Abstract
Dendritic cells (DCs) are known to produce C1q, the initiator of the classical complement pathway. We demonstrate that murine DCs deficient in C1q (C1qa−/−) are poorer than wild-type (WT) DCs at eliciting the proliferation and Th1 differentiation of antigen-specific T cells. These defects result from decreased production of IL-12p70 by C1qa−/− DCs and impaired expression of costimulatory molecules CD80 and CD86 in response to CD40 ligation. The defective production of IL-12p70 and the reduced expression of CD80 and CD86 by C1qa−/− DCs were specifically mediated via CD40 ligation, as normal levels of IL-12p70 and CD80/86 were observed after ligation of Toll-like receptors (TLRs) on C1qa−/− DCs. CD40 ligation on C1qa−/− DCs, but not TLR ligation, results in decreased phosphorylation of p38 and ERK1/2 kinases. A strong colocalization of CD40 and C1q was observed by confocal microscopy upon CD40 ligation (but not TLR ligation) on DCs. Furthermore, human DCs from 2 C1q-deficient patients were found to have impaired IL-12p70 production in response to CD40L stimulation. Our novel data suggest that C1q augments the production of IL-12p70 by mouse and human DCs after CD40 triggering and plays important roles in sustaining the maturation of DCs and guiding the activation of T cells.
Introduction
Successful induction of immune responses requires cross-talk between the innate and adaptive arms of the immune system. The complement system is an integral part of the effector arm of innate immunity, with prominent influence on adaptive immunity. The role of complement in humoral immunity is well characterized. It acts as a natural adjuvant, lowering the threshold for B-cell activation and favoring the maintenance of B-cell tolerance. Interestingly, recent research suggests that the complement system may also be involved in the regulation of T-cell responses.1,2
Effective T-cell responses require interaction of T cells with antigen-presenting cells (APCs). Dendritic cells (DCs) are professional APCs that specialize in antigen capture and T-cell priming. DCs express a large repertoire of complement receptors on their surface3 and are the source of many complement components. The first component of the classical complement pathway, C1q, is primarily produced by DCs and macrophages, whereas most other complement factors originate in the liver.4,5 C1q production can occur spontaneously from immature DCs as well as after Toll-like receptor (TLR) ligation.6-8 Many endogenous proteins secreted by DCs in response to inflammatory signals (eg, proinflammatory cytokines and high mobility group box 1 protein, HMGB1) influence DC maturation and subsequent T-cell responses.9 It is therefore not unlikely that complement components locally synthesized by DCs may exert similar modulatory roles on the interaction between DCs and T cells.
C1q is a unique molecule with multiple functions. On the one hand, C1q performs proinflammatory functions as a recognition molecule of innate immunity, activates complement cascade, and mediates rapid uptake of pathogens.10-13 On the other hand, C1q deficiency in humans and mice is associated with inflammation and autoimmune features similar to systemic lupus erythematosus (SLE),14,15 indicating that C1q may also have immunoregulatory roles. This dual behavior of C1q is thus not unlike the DC itself, a cell that is actively involved in the generation of effector T cells but that also controls the generation of regulatory T cells that suppress immune responses.16
The interaction between exogenous C1q and DCs has been previously explored. Addition of exogenous C1q has been shown to influence the maturation of human DCs and alter functions such as cytokine production and allogenic T-cell responses.7,17 However, the role of endogenous C1q derived from DCs in the regulation of antigen-specific T-cell responses has not been addressed. In addition, the mechanisms by which C1q influences the function of DCs and of antigen-specific T cells are yet to be understood. With this in view, we performed studies to determine whether C1q produced by DCs influences the activation and differentiation of antigen-specific T cells. To assess the effect of local production of C1q on the interaction between APCs and T cells, we used DCs deficient in C1q derived from C1q knockout (C1qa−/−) mice. We show that C1q functions primarily by modulating effects induced by CD40 ligation on DCs.
Methods
Murine DCs
Murine DCs were derived as previously described18 from bone marrow progenitors of C1qa−/− and WT mice matched for strain, age, and sex. The bone marrow cells were cultured for 5 to 7 days with 10 ng/mL recombinant murine GM-CSF (Invitrogen, Carlsbad, CA). In select experiments, DCs were generated using recombinant murine GM-CSF (Invitrogen) and 5 ng/mL IL-4 (R&D Systems, Minneapolis, MN). Splenic DCs were isolated by magnetic sorting for CD11c-positive cells (Miltenyi Biotec, Auburn, CA). DC phenotype was monitored by assessing the expression of CD40, CD80, CD86, and class II using fluorochrome-conjugated monoclonal antibodies (BD Biosciences Pharmingen, San Jose, CA) and flow cytometry. Maturation of DCs was induced by triggering TLRs with LPS (10-1000 ng/mL), lipoteichoic acid (1 μg/mL), CpG (1 μM), and Imidazoquinoline (Gardiquimod; 1 μg/mL; Invivogen, San Diego, CA). For CD40 ligation, purified antibodies against CD40 (4 μg/mL; Axxora, San Diego, CA) were added to DC cultures followed by overnight incubation. For intracellular staining, monensin was added for the last 8 hours of culture. The cells were then stained with APC-conjugated anti–mouse IL-12 antibody (BD Biosciences Pharmingen). CD40L-transfected and control fibroblasts (a kind gift from Patrick Hwu [M.D. Anderson Cancer Center, Houston, TX] and Caetano Reis e Sousa [Cancer Research UK, London, United Kingdom]) were irradiated and plated in 96-well plates overnight before addition of C1qa−/− and WT DCs. Where indicated, exogenous C1q (10-50 μg/mL) was added to the cultures. Supernatants were collected 24 to 48 hours later and frozen until analysis by enzyme-linked immunosorbent assay (ELISA).
Signaling
To evaluate activation of signaling pathways, DCs were treated with purified antibodies against CD40 and cells collected at various time points. The cells were fixed and stained with antibodies to phosphorylated ERK1/2 and p38 MAPK (BD Biosciences Pharmingen) as per the manufacturer's instructions. For Western blot analysis of p38 and ERK1/2 protein kinases, cells were collected at the specified time points and lysates prepared as previously described.9 Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes. Antibodies to phospho- and total p38 and ERK1/2 were from Cell Signaling Technology (Danvers, MA). Antibodies to β-actin were from Sigma-Aldrich (St Louis, MO). Detection was by enhanced chemiluminescence (ECL; Pierce, Rockford, IL).
In vivo assessment of IFN-γ production
Male splenocytes (10 × 106) were injected intraperitoneally into female WT and C1qa−/− mice. Ten days later, the mice were killed and spleen cell suspensions prepared. Assessment of intracellular cytokines was performed as previously described.19 In brief, the splenocytes (4 × 106/mL) from immunized female mice were cultured with irradiated B6 male splenocytes (4 × 106/mL), with hu-rIL-2 in 24-well plates. Seven days later, the viable activated T cells were restimulated with irradiated unpulsed or peptide-pulsed APCs (B6 female T cell–depleted splenocytes) in the presence of monensin. Cultured cells were stained with anti-CD4–PerCP and anti-CD8–PE, fixed, and permeabilized. Further staining was done with anti–IFN-γ–fluorescein isothiocyanate (FITC), anti–IL-2–APCs, or isotype-matched control antibodies followed by flow cytometric analysis. All animal studies were conducted under a project license (ppl 70/5946) approved by the United Kingdom Home Office.
Antigen presentation assays
WT and C1qa−/− DCs (2 × 106/mL) were incubated with apoptotic cells (4 × 106/mL) and plated out on 96-well plates in 1:2 serial dilutions. B9 cells (2 × 105/mL) or transgenic Marilyn CD4+ T cells (106/mL) were then added to the cultures for 24 (B9 cells) to 48 hours (Marilyn cells). Supernatants were collected and assessed for IFN-γ by ELISA. To assess T-cell proliferation, Marilyn T cells were labeled with CFSE before adding them to the DC cocultures. The T cells were collected after 3 days and analyzed using flow cytometry for the dilution of CFSE. The HY peptide–specific CD4+ T-cell clones (B9) and Marilyn cells were obtained using protocols previously described.19,20 The HYAbDby peptide NAGFNSNRANSSRSS (10 nm to 0.1 nM) and C1q (25 μg/mL) were added when indicated.
Confocal microscopy
DCs were incubated with various concentrations of C1q (5-100 μg/mL) for 30 minutes at 4°C. C1q binding was detected by staining with FITC-conjugated anti-C1q antibody (DAKO, Carpinteria, CA) and flow cytometry. DC viability was assessed using annexin V and propidium iodide (PI). For confocal microscopy, DCs were incubated with C1q (50 μg/mL) for 30 minutes at 4°C. Cells were then stained with FITC-conjugated anti-C1q antibodies followed by incubation with biotinylated anti-CD40 antibodies (BD Biosciences Pharmingen) and streptavidin–Alexa Fluor 633 conjugate (Invitrogen). Cells were washed and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and adhered to poly-lysinated glass slides. Nuclei were counterstained with Hoechst 33342 (Molecular Probes/Invitrogen, Eugene, OR). Images were visualized using a Leica TCS SP2 confocal microscope equipped with a 63x/1.4 NA oil-immersion objective lens (Leica, Heidelberg, Germany). Leica Confocal Software (LCS) was used to acquire images, and Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA) was used to process them.
Statistical analysis
Statistical analysis was performed using the 2-tailed Student t test for unpaired samples with unequal variance. P values less than .05 were considered statistically significant.
Results
Injection of male splenocytes in C1qa−/− mice elicits lower percentage of IFN-γ–producing T cells compared with WT mice
We have previously reported that intraperitoneal injection of KLH in C1q-deficient mice resulted in decreased IFN-γ production from antigen-specific T cells compared with WT mice.21 Here, we evaluated whether this phenomenon was true also for cell-associated antigens. WT and C1qa−/− female mice were immunized by intraperitoneal injection with male splenocytes (10 × 106). The mice were killed 10 days later and spleen cells were restimulated in vitro for 7 days with irradiated male splenocytes. The percentage of CD4+ T cells producing IFN-γ or IL-2 was quantified after further restimulation with APCs loaded with class II–restricted HYAbDby peptide. Interestingly, the percentage of peptide-specific CD4+ T cells producing IFN-γ or IL-2 was higher in WT animals compared with the C1qa−/− mice (Figure 1A,B). Similar experiments were performed to assess the response of CD8+ T cells after restimulation with APCs loaded with the class I–restricted peptide (HYDbUty, WMHHNMDLI). CD8+ T cells from WT mice also responded with a higher IFN-γ production compared with C1qa−/− mice (Figure 1C). IL-2 was not detected in the CD8+ T cells (not shown).
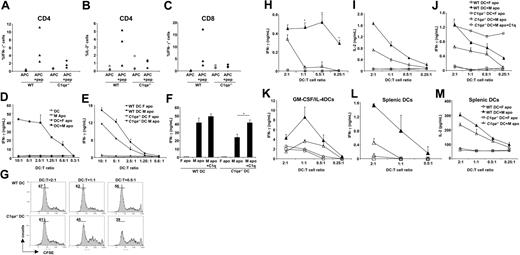
C1q is required for optimal IFN-γ production by HY-specific CD4+ T cells upon activation by DCs pulsed with male apoptotic cells. (A-C) WT and C1qa−/− mice immunized with intraperitoneal male splenocytes were killed and splenic cells restimulated in vitro for 7 days with irradiated male splenocytes. IFN-γ and IL-2 were detected by intracellular staining after another round of restimulation with peptide-pulsed (APC + pep) or unpulsed (APC) female APCs. (D) Immature DCs alone (DC) or pulsed with female/male apoptotic cells (DC + F apo and DC + M apo, respectively) were cultured with HY-specific CD4+ T-cell clone (B9 cells) for 48 hours and supernatants tested for IFN-γ. Male apoptotic cells (M apo) cultured with B9 cells in the absence of DCs were used as control. (E) B9 cells were cultured with WT or C1qa−/− DCs pulsed with male or female apoptotic cells and production of IFN-γ was evaluated by ELISA. (F) B9 cells were cultured with WT or C1qa−/− DCs pulsed with male or female apoptotic cells as described for panel E. C1q (25 μg/mL) was added to the coculture as indicated. (G) Marilyn T cells were stained with CFSE and cocultured with DCs (WT and C1q−/−) pulsed with male apoptotic cells for 72 hours. T-cell proliferation was assessed by CFSE dilution using flow cytometry. (H,I) WT and C1qa−/− DCs pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) were cultured with Marilyn cells for 48 hours and supernatants tested for IFN-γ (H) and IL-2 (I). (J) Marilyn T cells were cultured with WT or C1qa−/− DCs pulsed with male or female apoptotic cells. C1q (25 μg/mL) was added to the coculture when indicated. (K) WT and C1qa−/− DCs generated using GM-CSF and IL-4 were pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) and were cultured with Marilyn T cells for 48 hours. IFN-γ was measured in the supernatants. One representative experiment (mean ± SD of duplicate samples) of at least 3 independent experiments performed with cells from different mice is shown. (L,M) WT and C1qa−/− splenic DCs pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) were cultured with Marilyn cells for 48 hours and supernatants tested for IFN-γ and IL-2. *P < .05, statistically different from control.
C1q is required for optimal IFN-γ production by HY-specific CD4+ T cells upon activation by DCs pulsed with male apoptotic cells. (A-C) WT and C1qa−/− mice immunized with intraperitoneal male splenocytes were killed and splenic cells restimulated in vitro for 7 days with irradiated male splenocytes. IFN-γ and IL-2 were detected by intracellular staining after another round of restimulation with peptide-pulsed (APC + pep) or unpulsed (APC) female APCs. (D) Immature DCs alone (DC) or pulsed with female/male apoptotic cells (DC + F apo and DC + M apo, respectively) were cultured with HY-specific CD4+ T-cell clone (B9 cells) for 48 hours and supernatants tested for IFN-γ. Male apoptotic cells (M apo) cultured with B9 cells in the absence of DCs were used as control. (E) B9 cells were cultured with WT or C1qa−/− DCs pulsed with male or female apoptotic cells and production of IFN-γ was evaluated by ELISA. (F) B9 cells were cultured with WT or C1qa−/− DCs pulsed with male or female apoptotic cells as described for panel E. C1q (25 μg/mL) was added to the coculture as indicated. (G) Marilyn T cells were stained with CFSE and cocultured with DCs (WT and C1q−/−) pulsed with male apoptotic cells for 72 hours. T-cell proliferation was assessed by CFSE dilution using flow cytometry. (H,I) WT and C1qa−/− DCs pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) were cultured with Marilyn cells for 48 hours and supernatants tested for IFN-γ (H) and IL-2 (I). (J) Marilyn T cells were cultured with WT or C1qa−/− DCs pulsed with male or female apoptotic cells. C1q (25 μg/mL) was added to the coculture when indicated. (K) WT and C1qa−/− DCs generated using GM-CSF and IL-4 were pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) and were cultured with Marilyn T cells for 48 hours. IFN-γ was measured in the supernatants. One representative experiment (mean ± SD of duplicate samples) of at least 3 independent experiments performed with cells from different mice is shown. (L,M) WT and C1qa−/− splenic DCs pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) were cultured with Marilyn cells for 48 hours and supernatants tested for IFN-γ and IL-2. *P < .05, statistically different from control.
DCs present HY antigens derived from male apoptotic cells to antigen-specific CD4+ T-cell clone
HY antigens are male-specific minor histocompatibility (H) antigens encoded by genes on the Y chromosome.22 We assessed the presentation of HY antigen by DCs to a CD4 T-cell clone (B9) specific for an H2Ab-restricted epitope of the Dby gene.23 The B9 clone produces IFN-γ in response to class II–restricted antigen presentation either by female DCs pulsed with the HYAbDby peptide or by male bone marrow dendritic cells (BMDCs) (not shown). We evaluated whether DCs could also present HY antigens processed from male thymocytes rendered apoptotic by γ-irradiation. Female DCs fed with male apoptotic cells efficiently presented the HY antigen, inducing production of significant levels of IFN-γ by B9 cells (Figure 1D). This effect was antigen specific since female DCs cultured alone or fed with female apoptotic cells did not elicit any IFN-γ response from B9 cells. In addition, no IFN-γ was produced by B9 cells cultured with apoptotic male thymocytes alone, ruling out direct presentation of HY antigen by these apoptotic cells (Figure 1D).
C1q-deficient DCs fed with male apoptotic cells are poorer than WT DCs at eliciting IFN-γ from antigen-specific CD4+ T-cell clone
We then determined whether C1q influenced the presentation of antigen from apoptotic cells by DCs to T cells. Female C1qa−/− or WT DCs were cultured with male apoptotic cells and their ability to induce production of IFN-γ by B9 cells was evaluated. C1qa−/− DCs elicited lower levels of IFN-γ by B9 cells after presentation of apoptotic cell–associated HY antigen compared with WT DCs (Figure 1E). We then checked whether the ability of C1qa−/− DCs to elicit IFN-γ could be restored by exogenous C1q: indeed, this restored the production of IFN-γ elicited by C1qa−/− DCs to the levels observed with WT DCs (Figure 1F). Interestingly, addition of C1q had no significant effect on the ability of WT DCs to trigger IFN-γ from B9 cells after presentation of antigens from male apoptotic cells (Figure 1F).
C1qa−/− DCs are defective at eliciting production of IFN-γ from naive CD4+ T cells after presentation of antigens derived from apoptotic cells
We next evaluated whether DCs could present HY antigen derived from apoptotic cells to antigen-specific naive T cells. Naive CD4+ T cells were isolated from spleen and lymph nodes of mice expressing transgenic T-cell receptors specific for the same class II–restricted HY epitope as the B9 clone (Marilyn mice24 ). WT and C1qa−/− female DCs were incubated with male or female apoptotic thymocytes and then cocultured with naive transgenic CD4+ T cells labeled with CFSE. WT DCs incubated with male apoptotic cells induced robust proliferation of naive CD4+ T cells as well as IFN-γ production (Figure 1G,H). This effect was not observed with DCs that were exposed to female apoptotic thymocytes (Figure 1H and not shown). In keeping with our observations with the B9 cells (Figure 1E), C1qa−/− DCs were poorer at eliciting the proliferation of naive HY-specific T cells (Figure 1G) and elicited less IFN-γ from the CD4+ T cells compared with WT DCs (Figure 1H). In addition, the levels of IL-2 in the supernatants were lower after stimulation of T cells with C1qa−/− DCs compared with WT DCs (Figure 1I). Other cytokines such as IL-10 and IL-4 were not detected (not shown). The addition of exogenous C1q restored IFN-γ production elicited by C1qa−/− DCs to the level seen with WT DCs (Figure 1J). We next evaluated whether the differences observed between WT and C1qa−/− DCs were influence by the method used to generate the DCs. We tested WT and C1qa−/− DCs generated using GM-CSF and IL-4 (GM-CSF/IL-4 DCs) for their ability to elicit IFN-γ from CD4+ T cells in response to antigen derived from male apoptotic cells. In keeping with the results obtained using DCs generated with GM-CSF alone (GM-CSF DCs), the WT GM-CSF/IL-4 DCs pulsed with male apoptotic cells elicited more IFN-γ from Marilyn T cells than the C1qa−/− DCs (Figure 1K). We also performed antigen presentation assays with CD11c+ splenic DCs isolated from WT and C1qa−/− mice. Again, C1qa−/− splenic DCs pulsed with male apoptotic cells were poorer than WT splenic DCs in eliciting IFN-γ and IL-2 from Marilyn T cells (Figure 1L-M). These results demonstrate that the defect observed with C1qa−/− DCs is not restricted to the GM-CSF DCs alone, but extends to GM-CSF/IL-4 DCs as well as splenic CD11c+ DCs.
C1qa−/− and WT DCs display similar capacity to respond to TLR ligation and phagocytose apoptotic cells
We assessed whether lack of C1q resulted in any differences in expression of costimulatory and antigen-presenting molecules on DCs. Expression of CD86, CD80, and CD40 was equivalent in WT and C1qa−/− DCs (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, upon triggering TLR4 with lipopolysaccharide (LPS) the up-regulation of these markers was similar in the WT and C1qa−/− DCs. Similarly cell surface expression of class II molecules was equivalent in WT and C1qa−/− DCs (not shown). We also evaluated whether there was a difference in cytokine production after TLR ligation by C1qa−/− DCs. IL-12 p70 production by WT and C1qa−/− DCs was equivalent in response to TLR-4 and TLR-9 ligation (Figure S1B). This was true even when different doses of LPS were used to stimulate the cells. Moreover, no differences were observed in the production of other cytokines such as TNF-α, IL-10, and IL-6 in response to a panel of TLR ligands including LPS, lipoteichoic acid (LTA), gardiquimod, flagellin, and CpG (not shown).
C1q has been shown to increase uptake of apoptotic cells7,25 by human DCs. To explain the differences that we observed in the ability of C1qa−/− and WT DCs to stimulate T cells, we first checked whether C1qa−/− DCs displayed defects in the uptake of apoptotic cells. Under our experimental conditions, equivalent engulfment was noted for WT and C1qa−/− DCs cultured with apoptotic cells over various time intervals (Figure S1C). This observation ruled out a defect in phagocytosis of apoptotic cells as the cause of poorer induction of T-cell responses to apoptotic cell antigens by C1qa−/− DCs. The lack of effect of C1q on the phagocytosis of apoptotic cells by murine DCs might be attributed to intrinsic differences between murine and human DCs. Compensatory phagocytic mechanisms present in DCs from C1qa−/− mice might be another possible explanation. In addition, culturing immature murine DCs with apoptotic cells did not elicit any phenotypic changes or cytokine production from DCs (not shown).
C1qa−/− DCs are impaired at presenting the HY peptide to naive CD4+ T cells
Given the absence of a defect in the phagocytosis of apoptotic cells by C1qa−/− DCs, we next determined whether C1q influenced the presentation of cognate peptide to naive antigen-specific CD4+ T cells. WT and C1qa−/− DCs were pulsed with the class II–restricted HYAbDby peptide and cocultured with naive Marilyn CD4+ T cells. The CFSE dilution assay revealed good T-cell proliferation after stimulation with WT DCs pulsed with the peptide (Figure 2A top panels). In contrast, after activation by C1qa−/− DCs the proliferation of CD4+ naive T cells was reduced and lagged behind that induced by WT DCs (Figure 2A bottom panels). This observation was in keeping with the decreased ability of C1qa−/− DCs to trigger the proliferation of CD4+ naive T cells to antigens derived from apoptotic cells (Figure 1G). We also evaluated the production of IFN-γ by CD4+ naive T cells in response to DCs presenting the cognate HY peptide. The C1qa−/− DCs were found to be poorer than WT DCs at eliciting IFN-γ from T cells in this response (Figure 2B). This was also found to be the case when splenic CD11c+ DCs from C1qa−/− mice were compared with WT splenic DCs (Figure 2C). We next evaluated whether C1q was present in the cocultures of DC and T cells using ELISA. Significant levels of C1q were detected in the supernatants from cocultures of T cells with WT DCs, whereas no C1q was present when T cells were cultured with C1qa−/− DCs (Figure 2D). In keeping with previous reports that immature DCs secreted C1q,6-8 we detected C1q in cultures of DCs and T cells without the peptide. Interestingly, the presence of low amounts of peptide decreased the levels of C1q, and C1q levels then increased proportionately with increasing peptide concentrations.
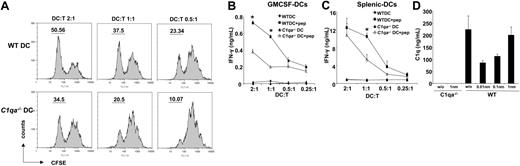
Peptide-pulsed C1qa−/− DCs are poorer at eliciting IFN-γ production from naive HY-specific CD4+ T (Marilyn) cells. (A) Marilyn T cells were stained with CFSE and cocultured with DCs (WT or C1qa−/−) pulsed with the HYAbDby peptide NAGFNSNRANSSRSS. T-cell proliferation was assessed by CFSE dilution using flow cytometry. (B) WT and C1qa−/− GM-CSF DCs pulsed with the peptide were cultured with Marilyn cells for 48 hours and supernatants tested for IFN-γ. (C) WT and C1qa−/− splenic DCs pulsed with the peptide were cultured with Marilyn T cells for 48 hours and supernatants tested for IFN-γ. (D) WT and C1qa−/− GM-CSF DCs pulsed with the peptide were cultured with Marilyn cells for 48 hours and supernatants tested for C1q by ELISA. One representative experiment (mean ± SD of duplicate samples) of at least 2 (splenic DCs) or 3 independent experiments performed with cells from different mice is shown. *P < .05, statistically different from control.
Peptide-pulsed C1qa−/− DCs are poorer at eliciting IFN-γ production from naive HY-specific CD4+ T (Marilyn) cells. (A) Marilyn T cells were stained with CFSE and cocultured with DCs (WT or C1qa−/−) pulsed with the HYAbDby peptide NAGFNSNRANSSRSS. T-cell proliferation was assessed by CFSE dilution using flow cytometry. (B) WT and C1qa−/− GM-CSF DCs pulsed with the peptide were cultured with Marilyn cells for 48 hours and supernatants tested for IFN-γ. (C) WT and C1qa−/− splenic DCs pulsed with the peptide were cultured with Marilyn T cells for 48 hours and supernatants tested for IFN-γ. (D) WT and C1qa−/− GM-CSF DCs pulsed with the peptide were cultured with Marilyn cells for 48 hours and supernatants tested for C1q by ELISA. One representative experiment (mean ± SD of duplicate samples) of at least 2 (splenic DCs) or 3 independent experiments performed with cells from different mice is shown. *P < .05, statistically different from control.
C1qa−/− DCs produce less IL-12p70 in response to CD40 ligation
These observations indicate that C1qa−/− DCs are defective in sustaining Th1-type polarization, irrespective of the source of antigen (apoptotic-cell associated or peptide). The efficient induction of Th1 effector T cells is dependent on the production of IL-12 by DCs. IL-12 production is sustained by triggering of CD40 on DCs by CD40L expressed on activated T cells.26,27 We therefore investigated the response of C1qa−/− DCs to ligation of CD40 molecules expressed on their surface using antibodies directed to CD40. WT DCs produced IL-12p40/p70 after CD40 ligation as detected by intracellular staining (Figure 3A), whereas C1qa−/− DCs produced significantly less (Figure 3A). We then performed experiments in which the ligation of CD40 on DCs was induced using fibroblasts transfected with CD40L.28 WT DCs stimulated with CD40L-expressing fibroblasts produced IL-12p70, whereas mock-transfected fibroblasts (SAMEN) had no effect (Figure 3B left panel). Again, C1qa−/− DCs produced significantly less IL-12p70 in response to CD40 ligation (Figure 3B middle panel). Furthermore, the defect in IL-12p70 production after CD40 ligation was reversed by addition of exogenous C1q and this reversal was dose dependent (Figure 3B middle panel). This impairment was restricted to CD40-CD40L interaction as WT and C1qa−/− DCs produced equivalent levels of IL-12 in response to TLR ligation with LPS or CpG (Figure S1B). The defect in IL-12p70 production was noted with GM-CSF/IL-4 C1qa−/− DCs as well (Figure 3B right panel).
C1q increases the production of IL-12p70 from DCs stimulated by CD40 ligation. (A) WT and C1qa−/− GM-CSF DCs were cultured alone or stimulated with antibodies against CD40 (4 μg/mL) for 16 hours. The production of the cytokine IL-12 was evaluated by intracellular staining and flow cytometry. Numbers in the square gates represent the percentage of IL-12–positive DCs. The bar graph on the right represents the mean (± SD) of 3 independent experiments (the response of the WT DCs to CD40 Ab is normalized to 100). (B) GM-CSF DCs were stimulated using CD40L-transfected or mock-transfected fibroblasts. IL-12p70 from DCs was induced only by the CD40L-transfected fibroblasts (left panel). WT and C1qa−/− GM-CSF DCs were cultured with CD40L-transfected fibroblasts and IL-12p70 was evaluated in the supernatants (middle panel). C1q (20-40 μg/mL) was added as indicated (middle panel). WT and C1qa−/− GM-CSF/IL-4 DCs were cultured with CD40L-transfected fibroblasts and IL-12p70 was evaluated in the supernatants (right panel). Results depicted are from 1 experiment of 3 similar ones performed with DCs from different mice (mean ± SD of duplicate samples). (C) WT or C1qa−/− GM-CSF DCs were cultured alone or in the presence of CD40Ab (4 μg/mL) for 48 hours and the expression of CD86 was evaluated by flow cytometry. The numbers adjacent to the rectangular gates represent the percentage of CD86+ cells. (D) DCs were cultured as described for panel C. The graphs display the percentage increase of CD86, CD80 (% positive cells), and class II (mean fluorescence intensity, MFI). Results represent mean (±SD) of at least 3 independent experiments performed with DCs from different mice. *P < .05, significantly different from control.
C1q increases the production of IL-12p70 from DCs stimulated by CD40 ligation. (A) WT and C1qa−/− GM-CSF DCs were cultured alone or stimulated with antibodies against CD40 (4 μg/mL) for 16 hours. The production of the cytokine IL-12 was evaluated by intracellular staining and flow cytometry. Numbers in the square gates represent the percentage of IL-12–positive DCs. The bar graph on the right represents the mean (± SD) of 3 independent experiments (the response of the WT DCs to CD40 Ab is normalized to 100). (B) GM-CSF DCs were stimulated using CD40L-transfected or mock-transfected fibroblasts. IL-12p70 from DCs was induced only by the CD40L-transfected fibroblasts (left panel). WT and C1qa−/− GM-CSF DCs were cultured with CD40L-transfected fibroblasts and IL-12p70 was evaluated in the supernatants (middle panel). C1q (20-40 μg/mL) was added as indicated (middle panel). WT and C1qa−/− GM-CSF/IL-4 DCs were cultured with CD40L-transfected fibroblasts and IL-12p70 was evaluated in the supernatants (right panel). Results depicted are from 1 experiment of 3 similar ones performed with DCs from different mice (mean ± SD of duplicate samples). (C) WT or C1qa−/− GM-CSF DCs were cultured alone or in the presence of CD40Ab (4 μg/mL) for 48 hours and the expression of CD86 was evaluated by flow cytometry. The numbers adjacent to the rectangular gates represent the percentage of CD86+ cells. (D) DCs were cultured as described for panel C. The graphs display the percentage increase of CD86, CD80 (% positive cells), and class II (mean fluorescence intensity, MFI). Results represent mean (±SD) of at least 3 independent experiments performed with DCs from different mice. *P < .05, significantly different from control.
In addition to IL-12 production, up-regulation of CD80 and CD86 molecules on DCs also occurs after ligation of CD40.29 Interestingly, the up-regulation of costimulatory molecules was significantly impaired in C1qa−/− DCs in comparison with WT DCs (Figure 3C,D) after stimulation with anti-CD40 antibodies. Again, this defect was specific for CD40 ligation, as stimulation with TLR ligands resulted in equivalent up-regulation of CD80 and CD86 by both C1qa−/− and WT DCs (Figure S1A and not shown). The up-regulation of class II molecules in C1qa−/− DCs was also decreased but did not reach statistical significance (Figure 3D).
CD40 ligation results in impaired phosphorylation of ERK-1/2 and p38 protein kinases in C1qa−/− DCs
CD40 engagement results in recruitment of membrane raft–associated Src family kinase Lyn, which initiates tyrosine phosphorylation of intracellular substrates.30 This triggers signaling events involving activation of MAP kinase ERK, which contributes to p38-dependent IL-12 production.30 Secretion of IL-12 induced by CD40 ligation on DCs has been demonstrated to be dependent on p38 MAP kinase activation.31 We therefore assessed the phosphorylation of ERK1/2 and p38 MAP kinases after CD40 ligation of WT and C1qa−/− DCs using flow cytometry. Phosphorylation of ERK1/2 and p38 was studied over a 30-minute period after DC stimulation (Figure 4A,B). Interestingly, the phosphorylation of both ERK1/2 and p38 in response to CD40 ligation was impaired in C1qa−/− DCs in comparison with WT cells (Figure 4A,B). This was in keeping with our observation that C1qa−/− DCs display defective production of IL-12 after CD40 ligation. Furthermore, this defect was specific to CD40 ligation as TLR4 ligation resulted in equivalent phosphorylation of ERK-1/2 and p38 in WT and C1qa−/− DCs (Figure 4A,B).
C1qa−/− DCs display impaired activation of MAP p38 and ERK1/2 protein kinase after CD40 ligation. GM-CSF DCs from WT and C1qa−/− mice were incubated alone or with CD40 antibodies (4 μg/mL) or LPS (10 ng/mL). (A) Intracellular staining with antibodies against phosphorylated ERK1/2 (P-ERK1/2) and p38 (P-p38) MAP kinases was evaluated at 10 minutes (LPS) and 30 minutes (CD40Ab) using flow cytometry. The gray histogram represents the phosphorylated form of the protein, whereas the black histogram represents staining with isotype-matched control antibodies. The numbers in the histograms represent the mean fluorescence intensity (MFI) of the antibody to the phosphorylated protein after subtraction of MFI of the isotype control antibody. (B) Phosphorylation of p38 (P-p38) and ERK1/2 (P-ERK1/2) MAP kinases at the indicated time points (0 to 30 minutes) from a different experiment is displayed. Results represent the MFI expressed as arbitrary units (au). Shown are results representative of 2 independent experiments performed with cells from different mice. (C) DCs were stimulated and lysates prepared at the indicated time points. Samples were analyzed by Western blotting using anti–phospho-p38 (P-p38), and anti–phospho-ERK1/2 (P-ERK). Total proteins were evaluated after stripping the membranes and reblotting with anti-p38 (p38), anti-ERK1/2 (ERK), and anti–β-actin (actin) antibodies. The numbers displayed above the bands represent densitometric quantification of the signals.
C1qa−/− DCs display impaired activation of MAP p38 and ERK1/2 protein kinase after CD40 ligation. GM-CSF DCs from WT and C1qa−/− mice were incubated alone or with CD40 antibodies (4 μg/mL) or LPS (10 ng/mL). (A) Intracellular staining with antibodies against phosphorylated ERK1/2 (P-ERK1/2) and p38 (P-p38) MAP kinases was evaluated at 10 minutes (LPS) and 30 minutes (CD40Ab) using flow cytometry. The gray histogram represents the phosphorylated form of the protein, whereas the black histogram represents staining with isotype-matched control antibodies. The numbers in the histograms represent the mean fluorescence intensity (MFI) of the antibody to the phosphorylated protein after subtraction of MFI of the isotype control antibody. (B) Phosphorylation of p38 (P-p38) and ERK1/2 (P-ERK1/2) MAP kinases at the indicated time points (0 to 30 minutes) from a different experiment is displayed. Results represent the MFI expressed as arbitrary units (au). Shown are results representative of 2 independent experiments performed with cells from different mice. (C) DCs were stimulated and lysates prepared at the indicated time points. Samples were analyzed by Western blotting using anti–phospho-p38 (P-p38), and anti–phospho-ERK1/2 (P-ERK). Total proteins were evaluated after stripping the membranes and reblotting with anti-p38 (p38), anti-ERK1/2 (ERK), and anti–β-actin (actin) antibodies. The numbers displayed above the bands represent densitometric quantification of the signals.
To confirm the results obtained by flow cytometry, we assessed the phosphorylation of ERK1/2 and p38 using Western blotting. WT and C1qa−/− DCs were incubated with CD40Ab or LPS for 15, 30, and 60 minutes and then evaluated for phosphorylated protein kinases. CD40 ligation induced p38 phosphorylation, which was higher in WT compared with C1qa−/− DCs, in keeping with the flow cytometry data (Figure 4C). Furthermore, ERK1/2 phosphorylation induced by CD40 ligation was higher in the WT cells at 15 minutes compared with C1qa−/− cells as seen in the flow cytometric assay (Figure 4C). In WT cells, the phosphorylated ERK1/2 decreased at 30 minutes and reappeared at the 60 minutes, whereas in C1qa−/− DCs the ERK1/2 phosphorylation took longer to decay and remained low at 60 minutes (Figure 4C). Notably, the phosphorylation of p38 and ERK1/2 after stimulation with LPS was not markedly different between WT and C1qa−/− DCs (Figure 4C), similar to the results obtained by flow cytometry. Total p38, ERK1/2, and β-actin showed no significant differences.
CD40 ligation induces colocalization of C1q and CD40 on the surface of DCs
Given the effects of C1q on DC function, we examined whether C1q could bind to the surface of DCs. C1q is known to bind to the surface of dying/dead cells.32 Using flow cytometry, we detected a strong binding of exogenous C1q to viable DCs (Figure 5A). This binding was dose dependent (not shown). Interestingly, viable T cells failed to bind C1q (Figure 5B), suggesting that effects of C1q on the interaction between DCs and T cells are mediated via the DC. We then evaluated the binding of C1q to DCs using confocal microscopy. C1q binding to both immature and LPS-stimulated DCs displayed a patchy pattern (Figure 5C and not shown). We next evaluated the expression of CD40 on DCs treated with LPS. CD40 was expressed diffusely on the surface of LPS treated DCs (Figure 5C), whereas immature DCs expressed negligible levels of CD40 (not shown). No colocalization of C1q and CD40 was noted on the surface of LPS-treated DCs (Figure 5C). We also cocultured DCs with Marilyn T cells in the presence of 10 nm HYAbDby peptide to trigger cognate CD40 ligation and evaluated the expression of CD40 and binding of exogenous C1q on DCs. CD40 expression by DCs increased dramatically after coculture with Marilyn T cells and peptide (Figure 5D). Interestingly, though C1q and CD40 did not colocalize on the DCs after LPS stimulation, both proteins were found to colocalize after ligation of CD40 (Figure 5D).
C1q colocalizes with CD40 after CD40 ligation on DCs. (A) GM-CSF DCs were incubated with exogenous C1q (50 μg/mL) and binding of C1q was detected by staining with FITC-conjugated anti-C1q antibodies (gray histogram) using flow cytometry. The black histogram represents control staining with the second step reagent only. (B) CD4+ T cells isolated from spleen and lymph nodes of WT mice were incubated with C1q (50 μg/mL) and bound C1q (gray histogram) was detected as described for panel A. The black profile represents control staining with the second step reagent only. (C) GM-CSF DCs were stimulated with LPS (10 ng/mL) for 48 hours to induce their maturation. The DCs were then incubated with C1q (50 μg/mL). Bound C1q was detected by staining with FITC-conjugated anti-C1q antibodies (green) and CD40 expression was detected using anti-CD40 biotinylated antibody and streptavidin-Alexafluor 633 (red). The nuclei were counterstained with Hoechst 33342 (blue) and analysis was done by confocal microscopy. Imaging was done using a Leica SP2 upright confocal microscope (Heidelberg, Germany). (D) GM-CSF DCs were loaded with HY peptide and then cocultured with Marilyn T cells for 48 hours to induce CD40 ligation on DCs. Then the DCs were incubated with C1q (50 μg/mL) and stained as described for panel C to analyze C1q binding by confocal microscopy. Results are representative of 2 independent experiments performed with DCs from different mice. (E) GM-CSF DCs or GM-CSF/IL-4 DCs were evaluated for the expression of calreticulin (CRT) by staining with anticalreticulin antibodies followed by FITC-conjugated secondary antibodies. (F) CRT expression was evaluated using confocal microscopy on DCs stained with anticalreticulin antibodies and FITC-conjugated secondary antibodies. (G) GM-CSF/IL-4 DCs were cultured alone or in the presence of LPS for 48 hours and CRT expression was assessed. (H) GM-CSF/IL-4 DCs were cocultured with Marilyn T cells in the presence or absence of peptide for 48 to 72 hours and stained with anti-CD11c and anticalreticulin antibodies. (I) DCs pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) were cultured with Marilyn T cells for 48 hours in the presence or absence of blocking anticalreticulin antibodies and supernatants tested for IL-2.
C1q colocalizes with CD40 after CD40 ligation on DCs. (A) GM-CSF DCs were incubated with exogenous C1q (50 μg/mL) and binding of C1q was detected by staining with FITC-conjugated anti-C1q antibodies (gray histogram) using flow cytometry. The black histogram represents control staining with the second step reagent only. (B) CD4+ T cells isolated from spleen and lymph nodes of WT mice were incubated with C1q (50 μg/mL) and bound C1q (gray histogram) was detected as described for panel A. The black profile represents control staining with the second step reagent only. (C) GM-CSF DCs were stimulated with LPS (10 ng/mL) for 48 hours to induce their maturation. The DCs were then incubated with C1q (50 μg/mL). Bound C1q was detected by staining with FITC-conjugated anti-C1q antibodies (green) and CD40 expression was detected using anti-CD40 biotinylated antibody and streptavidin-Alexafluor 633 (red). The nuclei were counterstained with Hoechst 33342 (blue) and analysis was done by confocal microscopy. Imaging was done using a Leica SP2 upright confocal microscope (Heidelberg, Germany). (D) GM-CSF DCs were loaded with HY peptide and then cocultured with Marilyn T cells for 48 hours to induce CD40 ligation on DCs. Then the DCs were incubated with C1q (50 μg/mL) and stained as described for panel C to analyze C1q binding by confocal microscopy. Results are representative of 2 independent experiments performed with DCs from different mice. (E) GM-CSF DCs or GM-CSF/IL-4 DCs were evaluated for the expression of calreticulin (CRT) by staining with anticalreticulin antibodies followed by FITC-conjugated secondary antibodies. (F) CRT expression was evaluated using confocal microscopy on DCs stained with anticalreticulin antibodies and FITC-conjugated secondary antibodies. (G) GM-CSF/IL-4 DCs were cultured alone or in the presence of LPS for 48 hours and CRT expression was assessed. (H) GM-CSF/IL-4 DCs were cocultured with Marilyn T cells in the presence or absence of peptide for 48 to 72 hours and stained with anti-CD11c and anticalreticulin antibodies. (I) DCs pulsed with male or female apoptotic thymocytes (DC + M apo and DC + F apo, respectively) were cultured with Marilyn T cells for 48 hours in the presence or absence of blocking anticalreticulin antibodies and supernatants tested for IL-2.
Calreticulin, a receptor for C1q, is expressed by dendritic cells
We next evaluated whether calreticulin (CRT), one of the receptors shown to bind C1q, is expressed by DCs using flow cytometry. Both GM-CSF and GM-CSF/IL-4 DCs expressed CRT (Figure 5E). A higher percentage of GM-CSF DCs expressed CRT compared with GM-CSF/IL-4 DCs. We also confirmed that DCs express CRT using confocal microscopy. Of note, CRT was distributed in a patchy fashion on the surface of DCs (Figure 5F), not unlike the pattern observed with C1q binding (Figure 5C,D). We then evaluated whether treatment of DCs with LPS alters the expression of CRT. Notably, LPS-treated DCs expressed lower levels of CRT compared with immature DCs (Figure 5G). We further assessed whether CRT expression by DCs is altered after CD40 ligation. Induction of CD40 ligation on DCs by culture with Marilyn T cells in the presence of HYAbDby peptide increased the percentage of CRT-expressing DCs compared with those cultured in the absence of the peptide (Figure 5H). Furthermore, blocking CRT using anti-CRT antibodies, when Marilyn T cells were challenged with DCs pulsed with male apoptotic cells, resulted in a marked decrease in IL-2 production (Figure 5I). Isotype control antibodies did not have any effect. These results suggest that CRT could be one of the receptors mediating the effects of C1q on DCs.
Human DCs deficient in C1q produce less IL-12p70 in response to CD40 ligation
Given our observations with C1q-deficient murine DCs, we then evaluated whether deficiency of C1q impacts on the CD40-CD40L pathway in human DCs. Human DCs were generated from peripheral blood monocytes of 2 C1q-deficient patients and healthy donors matched for age, sex, and race. Patient 1 was a 25-year-old female of South Asian descent who at the time of the investigation was not receiving any medication. Patient 2 was a 50-year-old white female on treatment with low doses of prednisolone (5 mg/day) and mycophenolate mofetil (500 mg/day). DCs derived from the 2 C1q-deficient patients expressed comparable levels of CD40, CD80, CD83, and CD86 to DCs from healthy donors (not shown). Furthermore, the up-regulation of these markers by DCs after stimulation with soluble CD40L or LPS was equivalent (not shown). In keeping with the results observed with murine DCs (Figure 3), IL-12 production by DCs in response to CD40 ligation was significantly impaired in both C1q-deficient donors (Figure 6A,B). Interestingly, the production of IL-12p70 by DCs in response to LPS was markedly impaired only in one patient (Figure 6B). Moreover, IL-10 secretion was not impaired in C1q-deficient human DCs upon stimulation with soluble CD40L (Figure 6A,B) or LPS (not shown).
Human DCs deficient in C1q have impaired production of IL-12p70 after CD40 ligation. (A,B) DCs from healthy donors (NDs 1 and 2) and C1q-deficient patients (C1q−/− PTs 1 and 2) were cultured alone or stimulated with soluble CD40L (1-2 μg/mL) or LPS (0.1-1 μg/mL) for 48 hours. IL-12p70 and IL-10 were evaluated in the supernatants by ELISA.
Human DCs deficient in C1q have impaired production of IL-12p70 after CD40 ligation. (A,B) DCs from healthy donors (NDs 1 and 2) and C1q-deficient patients (C1q−/− PTs 1 and 2) were cultured alone or stimulated with soluble CD40L (1-2 μg/mL) or LPS (0.1-1 μg/mL) for 48 hours. IL-12p70 and IL-10 were evaluated in the supernatants by ELISA.
Discussion
The current study clearly demonstrates that C1q is crucial to optimal generation of a Th1-type response characterized by proliferation and IFN-γ production. First, we showed that DCs from C1qa−/− mice have an impaired ability to stimulate the proliferation of major histocompatibility complex (MHC) class II–restricted antigen-specific T cells and to sustain their differentiation into Th1-type effectors. Second, we demonstrated that C1q boosts this differentiation by influencing the CD40-CD40L interaction between DCs and T cells. Third, we confirmed that human DCs deficient in C1q exhibit an impaired response to CD40 ligation.
The development of an effector T-cell response involves uptake of antigen by APCs such as DCs and presentation to naive T cells. The activation of T cells results in the expression of CD40L on their surface. The CD40L on T cells engages the CD40 molecules expressed by DCs. The CD40-CD40L interaction delivers signals that up-regulate the expression of costimulatory molecules such as CD80 and CD86 by DCs and sustains the production of inflammatory cytokines such as IL-12.27 Therefore, CD40 ligation on DCs increases their ability to prime CD4+ T cells.33 In addition, CD40-40L interaction delivers a retrograde signal into T cells that costimulate their proliferation and differentiation into Th1 effectors.34
Our results demonstrate that C1q is important for optimal IL-12p70 production after CD40 ligation on DCs. IL-12 production mediated via ligation of CD40 ligation involves the phosphorylation of p38 and ERK MAP kinases.30,31 In the absence of C1q, the phosphorylation of both these proteins was affected in DCs after CD40 ligation. However, in contrast, after TLR ligation IL-12 production and expression of costimulatory markers remained unchanged in murine C1qa−/− DCs. This suggests that CD40 ligation and the downstream signaling events form a very specific pathway influenced by C1q, whereas TLR-mediated responses of DCs are not affected by the absence of C1q. The colocalization of C1q and CD40 after ligation of CD40 on DCs by the CD40L on antigen-specific T cells lends further support to our hypothesis.
The confocal microscopy studies indicate that C1q does not bind directly to CD40 on the DCs as no colocalization was observed on LPS-stimulated DCs. Colocalization of C1q and CD40 occurred only after CD40 ligation. This suggests that after CD40 ligation, the CD40 molecules may cluster with C1q and its receptor to form a signaling complex (Figure 7). CRT is a well-characterized binding protein of C1q and is expressed on the surface of DCs (Zeng et al35 and Figure 5E). Moreover, CRT expression on DCs displays a patchy pattern (Figure 5F) similar to that observed with C1q. Interestingly, CRT was shown recently to be present on the surface of human neutrophils within lipid rafts in association with glycosylphosphatidylinositol (GPI)–anchored proteins.36 Thus, the site of aggregation of C1q/receptor complex and CD40 is likely to be the membrane rafts as CD40 signaling also occurs via proteins recruited to the membrane rafts.30 Of note, CD40 ligation on DCs induced by antigen-specific T cells increases the expression of CRT, whereas LPS has the opposite effect (Figure 5G,H). Moreover, blocking CRT decreases the production of IL-2 from antigen-specific T cells (Figure 5I). Together these observations suggest that CRT is at least one of the receptors mediating the effects of C1q on DCs.
C1q regulates CD40 pathway on DCs and drives the Th1 polarization of T cells. DCs secrete C1q, which binds to C1q receptors (C1qR) on the surface of the DC. When antigen-specific T cells recognize cognate antigen on DCs, it results in CD40 ligation on the DC by CD40L on activated T cells. This induces the up-regulation of CD40 expression on DCs and the colocalization of C1q with CD40 and formation of a signaling complex. Increased phosphorylation of p38 and ERK1/2 occurs, resulting in increased production of IL-12. This further sustains the IFN-γ production from T cells.
C1q regulates CD40 pathway on DCs and drives the Th1 polarization of T cells. DCs secrete C1q, which binds to C1q receptors (C1qR) on the surface of the DC. When antigen-specific T cells recognize cognate antigen on DCs, it results in CD40 ligation on the DC by CD40L on activated T cells. This induces the up-regulation of CD40 expression on DCs and the colocalization of C1q with CD40 and formation of a signaling complex. Increased phosphorylation of p38 and ERK1/2 occurs, resulting in increased production of IL-12. This further sustains the IFN-γ production from T cells.
C1q is not the only member of the complement family to influence T-cell activation by APCs. C5a and C3a locally produced by APCs and T cells have been recently shown to be involved in the T-cell activation process.37,38 Lack of C3 production by APCs also results in poor T-cell proliferation and IFN-γ production (Peng et al39 and P.B. and M.B., unpublished data). The ability of C1q to produce effects on DCs that are directly mediated by C1q receptors and not via the activation of classical complement pathway is still subject to an ongoing debate. DCs can generate several complement components including C1q, C3, C4b binding protein (C4BP), C7, and C8.40 In the absence of clear evidence of C1r and C1s production by DCs, it is probable that the effects of C1q are directly mediated via its receptors on DCs. Consistent with this, we were able to rectify all the defects observed in our experimental model by adding purified C1q. In addition, C1q-deficient DCs produce similar amounts of C3 as WT DCs (P.B. and M.B., unpublished data), providing further support to the idea of a specific role for C1q independent from complement activation.
Significantly, the effects of endogenous C1q on DC function are not limited to murine DCs. Production of IL-12p70 after CD40 stimulation was impaired in DCs derived from patients with C1q deficiency, whereas no impairment in production of IL-10 was noted, suggesting that C1q predominantly influences IL-12 production in DCs. Interestingly, in line with the results obtained with murine DCs, the second C1q-deficient patient exhibited a predominant defect in IL-12 secretion in response to CD40 stimulation and not to LPS. However, an involvement of C1q in TLR pathways in human DCs cannot be ruled out. Human DCs stimulated with LPS produce increased amounts of IL-12p70 in the presence of exogenous C1q.7,17 It is possible that exogenous and endogenous C1q can synergize with TLR signaling via unidentified pathways in human DCs.
Our present results demonstrate that C1q from DCs is required for optimum induction of IFN-γ–producing antigen-specific T cells in vitro and in vivo. Recently it has been demonstrated that IFN-γ, in addition to its classical role as the cytokine that drives Th1 cell–mediated immune responses, may also have anti-inflammatory or regulatory properties.41,42 Further evidence suggests that IFN-γ may also regulate the function of regulatory T cells. CD4+CD25+ regulatory T cells isolated from IFN-γ−/− mice or after in vivo neutralization of IFN-γ lose their suppressive function.43 The reduced IFN-γ production by T cells stimulated with C1q-deficient DCs may well translate into defective suppressor function and link to autoimmune phenomena observed in C1q-deficient mice and humans.
Our findings establish that C1q plays an important role in amplifying the signaling pathways triggered by CD40 ligation on DCs. This presumably occurs by the formation of a complex between CD40 and C1q and its receptor (Figure 7). The subsequent increased production of IL-12, a key cytokine for the induction of Th1 effectors, enables optimum generation of IFN-γ+ antigen-specific T cells. The link between CD40 triggering and C1q provides a novel mechanistic basis for explaining the effects of C1q on DC maturation and T-cell activation events.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff at the Biological Services Unit at our institution for the care of the animals involved in this study. We are very grateful to the C1q-deficient patients for their cooperation.
This work was supported by Wellcome Trust grant no. 071467 (London, United Kingdom).
Wellcome Trust
Authorship
Contribution: P.B. performed research, analyzed data, and wrote the paper; I.E.D. performed research and analyzed data; T.H.M. performed research; H.T.C. and J.D. designed research; D.S. contributed vital reagents; E.S. designed research and contributed vital reagents; and M.B. designed research and contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Botto, Molecular Genetics and Rheumatology Section, Faculty of Medicine, Imperial College London, Hammersmith Campus, Du Cane Rd, London W12 ONN, United Kingdom; e-mail: m.botto@imperial.ac.uk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal