss of CCR7, CD28, and CD27 occurs during CD8+ T-cell differentiation.7 At the time of PHI, HIV-specific CD8+ T cells were mainly CCR7− and CD28− (94% ± 6% and 79% ± 14% of cells were CCR7− and CD28−, respectively). In contrast, most cells expressed CD27 (90% ± 10%), reflecting an intermediate stage of differentiation. The majority of these cells coexpressed CD27 and CD45RO (77% ± 15%; Figure 1B). In untreated participants, after the acute phase of infection, the percentage of HIV-specific EM cells (CD27+ CD45RO+) fell from 78% (± 12%) to 52% (± 13%) at one year (M12; Figure 1C). A large proportion of HIV-specific CD8+ T cells switched to effector status, and 33% (± 12%) were CD27− at M12 and the majority of these cells remained CD45RO+. In contrast, in HAART-treated participants, the contraction of the HIV-specific CD27+ CD45RO+ CD8+ subset (43% ± 23% at M12) was associated with a parallel increase in a new CD27+ CD45RO− subset representing 42% (± 22%) of HIV-specific CD8+ T cells at M12 (Figure 1D). To confirm that the increased proportion of CD45RO− cells was the result of an increase in CD45RA+ cells, as suggested by the exclusive and reciprocal expression of the RA and RO CD45 isoforms,23 we analyzed the coexpression of CD27, CD45RA, and CD45RO (Figure S1): HIV-specific CD45RO− CD8+ T cells were CD45RA+ and, reciprocally, CD45RA− HIV-specific CD8+ T cells were CD45RO+. As most phenotypic analyses were performed with anti-CD45RO antibodies, the 2 subsets were hereafter referred to as CD45RO−/RA+ and CD45RO+ cells.
Phenotypic characterization of HIV-specific CD8+ T cells during primary HIV infection, in untreated and HAART-treated participants. (A) HIV-specific CD8+ T cells were gated based on HIV multimer and CD8 expression. (B-D) Representative phenotypes of HIV-specific CD8+ T cells are presented: most HIV-specific CD8+ T cells expressed CD27 and CD45RO at baseline during PHI (B); in untreated participants after PHI, HIV-specific CD8+ T cells were CD27+/− CD45RO+ (C); and HIV-specific CD8+ T cells in HAART-treated participants were CD27+ CD45RO+/− (D).
Phenotypic characterization of HIV-specific CD8+ T cells during primary HIV infection, in untreated and HAART-treated participants. (A) HIV-specific CD8+ T cells were gated based on HIV multimer and CD8 expression. (B-D) Representative phenotypes of HIV-specific CD8+ T cells are presented: most HIV-specific CD8+ T cells expressed CD27 and CD45RO at baseline during PHI (B); in untreated participants after PHI, HIV-specific CD8+ T cells were CD27+/− CD45RO+ (C); and HIV-specific CD8+ T cells in HAART-treated participants were CD27+ CD45RO+/− (D).
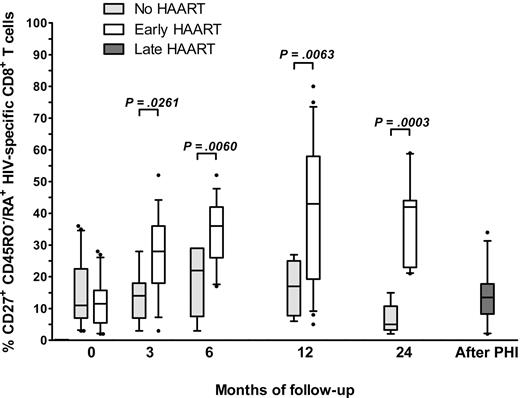
The CD27+ CD45RO−/RA+ subset increased rapidly after HAART initiation, as it accounted for 12% (± 7%) of HIV-specific CD8+ T cells at inclusion, 27% (± 13%) at M3, 34% (± 10%) at M6, 42% (± 22%) at M12, and 37% (± 14%) at M18. In untreated participants, the corresponding values were 15% (± 11%) at inclusion, 14% (± 8%) at M3, 18% (± 11%) at M6, 16% (± 8%) at M12, and 7% (± 5%) at M18 (Figure 2). This increase in the CD27+ CD45RO−/RA+ subset was observed whatever the multimeric MHC-peptide complex tested. Six of the 13 initially untreated patients started treatment at least 6 months after inclusion. When we tested their HIV-specific cells a median of 7 months (range 5-12 months) after the beginning of treatment, we found no increase in the CD45RO−/RA+ CD27+ subset (14% ± 9%, compared with 12% ± 11% before treatment and 16% ± 10% at inclusion). In summary, we observed a clear increase in an HIV-specific CD8+ T-cell subpopulation characterized by CD27 and CD45RA coexpression in participants treated during primary acute HIV infection. This subset, which remained low in untreated participants and in those treated later during the infection, may represent a stable quiescent memory pool.
Time course of CD45RO−/RA+ CD27+ HIV-specific CD8+ T cells in untreated and HAART-treated participants. We determined the phenotype of HIV-specific CD8+ T cells in 22 treated and 13 untreated participants during and after PHI. The percentage of HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells is represented at different time points (at inclusion and months 3, 6, 12, and 24 of HIV infection) in untreated participants ( ), HAART-treated participants (□), and participants treated after PHI (
), HAART-treated participants (□), and participants treated after PHI ( ) (median, range, and 25th-75th and 10th-90th percentiles). Statistical differences between groups are indicated above the bars.
) (median, range, and 25th-75th and 10th-90th percentiles). Statistical differences between groups are indicated above the bars.
Time course of CD45RO−/RA+ CD27+ HIV-specific CD8+ T cells in untreated and HAART-treated participants. We determined the phenotype of HIV-specific CD8+ T cells in 22 treated and 13 untreated participants during and after PHI. The percentage of HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells is represented at different time points (at inclusion and months 3, 6, 12, and 24 of HIV infection) in untreated participants ( ), HAART-treated participants (□), and participants treated after PHI (
), HAART-treated participants (□), and participants treated after PHI ( ) (median, range, and 25th-75th and 10th-90th percentiles). Statistical differences between groups are indicated above the bars.
) (median, range, and 25th-75th and 10th-90th percentiles). Statistical differences between groups are indicated above the bars.
Differentiation, activation, and cycling status of HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells
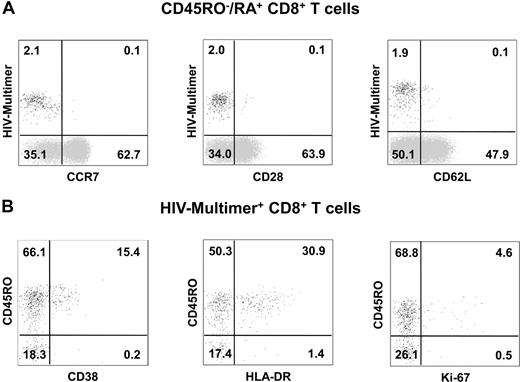
To exclude the possibility that these cells were naive T cells, we first evaluated the expression levels of CCR7, CD28, and CD62L. As shown in Figure 3A, HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells expressed low levels of these markers (11% ± 11%, 11% ± 9%, and 18% ± 7% for CCR7, CD28, and CD62L, respectively), suggesting that multimer-positive HIV-specific CD8+ T cells are antigen-experienced and not naive. Therefore, as this subset had phenotypic characteristics of both memory and effector cells, we compared HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells with their CD27+ CD45RO+ counterparts.
Differentiation and activation of CD45RO−/RA+ CD27+ HIV-specific CD8+ T cells in HAART-treated participants. (A) PBMCs were isolated from HAART-treated participants and labeled with HIV-multimer-PE; CCR7-FITC, CD28-FITC, or CD62L-FITC; CD45RO-ECD and CD8-PC5. Representative phenotypes gated on CD45RO−/RA+ CD8+ T cells. The majority of CD45RO−/RA+ HIV-specific CD8+ T cells did not express CCR7, CD28, or CD62L as opposed to global CD45RO−/RA+ HIV-multimer negative CD8+ T cells. (B) PBMCs were isolated from HAART-treated participants and stained with HIV-multimer-PE; CD38-FITC, HLA-DR-FITC, or Ki-67-FITC; CD45RO-ECD and CD8-PC5. Representative phenotypes gated on HIV-specific CD8+ T cells.
Differentiation and activation of CD45RO−/RA+ CD27+ HIV-specific CD8+ T cells in HAART-treated participants. (A) PBMCs were isolated from HAART-treated participants and labeled with HIV-multimer-PE; CCR7-FITC, CD28-FITC, or CD62L-FITC; CD45RO-ECD and CD8-PC5. Representative phenotypes gated on CD45RO−/RA+ CD8+ T cells. The majority of CD45RO−/RA+ HIV-specific CD8+ T cells did not express CCR7, CD28, or CD62L as opposed to global CD45RO−/RA+ HIV-multimer negative CD8+ T cells. (B) PBMCs were isolated from HAART-treated participants and stained with HIV-multimer-PE; CD38-FITC, HLA-DR-FITC, or Ki-67-FITC; CD45RO-ECD and CD8-PC5. Representative phenotypes gated on HIV-specific CD8+ T cells.
To determine their activation status, we measured the expression of CD38 and HLA-DR and that of the cell cycle marker Ki-67. During PHI, HIV-specific cells are highly activated and cycling: in our participants, CD38, HLA-DR, and Ki-67 were expressed by, respectively, 82% (± 16%), 72% (± 17%), and 51% (± 28%) of HIV-specific CD8+ T cells (data not shown). HAART initiation was associated with a decrease in the expression of these markers, but the expression levels were significantly lower in the CD27+ CD45RO−/RA+ subset than in the CD27+ CD45RO+ subset: CD38 was expressed by 12% (± 10%) of CD45RO−/RA+ cells and 17% (± 10%) of CD45RO+ cells (P = .002); HLA-DR by 17% (± 13%) and 31% (± 19%; P < .001); and Ki-67 by 3% (± 4%) and 6% (± 5%; P = .03; Figure 3B).
These results suggested that the CD27+ CD45RO−/RA+ subset was in a quiescent state, and we therefore investigated its resistance to apoptosis.
Resistance to apoptosis of HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells
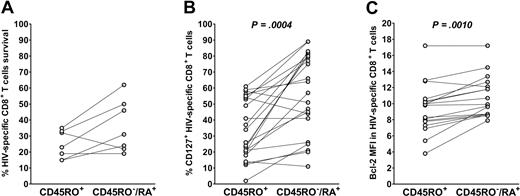
After separation of cell populations with CD45RA-coated magnetic microbeads, we first examined the ability of isolated CD45RA+/RO− and CD45RA−/RO+ cell fractions (hereafter referred to as CD45RA+ and CD45RO+ fractions for the sake of clarity in experiments with magnetic bead separation) to survive in culture in the absence of stimuli. Absolute numbers of HIV-specific CD8+ T cells were counted on day 0 and day 6 of culture. In 6 of 7 experiments with cells from different donors, the CD45RA+ fraction survived better than the CD45RO+ fraction (Figure 4A).
HIV-specific CD45RO−/RA+ CD8+ T cells are more resistant to apoptosis than the CD45RO+ subset. (A) PBMCs from treated participants were magnetically separated based on CD45RA expression and then cultured in the absence of exogenous mediators. The absolute number of HIV-specific CD8+ T cells was calculated before and after culture. The percentage survival of HIV-specific CD45RO+ and CD45RA+ CD8+ T cells after 6 days in culture is presented for cells from 7 participants. (B,C) The relative percentage of CD127+ cells and the relative Bcl-2 mean fluorescence intensity in the CD45RO+ and CD45RO−/RA+ HIV-specific CD8+ subsets are presented in 11 and 10 participants, respectively.
HIV-specific CD45RO−/RA+ CD8+ T cells are more resistant to apoptosis than the CD45RO+ subset. (A) PBMCs from treated participants were magnetically separated based on CD45RA expression and then cultured in the absence of exogenous mediators. The absolute number of HIV-specific CD8+ T cells was calculated before and after culture. The percentage survival of HIV-specific CD45RO+ and CD45RA+ CD8+ T cells after 6 days in culture is presented for cells from 7 participants. (B,C) The relative percentage of CD127+ cells and the relative Bcl-2 mean fluorescence intensity in the CD45RO+ and CD45RO−/RA+ HIV-specific CD8+ subsets are presented in 11 and 10 participants, respectively.
We then determined the expression levels of the IL-7 receptor-α (CD127), and the antiapoptotic marker Bcl-2. IL-7Rα is expressed on memory cells, and signal transduction by this receptor enhances survival and homeostatic proliferation, partly through an increase in Bcl-2 expression. The CD45RO−/RA+ HIV-specific CD8+ T-cell subset expressed higher levels of CD127 than its CD45RO+ counterpart (57% ± 25% and 34% ± 19%, respectively; P < .001; Figure 4B). In line with these results, intracellular staining of Bcl-2 was more intense in the CD45RO−/RA+ subset than in the CD45RO+ subset (mean fluorescence intensity, 10.9 ± 2.5 and 9.4 ± 3.1, respectively, P = .001; Figure 4C).
These results show that the CD45RO−/RA+ HIV-specific CD8+ T subset is resistant to apoptosis and possesses characteristics of memory cells.
Functional capacities of HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells
Changes in CD45 isoform expression.
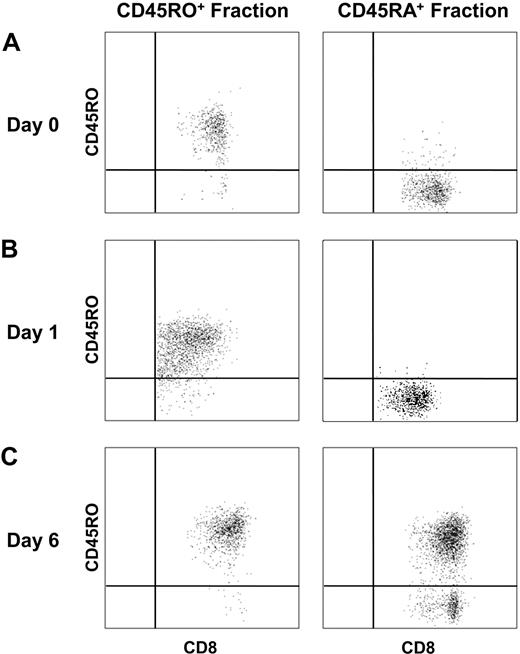
Because functional analyses often require cell culture, we first explored possible variations in CD45RO/CD45RA expression with the time in culture. The CD45RA+ and CD45RO+ CD8+ fractions were isolated with CD45RA microbeads. The subpopulations were relatively pure, as a median of 98% of cells were CD45RA+ in the positive fraction, whereas 88% were CD45RO+ in the negative fraction (Figure 5A). After short (15-hour) TCR stimulation with anti-CD3, each fraction retained its original phenotype, with 94% (± 6%) of cells still being negative for CD45RO expression in the CD45RA+ fraction and 90% (± 6%) of cells still being CD45RO in the CD45RO+ fraction (Figure 5B). In contrast, after 6 days of culture with antigen, almost all initially CD45RA+ HIV-specific CD8+ T cells had switched to CD45RO expression (91% ± 7%), whereas nearly all initially CD45RO+ HIV-specific CD8+ T cells continued to express CD45RO (97% ± 3%; Figure 5C). These data suggested that functional analyses requiring short-term culture could be performed directly by flow cytometry without the need for prior subset separation. By contrast, assays requiring 6 days of culture necessitated initial purification of the 2 cell subsets.
Phenotypic changes in the HIV-specific CD45RA+ CD8+ T-cell subset after activation. PBMCs were separated with CD45RA magnetic microbeads, and the isolated CD45RA+ and CD45RO+ fractions were cultured with anti-CD3 overnight or with a specific HIV peptide for 6 days. HIV-multimer and CD45RO staining were assessed on days 0, 1, and 6 by flow cytometry. (A-C) CD45RO expression on HIV-specific CD8+ T cells from negative fraction CD45RO+ and positive fraction CD45RA+ on days 0, 1, and 6, respectively.
Phenotypic changes in the HIV-specific CD45RA+ CD8+ T-cell subset after activation. PBMCs were separated with CD45RA magnetic microbeads, and the isolated CD45RA+ and CD45RO+ fractions were cultured with anti-CD3 overnight or with a specific HIV peptide for 6 days. HIV-multimer and CD45RO staining were assessed on days 0, 1, and 6 by flow cytometry. (A-C) CD45RO expression on HIV-specific CD8+ T cells from negative fraction CD45RO+ and positive fraction CD45RA+ on days 0, 1, and 6, respectively.
Antigen-specific cytokine production.
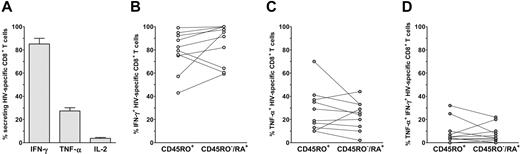
We then investigated the capacity of the HIV-specific CD8+ subsets to produce IFN-γ, TNF-α, and IL-2 (Figure 6). After specific stimulation, 85% (± 15%) of HIV-specific CD8+ T cells produced IFN-γ (85% ± 17% and 79% ± 17% for CD45RO−/RA+ and CD45RO+ subsets, respectively). In the same way, both subsets of HIV-specific CD8+ T cells produced TNF-α (27% ± 16% overall; 24% ± 12% of CD45RO−/RA+ cells and 28% ± 19% of CD45RO+ cells). In contrast, the frequency of IL-2-producing HIV-specific CD8+ T cells was very low (6% ± 3% overall), and it was not possible to precisely measure the frequency of such cells in each subset.
Cytokine production by CD45RO+ and CD45RO−/RA+ HIV-specific CD8+ T cells. PBMCs were stimulated overnight with HIV peptide, and cytokine secretion (IFN-γ, TNF-α, and IL-2) was blocked after 1 hour of activation. (A) The percentage of responding CD8+ T cells was measured by flow cytometry after costaining with IFN-γ-FITC/multimer-PE, TNF-α-FITC/IFN-γ-PE, or IFN-γ-FITC/IL-2-PE. The total of the 3 quadrants FITC+ PE−, FITC+ PE+, and FITC− PE+ was considered to be HIV-specific CD8+ T cells. (B-D) The relative percentages of CD45RO−/RA+ and CD45RO+ responding cells among HIV-specific cells were calculated and are shown for IFN-γ production, TNF-α production, and simultaneous IFN-γ and TNF-α production.
Cytokine production by CD45RO+ and CD45RO−/RA+ HIV-specific CD8+ T cells. PBMCs were stimulated overnight with HIV peptide, and cytokine secretion (IFN-γ, TNF-α, and IL-2) was blocked after 1 hour of activation. (A) The percentage of responding CD8+ T cells was measured by flow cytometry after costaining with IFN-γ-FITC/multimer-PE, TNF-α-FITC/IFN-γ-PE, or IFN-γ-FITC/IL-2-PE. The total of the 3 quadrants FITC+ PE−, FITC+ PE+, and FITC− PE+ was considered to be HIV-specific CD8+ T cells. (B-D) The relative percentages of CD45RO−/RA+ and CD45RO+ responding cells among HIV-specific cells were calculated and are shown for IFN-γ production, TNF-α production, and simultaneous IFN-γ and TNF-α production.
In summary, both the CD45RO−/RA+ and CD45RO+ HIV-specific subsets produce IFN-γ and TNF-α, but very few cells of either subset produce IL-2. These results show that CD45RO−/RA+ and CD45RO+ HIV-specific CD8+ T cells have similar functional capacities in terms of cytokine production.
Antigen-specific proliferation.
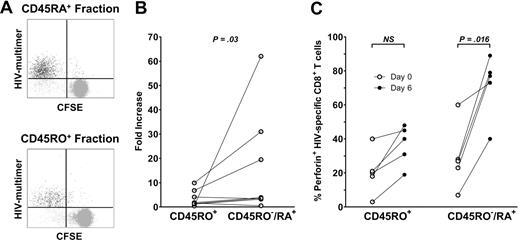
The capacities of CD45RA+ and CD45RO+ HIV-specific CD8+ T cells to proliferate were evaluated in the CFSE test. The 2 subsets were first isolated with microbeads as described in “Methods,” then stained with CFSE and stimulated with an HIV peptide for 6 days. At the end of culture, the proportion of CFSELow proliferating HIV-specific CD8+ T cells was measured by flow cytometry (Figure 7A). The CD45RA+ subset proliferated slightly more than the CD45RO+ subset, without reaching significance (84.2% ± 14.9% vs 69.2% ± 29.2%, respectively). These results were in line with the 16-fold (± 21-fold) increase in HIV-specific CD45RA+ cells and the 3-fold (± 3-fold) increase in CD45RO+ cells observed after 6 days of culture with the relevant peptide (P = .04; Figure 7B).
HIV-specific CD45RA+ CD8+ T cells are able to proliferate and to acquire cytotoxic potential after culture. CD45RA+ and CD45RO+ fractions were obtained after magnetic separation and cultured with the appropriate peptide during 6 days. (A) A representative dot plot obtained after CFSE staining and culture of each fraction. (B) The fold increase of HIV-specific CD8+ T cells was calculated for each fraction. (C) Perforin expression by HIV-specific CD8+ T cells was measured before (white plots) and after (black plots) culture of the isolated CD45RO+ and CD45RA+ subsets. The percentage of positive cells is represented for each subset on day 0 and day 6. Statistical differences between groups are indicated above the bars.
HIV-specific CD45RA+ CD8+ T cells are able to proliferate and to acquire cytotoxic potential after culture. CD45RA+ and CD45RO+ fractions were obtained after magnetic separation and cultured with the appropriate peptide during 6 days. (A) A representative dot plot obtained after CFSE staining and culture of each fraction. (B) The fold increase of HIV-specific CD8+ T cells was calculated for each fraction. (C) Perforin expression by HIV-specific CD8+ T cells was measured before (white plots) and after (black plots) culture of the isolated CD45RO+ and CD45RA+ subsets. The percentage of positive cells is represented for each subset on day 0 and day 6. Statistical differences between groups are indicated above the bars.
Cytotoxicity.
Coexpression of intracellular IFN-γ and TNF-α after specific stimulation has been described as a marker of cytotoxicity.24 Similar proportions of HIV-specific CD8+ T cells producing both IFN-γ and TNF-α were found in the 2 CD45RO−/RA+ and CD45RO+ subsets (8% ± 7% and 9% ± 11% in the CD45RO−/RA+ and CD45RO+ subsets, respectively). A minority of HIV-specific CD8+ T cells expressed perforin ex vivo (25% ± 18% overall), but only 1.8% (± 1.9%) of these cells expressed the high levels usually associated with efficient cytotoxicity. Perforin expression was similar in the CD45RO−/RA+ and CD45RO+ HIV-specific CD8+ subsets (29% ± 19% and 21% ± 13%, respectively, for overall expression). However, after 6 days of culture with the cognate peptide, the CD45RA+ HIV-specific CD8+ T-cell fraction expressed higher levels of perforin than the CD45RO+ fraction (72% ± 19% compared with 37% ± 12%, respectively, P = .047; Figure 7C). The proportion of perforin+ HIV-specific CD8+ T cells increased by 43% (± 20%) in the CD45RA+ fraction and by 16% (± 9%) in the CD45RO+ fraction (P = .013). These results suggest that the CD45RA+ HIV-specific CD8+ subpopulation has little cytotoxic capacity ex vivo but that these cells can efficiently acquire this capacity on exposure to their cognate antigen.
Comparison of HIV- and EBV-specific CD8+ T cells in healthy and HIV-infected participants
The frequency of CD45RO−/RA+ CD8+ T cells has been shown to increase in EBV infection after resolution of acute infectious mononucleosis.25 To assess whether the increase observed after early treatment was HIV-specific, we therefore extended the analysis to EBV-specific CD8+ T cells in healthy and HIV-infected participants. As described in Figure S2A, the proportion of EBV-specific CD45RO−/RA+ in CD27+ T cells was slightly, but not significantly, decreased during PHI compared with noninfected participants (33% ± 18% during PHI and 51% ± 22% in noninfected participants). These values remained stable thereafter whether patients were treated (39% ± 28%) or not (41% ± 21%). In HAART-treated HIV-infected participants, cytokine production and proliferative capacity by EBV- and HIV-specific CD8+ T cells were similar (Figure S2B-E). For EBV-specific CD8+ T cells, IFN-γ production was comparable in HAART-treated and noninfected participants, whereas TNF-α and IL-2 production were decreased in HIV-infected participants. EBV-specific proliferation was identical in noninfected and HAART-treated patients (88% ± 8% and 84% ± 11%, respectively; Figure S2B-E). The increase in CD45RO−/RA+ CD8+ T-cell frequency is specific to HIV and is not observed among EBV-specific CD8+ T cells after early treatment of HIV-infected participants.
Discussion
We describe here a particular subset of HIV-specific CD8+ T cells expressing a CD27+ CD45RO− phenotype. This minor subset increased in patients treated with HAART early during primary acute HIV infection. We focused on its differentiation, activation, survival, and functions and obtained evidence that it might be a stable quiescent memory T-cell pool.
This subset represents a minority of both total and virus-specific CD8+ T cells and is poorly defined. Some relevant studies have been performed in other settings, such as EBV infection,25 cytomegalovirus infection,26 vaccinia virus immunization,27 and also with antitumoral CTL clones.28 However, no consensus definition of this subpopulation has been reached.
We first showed that HIV-specific CD45RO− are CD45RA+, as expected based on the mutual and exclusive expression of the RO and RA isoforms of the CD45 marker.23 We then showed that these CD27+ CD45RO−/RA+ CD8+ T cells are real antigen-experienced cells and not naive, as they only express very low levels of the CCR7, CD28, and CD62L markers, which are classically present on naive cells. This HIV-specific CD8+ T-cell subset was very minor during the acute phase of HIV infection and increased rapidly during early therapy, reaching levels close to those we and others25 have found for EBV-specific CD8+ T cells, either in HIV-uninfected persons or in early HAART-treated HIV-infected persons. This increase in CD45RO−/RA+ CD8+ T-cell frequency is specific to HIV-specific CD8+ T cells and is not observed among EBV-specific CD8+ T cells, suggesting that it is specifically related to the decrease of HIV antigenic burden induced by HAART.
This increase may correspond to a change in the CD45 isoform. The CD45RO−/RA+ subset would then derive from the CD45RO+ counterpart, as previously demonstrated in human EBV infection25,29 and in antitumoral clones in vitro28 and as suggested by common Vβ usage by the 2 subsets (data not shown). Alternatively, the fall in HIV-specific CD8+ T cells usually observed after initiation of HAART30 may preferentially affect the CD45RO+ subset, being more activated and more susceptible to apoptosis, leading to a relative increase in the CD45RO−/RA+ subset.
We then demonstrated that these CD27+ CD45RO−/RA+ cells are quiescent. Although the global immune activation generally observed during acute PHI31,32 was drastically attenuated after HAART-induced viral suppression, expression of the cycling protein Ki-67, and of the activation markers CD38 and HLA-DR was more strongly reduced in CD45RO−/RA+ than CD45RO+ CD8+ T cells. This has also been observed in EBV infection, after acute infectious mononucleosis: CD8+ T cells specific for EBV lytic epitopes, which are no longer presented, reexpress CD45RA, whereas CD8+ T cells specific for latent and persistent epitopes still express CD45RO.25
In line with their resting state, HIV-specific CD45RO−/RA+ CD8+ T cells were resistant to apoptosis. This was shown by the higher expression of the antiapoptotic marker Bcl-2 and of IL-7Rα (CD127) by the CD45RO−/RA+ subset than by the CD45RO+ subset. IL-7Rα (CD127) has been used to distinguish between short-lived effectors, which are negative, and effectors that differentiate into long-lived memory cells, which are positive. The latter contain high levels of antiapoptotic molecules, such as Bcl-2 and Bcl-XL, and are able to respond to homeostatic cytokines for survival and self-renewal. Our survival results are in line with these characteristics.33
Thus, HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells are quiescent, resistant to apoptosis, and possibly able to acquire a long-lived memory phenotype and to respond to homeostatic cytokines. This confirms the findings of Dunne et al25 in EBV infection but tends to conflict with the conclusion of Rufer et al26 that these cells are at a pre-effector stage. Our findings suggest that these cells may be considered as true resting long-lived memory cells.
Such resting cells should be able to react rapidly on reexposure to their cognate antigen, usually by expanding and acquiring effector functions.34 We therefore explored the capacity of HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells to respond to antigen in vitro.
Both the CD45RO−/RA+ and CD45RO+ subsets produced TNF-α and IFN-γ after short specific stimulation, whereas IL-2 production was negligible. No proliferation or differentiation occurred during this short stimulation, as suggested by the maintenance of CD45 isoform expression. These results confirm data from Rufer et al,26 who demonstrated that CCR7− CD27+ CD45RA+ CD8+ T cells from healthy donors express genes associated with effector functions, such as IFN-γ and TNF-α, in vitro, without stimulation. In addition, Precopio et al27 showed that vaccinia virus-specific CD8+ T cells obtained after effective vaccinia virus immunization expressed a particular phenotype (CD27+ CD45RA+ CCR7−) and that these cells exhibited polyfunctional responses with IFN-γ, TNF-α, MIP-1β, and IL-2 production.
Both the CD45RO−/RA+ and CD45RO+ subsets were able to expand after specific stimulation, although the capacity of the CD45RO−/RA+ subset was slightly higher. The strong proliferative potential of the CD45RO−/RA+ subset has also been described in EBV infection25 and in the case of CD45RA+ antitumoral CTL clones.28 This potential may be the result of telomeric reconstitution by telomerase.35 Indeed, several studies have shown that the telomeres of CD27+ CD45RA+ CD8+ T cells are longer than those of their CD45RO+ counterparts but shorter than those of naive T cells.25,26
The CD45RO−/RA+ subset has little, if any, spontaneous cytotoxic activity. This is suggested by gene analysis of CD27+ CD45RA+ CD8+ T cells in healthy donors. Indeed, these cells contain few, if any, perforin and granzyme B transcripts ex vivo.36 We confirmed their low cytotoxic capacity, as suggested by the low numbers of HIV-specific CD8+ T cells expressing perforin, the weak intensity of this expression, and the moderate numbers of cells able to produce TNF-α and IFN-γ, which has been proposed as a surrogate marker of cytotoxicity. However, these cells may acquire cytotoxic potential on exposure to antigen, as suggested by the increased expression of perforin, which was significantly higher in the CD45RA+ isolated population. Similarly, Dunne et al25 observed strong lytic capacity in a standard chromium release assay after expansion of CD45RA+ EBV-specific CD8+ T cells. In the same way, CD45RA+ vaccinia virus-specific CD8+ T cells observed after immunization also show strong CD107a expression and high granzyme A, granzyme B, and perforin contents after stimulation.27
We found that isolated HIV-specific CD45RA+ T cells acquired the CD45RO+ phenotype after lengthy specific stimulation, confirming observations by Dunne et al25 in EBV infection and by Carrasco et al28 in antitumoral CD8+ T-cell clones. The molecular significance of the 2 isoforms of CD45 is unclear. The CD45 molecule is a transmembrane tyrosine phosphatase generated by alternative splicing of exons A, B, and C, leading to the expression of the short isoform CD45RO (the low-molecular-weight form contains none of the variable exons) or the long isoform CD45RA (the high-molecular-weight form contains exons A and B and/or C). CD45 regulates signaling through the TCR-CD3 complex. The smaller size of the CD45RO extracellular domain could facilitate dimerization, making this isoform less efficient in its support for TCR-CD3 signaling.37,38 The precise mechanisms regulating the switch between the 2 isoforms are unclear, but the Carrasco et al study of antitumoral CTL clones28 suggests that CD45RA expression by specific CD8+ T cells is related to the time elapsed in the absence of the antigen. The emergence of this subset during HAART-treated primary HIV infection would fit with this hypothesis.
Taken together, these results confirm that the CD27+ CD45RO−/RA+ CD8+ T-cell subset is resting, resistant to apoptosis, and able to survive and to react on antigen reexposure by marked expansion and acquisition of effector capacities, such as cytokine secretion and cytotoxic potential. These properties are in line with the functional definition of memory cells.39,40 Indeed, we have shown that the functional profile of CD45RO−/RA+ HIV-specific CD8+ T cells is close to that of EBV-specific CD8+ T cells, either in HIV-uninfected persons or in early HAART-treated HIV-infected persons.
Recent studies of HIV-specific CD8+ T cells in participants with different virologic status have highlighted the presence of HIV-specific CCR7− CD45RA+ CD8+ T cells in participants controlling viral replication, either during or after therapy.18,19 These cells were described as fully differentiated effectors, and it was suggested that they might play a role in the control of viremia. However, as already mentioned, a variety of markers are used to define T-cell subsets. In particular, the CCR7− CD45RA+ CD8+ T-cell subset is heterogeneous and includes the CCR7− CD45RA+ CD27− subset (clearly defined as terminally differentiated effector cells) and the less well-defined CCR7− CD45RA+ CD27+ subset that we describe here. Most of the aforementioned studies did not use the CD27 marker to differentiate between these subsets, and it is therefore difficult to attribute a precise role to either subset. Only Tussey et al41 have described the CD27+ CD45RA+ subset in HAART-treated participants, considering it as an intermediary between acute and memory subsets defined, respectively, as CD45RO+ CD28− CD27+ and CD45RO+ CD28+ CD27+, with gradual reexpression of CD28. Like Tussey et al,41 we observed an increase in the CD27+ CD45RO−/RA+ subset in treated patients, probably because of the reduced antigen burden. However, in contrast to this latter study, CD28 was not expressed in the subset that we describe. In addition, we did not observe an increase of the CD27+ CD45RO−/RA+ subset in patients in whom HAART was started later, after the acute primary infection. This may be related to a longer period with viremia, as Carrasco et al have reported that the timing of exposure/nonexposure to antigen may play a role in the CD45 isoform switch.28
HIV-specific CCR7− CD27+ CD45RO−/RA+ CD8+ T cells did not appear in untreated participants or in persons treated at a later stage of infection. Likewise, Sabbaj et al42 reported that HIV-specific CD8+ CD127+ T cells were only maintained in participants who received early HAART.43,44 Several reasons can be advanced to explain why this particular subset only emerges when viral replication is controlled early during PHI. As discussed, the timing of exposure/nonexposure to antigen may be involved in the increase in the CD45RO−/RA+ subset. Alternatively, broad-ranging immune activation occurs during PHI and includes not only HIV-specific CD8+ T cells but also a large variety of other virus-specific CD8+ T cells.31,32 This generalized, combined specific and bystander activation has been shown to be deleterious for the immune system45-47 as demonstrated by the poor proliferative capacity of viral-specific cells in untreated viremic patients, whether they are HIV- or EBV-specific. Therefore, an early and rapid deactivation of CD8+ T cells could allow the emergence of quiescent cells reprogrammed to differentiate into memory cells. Finally, CD27+ CD45RO−/RA+ specific CD8+ T cells may be considered as true memory cells. Emergence of such cells has been linked to optimal numbers and functions of both dendritic cells and viral-specific CD4+ T cells.48-50 Yet HIV-specific CD4+ T-cell responses are preserved in persons with early control of viral replication,30,51,52 and it has also been shown that early therapy improves the number and functional status of dendritic cells, which were diminished during PHI.53
A final question raised by our findings concerns the role of the HIV-specific CD27+ CD45RO−/RA+ CD8+ T-cell subset. In vitro, these cells present several characteristics reported to be beneficial in HIV infection. They are resting, and it is increasingly clear that immune activation is deleterious.45-47 They are able to react to antigenic stimulation by expanding and acquiring several functions, and polyfunctionality is a major characteristic of efficient T cells.24,27,54 However, they produce only very small amounts of IL-2, a cytokine that appears to be essential.55,56 It has been found that HIV-specific CD8+ T cells with the CCR7− CD45RA+ phenotype probably emerge in persons who control viral replication after structured treatment interruption.19 However, the latter study did not examine whether these cells were CD27− (and thus probably effectors) or CD27+ (and thus probably memory cells). In the present study, only 3 participants interrupted their therapy, and all had a viral rebound. However, they were treated for rather short periods, and longer treatment might combine the benefits of a better immune reconstitution and efficient depletion of the viral reservoir.
In conclusion, we observed the development of CD27+ CD45RO−/RA+ HIV-specific CD8+ T cells in participants treated early during the primary infection. This subset is stable, nonactivated, and resting, presents memory characteristics, and can develop effector capacities after stimulation. The possible role of this memory HIV-specific CD27+ CD45RO−/RA+ CD8+ T cells in the long-term control of HIV infection remains to be demonstrated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinicians and the patients from all of the participating centers of the French PRIMO cohort (Agence Nationale de Recherche sur le SIDA, CO6-PRIMO Cohort) for their efficient collaboration, David Young who edited the English text, and Christine Bourgeois for helpful discussions.
This work was supported in part by institutional grants from Inserm, Agence Nationale de Recherche sur le SIDA, and Sidaction. C.L. is supported by grants from Ministère de l'Enseignement Supérieur et de la Recherche and Sidaction.
Authorship
Contribution: M.S. and A.V. managed and designed the research and participated in writing the paper; C.D., C.G., and L.M. participated in data centralization and coordination of the French PRIMO cohort; I.G., A.U., and J.-M.D. participated in performing the research and analyzing data; and C.L. performed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Camille Lécuroux, Inserm U802, Laboratoire d'Immunologie antivirale Systémique et Cérébrale, Faculté de Médecine Paris-Sud, 63, rue Gabriel Péri, 94276 Le Kremlin-Bicêtre, France; e-mail: camille.lecuroux@u-psud.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal