Abstract
Human neutrophil Siglec-9 is a lectin that recognizes sialic acids (Sias) via an amino-terminal V-set Ig domain and possesses tyrosine-based inhibitory motifs in its cytoplasmic tail. We hypothesized that Siglec-9 recognizes host Sias as “self,” including in cis interactions with Sias on the neutrophil's own surface, thereby dampening unwanted neutrophil reactivity. Here we show that neutrophils presented with immobilized multimerized Siaα2-3Galβ1-4GlcNAc units engage them in trans via Siglec-9. The sialylated capsular polysaccharide of group B Streptococcus (GBS) also presents terminal Siaα2-3Galβ1-4GlcNAc units, and similarly engages neutrophil Siglec-9, dampening neutrophil responses in a Sia- and Siglec-9–dependent manner. Reduction in the neutrophil oxidative burst, diminished formation of neutrophil extracellular DNA traps, and increased bacterial survival are also facilitated by GBS sialylated capsular polysaccharide interactions with Siglec-9. Thus, GBS can impair neutrophil defense functions by coopting a host inhibitory receptor via sialoglycan molecular mimicry, a novel mechanism of bacterial immune evasion.
Introduction
Although antimicrobial properties of vertebrate innate immune cells are extensively studied, less is understood about mechanisms dampening inflammatory responses. Such negative regulatory systems can be subverted by microbes. Of relevant interest is the “molecular mimicry” of mammalian sialic acid (Sia)–terminated sialoglycans by microbes that are obligate commensals or potential pathogens of humans.1 Surface Sia expression can blunt alternative pathway complement activation and reduce immunogenicity.1 However, this may not fully explain convergent bacterial evolution of near-perfect mimicry of vertebrate sialoglycans. For example, the human-specific commensal/pathogen group B Streptococcus (GBS) has a capsular polysaccharide (CPS) that displays the structure Siaα2-3Galβ1-4GlcNAc,2 a sequence identical to one common at termini of human glycoproteins.
Sia-recognizing immunoglobulin superfamily lectins (Siglecs) are type I transmembrane proteins expressed on immune cells.3,4 The rapidly evolving subgroup of CD33-related Siglecs (CD33rSiglecs) are postulated (but not proven) to negatively regulate inflammatory responses by recognizing host sialoglycans.3 Many CD33rSiglecs have conserved cytoplasmic tyrosine-based motifs, comprising a membrane-proximal immunoreceptor tyrosine-based inhibitory motif (ITIM) and a membrane-distal ITIM-like motif.3,4 The wide expression of host Sias and the prominence of cognate ITIM-bearing CD33rSiglecs on immune cells suggest that they may function in “self”-recognition, dampening innate immune responses to prevent autoreactivity.3
The functional outcome of CD33rSiglec binding to sialylated ligands remains poorly understood. Cross-linking antibodies and/or Siglec transfection into Siglec-deficient cell lines has demonstrated the importance of the ITIM and ITIM-like motifs for inhibiting cellular activation and proliferation,5-12 and even inducing apoptosis.13,14 However, interpretation of such data is limited by the use of nonnative transfected cells and/or anti-Siglec antibodies that are unnatural ligands.
Although CD33r Siglecs recognize Sias on the same cell surface (so-called cis interactions),15 high densities of Sias on adjacent cell surfaces or multimerized polyvalent probes,16 heavily sialylated glycoproteins,17-19 or bacterial CPSs20 can engage Siglecs in trans, presumably outcompeting the cis-Sia ligands at contact sites.
Methods
Bacteria
GBS COH1, a highly encapsulated serotype III strain, was propagated in Todd-Hewitt broth at 37°C to logarithmic growth phase.
Neutrophils
Normal human volunteers donated small blood samples for the isolation of neutrophils, with informed consent obtained in accordance with the Declaration of Helsinki, under protocols approved by the University of California, San Diego Human Subjects Institutional Review Board. Isolation was performed using Polymorphprep (Axis-Shield, Oslo, Norway). For prelabeling, neutrophils were resuspended in 500 μL of Hanks balanced salt solution (HBSS), Ca−, Mg−+/−calcein-AM (Invitrogen, Carlsbad, CA) for 30 minutes at 37°C, washed 3 times, and resuspended in 1.5 mL HBSS.
Neutrophil binding to glycans
Natural or synthetic glycans in carbonate buffer, pH 9.4, were added (100 μL) to 96-well Immulon 4HBX plates (Thermo Electron, Waltham, MA), incubated overnight at 4°C, washed 3 times, and blocked at room temperature with phosphate-buffered saline (PBS) plus 3% bovine serum albumin (BSA) for 1 hour. Labeled neutrophils were added, and initial fluorescence intensity (FI) was measured. After 30 minutes, nonadherent neutrophils were removed with PBS using a 12-well multichannel pipetter, and final FI was measured.
Antibody inhibition of Siglec-9
Neutrophils were incubated for 5 minutes in the presence or absence of the NBSAb (murine IgG1 clone E10-286; BD Biosciences Pharmingen, San Diego, CA) or BSAb (murine IgG2a clone 191240; R&D Systems, Minneapolis, MN) at 1:200 dilution and washed with HBSS plus calcium and magnesium plus 3% BSA before use in various assays.
Results and discussion
Siglec-9–dependent adherence of human neutrophils to immobilized α2-3–linked Sias
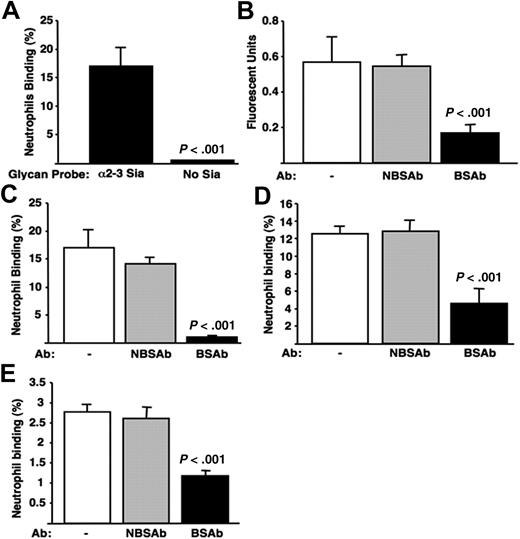
Neutrophils showed specific binding to polyacrylamide arrays bearing multiple copies of Siaα2-3Galβ1-4GlcNAcβ1, even without prior removal of competing cell surface Sias (Figure 1A). Siglec-9 is the dominant CD33rSiglec on neutrophils that recognizes α2-3–linked Sias and could be involved in binding. To study this, we validated anti–Siglec-9 IgG mouse monoclonal antibodies reacting with the Sia-binding site (BSAb) or not reacting with the Sia-binding site (NBSAb) as functional reagents, using competition assays with Siglec-9-Fc chimeric protein. The BSAb, but not the NBSAb, inhibited Siglec-9-Fc binding to Siaα2-3Galβ1-4GlcNAcβ1–coated wells (Figure 1B). The 2 antibodies are thus validated as functional reagents to dissect differences between blocking or not blocking the Sia-binding site. The BSAb also selectively reduced neutrophil interactions with Sia-containing glycan probes (Figure 1C), confirming that this CD33rSiglec can mediate adhesive interactions with sialoglycans in trans, without removal of competing cis Sias. The high density of sialoglycans and/or their arrangement allows them to defeat competition by cell surface sialoglycans.
Human neutrophil cell surface Siglec-9 interacts with sialic acids presented in trans. (A) Neutrophils bind to immobilized α2-3–linked Sias. Wells were coated with polyacrylamide arrays carrying multiple copies of Siaα2-3Galβ1-4GlcNAc or Galβ1-4GlcNAc (100 μL of 1 mg/mL stock diluted 1:100; GlycoTech, Gaithersburg, MD). Fluorescently labeled neutrophils were added, allowed to adhere, and unbound cells then washed away. The percentage of adherent neutrophils was determined by dividing the final FI by initial FI and multiplying by 100. (B) Validation of IgG anti–Siglec-9 Sia binding site (BSAb) and Sia nonbinding site (NBSAb) antibodies as functional reagents; 96-well plates were coated with 100 μL protein A in carbonate buffer (2 μg/mL) overnight at 4°C. Recombinant soluble Siglec-9-Fc chimera was then added in 100 μL enzyme-linked immunosorbent assay (ELISA) buffer (2 μg/mL) overnight at 4°C. The wells were washed, pretreated with BSAb, NBSAb, or PBS for 30 minutes at room temperature, and then washed 3 times with ELISA buffer. Wells were then incubated with biotinylated Siaα2-3Galβ1-4GlcNAc polyacrylamide array probes for 1 hour at room temperature, washed 3 times, incubated with streptavidin-alkaline phosphatase diluted 1:1000 in ELISA buffer, and washed again, and wells were developed with 100 μL substrate/well. (C) Neutrophil binding to immobilized α2-3–linked sialic acids requires Siglec-9. Neutrophils were incubated with BSAb, NBSAb, or PBS before carrying out the binding assay, as described in panel A. (D) Neutrophils bind to the α2-3–linked Sias of the GBS capsular polysaccharide via Siglec-9. Cell wall extracts were isolated from GBS strain COH1 using mutanolysin (Sigma-Aldrich, St Louis, MO) digestion as described.23 Further purification of CPS from cell wall preparations was performed using ion exchange and size exclusion chromatography as described.2 The purified CPS was deposited on microtiter wells as in panel A. Neutrophils pretreated with the BSAb or NBSAb were added and allowed to adhere before washing away nonadherent cells. (E) Neutrophils bind to GBS cell surface extracts via Siglec-9. The unpurified type III GBS (COH1) cell surface extract, which includes the sialylated CPS, was immobilized in ELISA wells and studied for binding of neutrophils pretreated with the BSAb or NBSAb as in panel A. All results are representative of at least 3 experiments performed in triplicate. Neutrophil viability was approximately 85% at the end of the 30-minute time period of the assays, and the antibodies had no major effects on viability or activation (data not shown). Error bars represent SEM.
Human neutrophil cell surface Siglec-9 interacts with sialic acids presented in trans. (A) Neutrophils bind to immobilized α2-3–linked Sias. Wells were coated with polyacrylamide arrays carrying multiple copies of Siaα2-3Galβ1-4GlcNAc or Galβ1-4GlcNAc (100 μL of 1 mg/mL stock diluted 1:100; GlycoTech, Gaithersburg, MD). Fluorescently labeled neutrophils were added, allowed to adhere, and unbound cells then washed away. The percentage of adherent neutrophils was determined by dividing the final FI by initial FI and multiplying by 100. (B) Validation of IgG anti–Siglec-9 Sia binding site (BSAb) and Sia nonbinding site (NBSAb) antibodies as functional reagents; 96-well plates were coated with 100 μL protein A in carbonate buffer (2 μg/mL) overnight at 4°C. Recombinant soluble Siglec-9-Fc chimera was then added in 100 μL enzyme-linked immunosorbent assay (ELISA) buffer (2 μg/mL) overnight at 4°C. The wells were washed, pretreated with BSAb, NBSAb, or PBS for 30 minutes at room temperature, and then washed 3 times with ELISA buffer. Wells were then incubated with biotinylated Siaα2-3Galβ1-4GlcNAc polyacrylamide array probes for 1 hour at room temperature, washed 3 times, incubated with streptavidin-alkaline phosphatase diluted 1:1000 in ELISA buffer, and washed again, and wells were developed with 100 μL substrate/well. (C) Neutrophil binding to immobilized α2-3–linked sialic acids requires Siglec-9. Neutrophils were incubated with BSAb, NBSAb, or PBS before carrying out the binding assay, as described in panel A. (D) Neutrophils bind to the α2-3–linked Sias of the GBS capsular polysaccharide via Siglec-9. Cell wall extracts were isolated from GBS strain COH1 using mutanolysin (Sigma-Aldrich, St Louis, MO) digestion as described.23 Further purification of CPS from cell wall preparations was performed using ion exchange and size exclusion chromatography as described.2 The purified CPS was deposited on microtiter wells as in panel A. Neutrophils pretreated with the BSAb or NBSAb were added and allowed to adhere before washing away nonadherent cells. (E) Neutrophils bind to GBS cell surface extracts via Siglec-9. The unpurified type III GBS (COH1) cell surface extract, which includes the sialylated CPS, was immobilized in ELISA wells and studied for binding of neutrophils pretreated with the BSAb or NBSAb as in panel A. All results are representative of at least 3 experiments performed in triplicate. Neutrophil viability was approximately 85% at the end of the 30-minute time period of the assays, and the antibodies had no major effects on viability or activation (data not shown). Error bars represent SEM.
Sialic acid–dependent engagement of Siglec-9 by GBS
The CPS of serotype III GBS presents terminal Siaα2-3Galβ1-4GlcNAc-glycans, a sequence similar to the synthetic glycan–coated wells and to native human cell surface glycans, which can interact with recombinant Siglec-9-Fc.20 To determine whether GBS serotype III CPS can engage human neutrophils via such molecular mimicry, we deposited purified CPS in microtiter wells and treated a subset with sialidase. Neutrophils adhered to native, but not desialylated, CPS (data not shown). Binding was diminished by the BSAb and not by NBSAb (Figure 1D). As with the immobilized synthetic sialoglycans, the dense Siaα2-3Galβ1-4GlcNAc presentation on the GBS serotype III CPS engaged Siglec-9 on neutrophils in trans. Surface extracts of type III GBS that include the GBS CPS in a more native context were isolated by mutanolysin cleavage of peptidoglycan backbones. Again, neutrophil binding to these extracts was Sia-dependent (not shown) and selectively blocked by the BSAb (Figure 1E), confirming Siglec-9 dependency.
GBS engagement of Siglec-9 attenuates human neutrophil responses
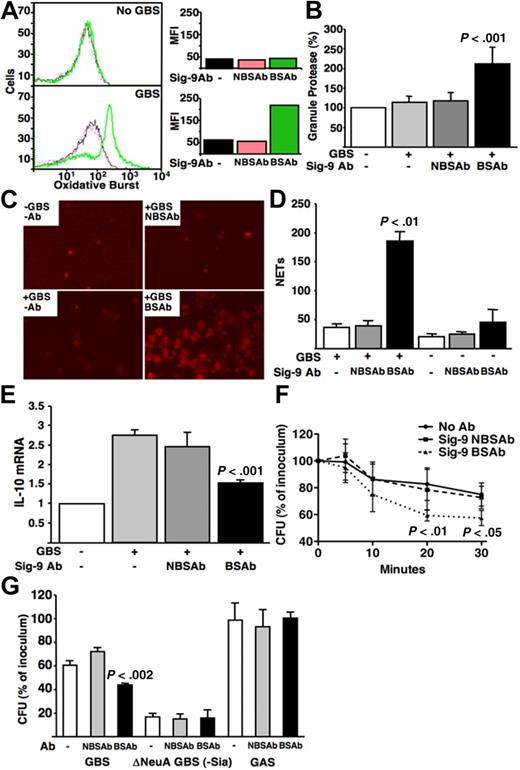
Neutrophils are specialized granulocytes that recognize and directly kill microorganisms. Because they interact with the sialylated serotype III GBS CPS in a Siglec-9–dependent manner, we tested the capacity of intact live GBS to alter functional responses. Neutrophils activated by bacterial recognition produce reactive oxidative species and release granule proteins and chromatin that form neutrophil extracellular traps (NETs), which ensnare and kill bacteria.21 Neutrophils interacting with live GBS in the presence of the BSAb produced stronger oxidative bursts and released more granule protease, compared with those in NBSAb (Figure 2A,B). Likewise, neutrophils stimulated by GBS generated more NETs in the presence of the BSAb compared with NBSAb (Figure 2C,D). Thus, Siglec-9 engagement by GBS CPS sialoglycans blunts key aspects of neutrophil activation in response to bacterial recognition.
GBS engagement of Siglec-9 attenuates human neutrophil immune functions. (A) GBS attenuates neutrophil oxidative burst via engagement of Siglec-9. BSAb- or NBSAb-treated neutrophils were labeled with dichlorofluorescin-diacetate at a final concentration of 20 μM and incubated for 20 minutes at 37°C. GBS COH1 WT was added at a multiplicity of infection (MOI) of 10 and incubated for 30 minutes at 37°C. Oxidative burst was measured using FACS analysis. MFI indicates mean fluorescence intensity. (B) GBS reduces neutrophil release of granule proteases by engagement of Siglec-9. Neutrophils were pretreated with BSAb or NBSAb (106 cells in 50 μL mixed with GBS COH1 bacteria at an MOI of 10 in triplicates) and incubated at 37°C for 30 minutes. Tubes were centrifuged at 1000g for 5 minutes and the supernatant collected into wells of a 96-well plate; 0.5 μL of MeOSuc-Ala-Ala-Pro-ValNmec dissolved in dimethyl sulfoxide at 20 mM was added to each well. After incubating at room temperature for 20 minutes, hydrolysis of the substrate was monitored spectrofluorometrically by change in absorbance at 405 nm. Baseline release of elastase by unstimulated neutrophils is set to 100%, and the graph shows a relative percentage release. (C,D) Production of NETs is attenuated GBS CPS engagement of Siglec-9. Antibody-treated neutrophils were incubated with bacteria at MOI 100 for 30 minutes at 37°C. NETs were stained using Cytox Orange and counted across the central transect of each well under the fluorescent microscope. The y-axis shows the number of NETs counted per transect of the wells. (E) Siglec-9 engagement by GBS blunts the up-regulation of mRNA expression of the inhibitory cytokine IL-10. GBS COH1 WT was added to antibody-pretreated neutrophils at an MOI 10 in a 6-well plate for 30 minutes at 37°C, and expression of IL-10 mRNA quantified with reverse-transcribed polymerase chain reaction primers forward (AACCTCCTCTCTGCCATCAA) and reverse (GGAAGACCCCTCCCAGATAG). (F) GBS engagement of Siglec-9 via its capsular Sia promotes resistance to neutrophil total killing. The y-axis shows percentage survival, ie, recovered colony-forming units (cfu) divided by inoculum cfu multiplied by 100. (G) GBS engagement of Siglec-9 via CPS Sia promotes resistance to neutrophil extracellular killing. Total and extracellular neutrophil killing assays were as described.24 Cytochalasin D was added to antibody-treated neutrophils and mixed with GBS COH1 WT, GBS COH1 deltaNeuA (Sia minus),25 or group A Streptococcus M1 strain at MOI 10, and incubated at 37°C. Part of each sample was plated at 5, 10, 20, and 30 minutes, and the surviving bacteria cfu counted the next day. The graph shows surviving cfu percentage at 30 minutes. Similar differences were seen at other time points.
GBS engagement of Siglec-9 attenuates human neutrophil immune functions. (A) GBS attenuates neutrophil oxidative burst via engagement of Siglec-9. BSAb- or NBSAb-treated neutrophils were labeled with dichlorofluorescin-diacetate at a final concentration of 20 μM and incubated for 20 minutes at 37°C. GBS COH1 WT was added at a multiplicity of infection (MOI) of 10 and incubated for 30 minutes at 37°C. Oxidative burst was measured using FACS analysis. MFI indicates mean fluorescence intensity. (B) GBS reduces neutrophil release of granule proteases by engagement of Siglec-9. Neutrophils were pretreated with BSAb or NBSAb (106 cells in 50 μL mixed with GBS COH1 bacteria at an MOI of 10 in triplicates) and incubated at 37°C for 30 minutes. Tubes were centrifuged at 1000g for 5 minutes and the supernatant collected into wells of a 96-well plate; 0.5 μL of MeOSuc-Ala-Ala-Pro-ValNmec dissolved in dimethyl sulfoxide at 20 mM was added to each well. After incubating at room temperature for 20 minutes, hydrolysis of the substrate was monitored spectrofluorometrically by change in absorbance at 405 nm. Baseline release of elastase by unstimulated neutrophils is set to 100%, and the graph shows a relative percentage release. (C,D) Production of NETs is attenuated GBS CPS engagement of Siglec-9. Antibody-treated neutrophils were incubated with bacteria at MOI 100 for 30 minutes at 37°C. NETs were stained using Cytox Orange and counted across the central transect of each well under the fluorescent microscope. The y-axis shows the number of NETs counted per transect of the wells. (E) Siglec-9 engagement by GBS blunts the up-regulation of mRNA expression of the inhibitory cytokine IL-10. GBS COH1 WT was added to antibody-pretreated neutrophils at an MOI 10 in a 6-well plate for 30 minutes at 37°C, and expression of IL-10 mRNA quantified with reverse-transcribed polymerase chain reaction primers forward (AACCTCCTCTCTGCCATCAA) and reverse (GGAAGACCCCTCCCAGATAG). (F) GBS engagement of Siglec-9 via its capsular Sia promotes resistance to neutrophil total killing. The y-axis shows percentage survival, ie, recovered colony-forming units (cfu) divided by inoculum cfu multiplied by 100. (G) GBS engagement of Siglec-9 via CPS Sia promotes resistance to neutrophil extracellular killing. Total and extracellular neutrophil killing assays were as described.24 Cytochalasin D was added to antibody-treated neutrophils and mixed with GBS COH1 WT, GBS COH1 deltaNeuA (Sia minus),25 or group A Streptococcus M1 strain at MOI 10, and incubated at 37°C. Part of each sample was plated at 5, 10, 20, and 30 minutes, and the surviving bacteria cfu counted the next day. The graph shows surviving cfu percentage at 30 minutes. Similar differences were seen at other time points.
Besides releasing bactericidal factors, neutrophils also synthesize cytokines that amplify or dampen immune responses. Others showed that transfection of Siglec-9 into macrophage cell lines increases production of the anti-inflammatory cytokine interleukin-10 (IL-10).22 We found that neutrophils that cannot engage sialylated GBS via Siglec-9 resulting from the BSAb produced less IL-10 mRNA (Figure 2E). Thus, bacterial sialoglycans signal through neutrophil Siglec-9 to increase expression of an anti-inflammatory cytokine.
Thus, a novel functional consequence of GBS sialoglycan molecular mimicry is interaction with neutrophil Siglec-9, blocking these cells from responding normally. This could contribute to GBS pathogenicity by promoting evasion of neutrophil-based killing. Indeed, neutrophils kill more GBS on coincubation with BSAb compared with NBSAb (Figure 2F). The blocking effect of the BSAb is dependent on bacterial cell surface sialoglycan expression, as it does not occur with either Sia-negative GBS or another nonsialylated bacterium, group A Streptococcus (Figure 2G).
Taken together, the data demonstrate, for the first time, that microbial evasion of neutrophil responses can occur via engagement of a host inhibitory receptor by precise molecular mimicry of a host sialoglycan. This property of GBS may assist it during invasive blood-borne infections in susceptible persons, such as newborn infants, pregnant women, and the elderly.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by National Institutes of Health grants P01HL57345 (A.V.) and HD051796 (V.N.).
National Institutes of Health
Authorship
Contribution: A.F.C., S.U., and Y.-C.C. performed the research, prepared figures and legends, and read the paper; and A.F.C., A.L.L., V.N., and A.V. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ajit Varki, 9500 Gilman Dr, University of California, San Diego, La Jolla, CA 92093-0687; e-mail: a1varki@ucsd.edu.
References
Author notes
*A.F.C. and S.U. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal