Abstract
IL-25, a member of the IL-17 cytokine family, is known to enhance Th2-like responses associated with increased serum levels of IgE, IgG1, IgA, blood eosinophilia, and eosinophilic infiltrates in various tissues. However, IL-25 also abrogates inflammatory responses driven by Th17 cells. However, the cell types that respond to IL-25 and the mechanisms by which IL-25 differentially regulates immune reactions are not well explored. To identify potential targets of IL-25, we initially examined IL-25 receptor (IL-25R) in human peripheral blood cells. IL-25R was predominantly expressed by CD14+ cells. We next assessed the functional role of IL-25 in modulating the response of CD14+ cells to various inflammatory signals. CD14+ cells responded to IL-25 by down-regulating the synthesis of inflammatory cytokines induced by toll-like receptor (TLR) ligands and inflammatory cytokines. Inhibition of cytokine response by IL-25 occurred via a p38 Map kinase–driven Socs-3–dependent mechanism. In vivo, IL-25 inhibited monocyte-derived cytokines and protected against LPS-induced lethal endotoxemia in mice. These data indicate that IL-25 is a negative regulator of monocyte proinflammatory cytokine responses, which may have therapeutic implications.
Introduction
IL-25 (also known as IL-17E) is a recently described member of the IL-17 cytokine gene family. IL-25 is made by several cell types, including T lymphocytes, mast cells, eosinophils, and basophils.1,2 IL-25 can also be produced by lung epithelial cells and alveolar macrophages on allergen stimulation.3,4 Unlike other members of the IL-17 family, IL-25 facilitates pathogenic Th2 cell responses. Studies in mice have shown that transgenic expression of IL-25, or systemic administration of recombinant cytokine, increases the production of IL-4, IL-5, and IL-13. The induction of these cytokines is also associated with increased serum levels of IgE, IgG1, IgA, blood eosinophilia, and eosinophilic infiltrates in various tissues.4-7 However, studies in murine models of autoimmunity have shown that IL-25 can negatively regulate the development, amplification, or both of Th17-mediated disorders,8 thus suggesting that IL-25 may both trigger and abrogate specific inflammatory responses. However, the cell types that respond to IL-25 and the mechanisms by which IL-25 can differentially regulate immune reactions are not well known
IL-25 biologic activity is mediated by a transmembrane receptor (IL-25R), also called IL-17BR or IL-17R homolog 1, that is constitutively expressed in the liver, kidney, and intestine.9 Although T cells, and particularly Th2 memory cells, express high levels of IL-25R,10 a non–B-/non–T-cell population is also a major target of IL-25, because this cytokine can boost robust cytokine responses in recombinase-activating gene (RAG) knockout mice.5 Moreover, studies with RAG knockout splenocytes showed that the IL-25–responding cell is an accessory cell that expresses high levels of MHC class II and low levels of CD11c,5 raising the possibility that IL-25 may control the activity of antigen-presenting cells. In this study, we aimed at further characterizing the cellular targets of IL-25R. We show that IL-25R is highly expressed by human blood CD14+ cells, and that these cells respond to IL-25 by down-regulating the expression of inflammatory cytokines induced by toll-like receptor (TLR) ligands, such as lipopolysaccharide (LPS) and peptidoglycan (PGN) or inflammatory cytokines (ie, tumor necrosis factor α [TNF-α] and interferon γ [(IFN)-γ]). Inhibition of cytokine responses by IL-25 occurs via a p38 mitogen-activated protein (Map) kinase–driven suppressor of cytokine signaling 3 (Socs-3) induction-dependent mechanism. We also show that IL-25 inhibits inflammatory cytokines in vivo and protects against LPS-induced lethal endotoxemia. Overall, these data indicate that IL-25 is a negative regulator of CD14+ cell cytokine response and that IL-25 therapy may be useful for attenuating monocyte-mediated disorders.
Methods
Cell isolation and culture
All reagents were from Sigma-Aldrich (Milan, Italy) unless specified. Human peripheral blood mononuclear cells (PBMCs) were isolated from enriched buffy coats of healthy volunteer donors by Ficoll gradients and used to assess IL-25R. PBMCs were also used to purify CD14+ cells, either positively using CD14 magnetic beads (Miltenyi Biotec, Bologna, Italy) or negatively using CD3, CD20, and CD56 magnetic beads (Miltenyi Biotec). Cell purity was routinely evaluated by flow cytometry and ranged between 94% and 98%. An aliquot of CD14+ cells was used to extract RNA, and the remaining cells were resuspended in RPMI 1640 medium, supplemented with 10% inactivated fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL; Life Technologies-GibcoCRL, Milan, Italy), and seeded in 48-well culture dishes (1 × 106 cells/well). CD14+ cells were incubated with human recombinant IL-25 (10-100 ng/mL; R&D Systems, Minneapolis, MN) for 30 minutes and then stimulated with LPS (1-1000 ng/mL) or PGN (1-100 μg/mL), or TNF-α (1-10 ng/mL; R&D Systems), or IFN-γ (50 ng/mL; PeproTech, London, United Kingdom) for 30 minutes to 48 hours. Cells were then used to extract RNA or analyzed for cell-surface proteins by flow cytometry, and cell-free supernatants were analyzed by enzyme-linked immunoabsorbent assay (ELISA). To examine the effect of IL-25 on Map kinase, CD14+ cells were stimulated with IL-25 (50 ng/mL) for 5 to 30 minutes, then lysed, and total extracts were analyzed for the content of both phosphorylated and total Map kinases and Map kinase phosphatase 1 (MKP-1) by Western blotting. In parallel, cells were preincubated with IL-25 for 30 minutes and then stimulated with LPS/PGN for a further 15 to 30 minutes. To assess the role of Map kinases on IL-25–induced Socs-3 expression, cells were preincubated with PD98059 (50 μM), or SB202190 (10 μM), or 420116 (5 μM), or AG490 (100 μM; all from Inalco, Milan, Italy), or DMSO (vehicle) for 30 minutes before adding IL-25, or LPS/PGN, or both. Cells were also preincubated with SB202190 or DMSO, then treated with or without IL-25 (30 minutes) and finally stimulated with LPS/PGN for 6 hours. To examine the role of p38 and Socs-3 in the IL-25–mediated negative regulation of CD14+ cell cytokine response, CD14+ cells were cultured with or without human p38 and Socs-3 siRNA or control siRNA according to the manufacturer's instructions (Santa Cruz Biotechnology, Santa Cruz, CA). In brief, CD14+ cells were resuspended in RPMI 1640 containing 10% FBS and 1% penicillin/streptomycin, and seeded in 6-well culture dishes (3 × 106 cells/well). At 40% to 50% confluence, cells were washed with 2 mL transfection medium (sc-3686; Santa Cruz Biotechnology) and a mixture of 20 to 50 pmol siRNA and 6 μL transfection reagent (sc-29528; Santa Cruz Biotechnology), diluted in siRNA transfection medium (1 mL /sample), was added into each well. After incubation at 37°C for 7 hours, wells were added with another 1 mL of RPMI 1640 containing FBS and penicillin/streptomycin (2×). After a further 18 hours of incubation, the medium was replaced with fresh RPMI 1640 containing FBS and penicillin/streptomycin, and cultures were maintained for another 48 hours. At the end, cells were washed and cultured with or without IL-25 for 30 minutes and then stimulated with LPS or PGN for a further 2 to 6 hours. To evaluate the siRNA transfection efficiency and viability of transfected cells, CD14+ cells were transfected with a fluorescein-conjugate control siRNA, according to the manufacturer's instructions (Santa Cruz Biotechnology) for 16 to 48 hours. The percentage of fluorescein or propidium iodide (PI)–positive cells was then assessed by flow cytometry.
Flow cytometry
To characterize IL-25R expression in freshly obtained blood samples, total PBMCs, and purified blood CD14+ cells, the following monoclonal anti–human antibodies were used: CD56 FITC (1:10 final dilution; Diaclone Research, Milan, Italy), CD3 PerCP (1:50 final dilution; Becton Dickinson, Milan, Italy), CD14 FITC (1:50 final dilution; Immunotools, Friesoythe; Germany), CD16 FITC (1:50 final dilution, Immunotools), CD19 (1:50 final dilution, Immunotools), isotype control IgGs (Becton Dickinson), IL-25R PE (1:40 final dilution; R&D Systems; catalog no. FAB1207P). TLR-2 and TLR-4 were evaluated with the use of an anti–human TLR-4 PE or TLR2 PE antibody (both at 1:5 final dilution; eBioscience, San Diego, CA). To assess cell apoptosis, cells were cultured as indicated, and the percentage of annexin V (AV)– or PI-positive cells or both was then assessed by flow cytometry according to the manufacturer's instructions (Immunotech, Marseille, France).
Real-time polymerase chain reaction
Complementary DNA was amplified with the following conditions: denaturation 1 minute at 95°C; annealing 30 seconds at 58°C for human IL-8 and mouse TNF-α; at 60°C for human and mouse IL-6; at 62°C for human TNF-α, human IL-10, human IL-25R, human and murine β-actin; followed by 30 seconds of extension at 72°C. Primer sequence was as follows: human IL-6: FWD, 5′-CCACTCACCTCTTCAGAACG-3′, and REV, 5′-GCCTCTTTGCTGCTTTCACAC-3′; human IL-8: FWD, 5′-AGGAACCATCTCACTGTGTG-3′, and REV, 5′-CCACTCTCAATCACTCTCAG-3′; human IL-10: FWD, 5′-GGCACCCAGTCTGAGAACAG-3′, and REV, 5′-CTTGGCAACCCAGGTAACCC-3′; human TNF-α: FWD, 5′-AGGCGGTGCTTGTTCCTCAG-3′, and REV, 5′-GGCTACAGGCTTGTCACTCG-3′; human IL-25R: FWD, 5′-CCTCCGAGTAGAACCTGTTAC-3′, and REV, 5′-AGTTGCTTTTGCCCGTCACAC-3′; mouse IL-6: FWD, 5′-AGCCAGAGTCCTTCAGAGAG-3′, and REV, 5′-GATGGTCTTGGTCCTTAGCC-3′; mouse TNF-α: FWD, 5′-ACCCTCACACTCAGATCATC-3′, and REV, 5′-GAGTAGACAAGGTACAACCC-3′. Human IL-12/p40, human IL-12/p35, human IL-1β, and human Socs-1 and Socs-3 were evaluated with commercially available TaqMan probes (Applied Biosystems, Foster City, CA). β-Actin (FWD, 5′-AAGATGACCCAGATCATGTTTGAGACC-3′; and REV, 5′-AGCCAGTCCAGACGCAGGAT-3) was used as an internal control. Cytokine and IL-25R RNA expression was calculated relative to the housekeeping β-actin gene on the base of the ddCt algorithm.
Systemic administration of LPS, PGN, and IL-25
Balb/c mice were given intraperitoneal injections of murine recombinant IL-25 (R&D Systems; 10 μg/mouse) followed 1 hour later by intraperitoneal injection of LPS (300 μg/mouse) or PGN (300 μg/mouse). Serum samples were collected at 5 hours. Survival of mice was monitored over the next 7 days. CD11c+ cells were purified by splenocytes of Balb/c mice by positive magnetic-activated cell sorting (MACS) separation (Miltenyi Biotec) and cultured with or without murine recombinant IL-25 (50 ng/mL) for 30 minutes followed by PGN or LPS stimulation for 6 hours. At the end, cytokine RNA expression was evaluated by real-time polymerase chain reaction (PCR). This study received ethical approval for the use of mice from the University Tor Vergata of Rome.
Western blotting and ELISA
All primary antibodies were from Santa Cruz Biotechnology, unless specified. Cell extracts were prepared as previously described.11 The membranes were blocked with Tris-buffered saline containing 0.05% Tween 20 and 5% nonfat dry milk and then incubated, depending on the experiment, with the following antibodies: anti–p-ERK1/2, anti–total ERK1/2, anti–p-p38, anti–total p38, anti–p-JNK, anti–total JNK, anti–Socs-3 (all used at 1:500 final dilution) and anti–p-MKP-1 (1:1000 final dilution; Cell Signaling, DBA Italia, Milan, Italy). The anti–p-MKP-1 specifically recognizes phosphorylation of MKP-1 on serine 369 residue. Appropriate horseradish peroxidase–conjugated secondary antibodies (Dako, Milan, Italy) were then used, and bound antibodies were visualized with the use of enhanced chemiluminescence (Pierce, S.I.A.L., Rome, Italy).
IL-1β, TNF-α, IL-6 (all from R&D Systems), and IL-12p40 (PeproTech) were analyzed in cell-free culture supernatants of CD14+ cells preincubated with IL-25 (50 ng/mL) for 30 minutes and then stimulated with LPS (100 ng/mL) or PGN (10 μg/mL) for further 48 hours. IL-12p70, IL-6, TNF-α, IL-17A (all from R&D Systems), and IL-4 (PeproTech) were analyzed in serum samples from mice injected with LPS or PGN and treated with PBS or IL-25 by ELISA.
Data analysis
Differences between groups were compared with either the Mann-Whitney U test or the Student t test. The survival curves from experiments with systemic injection of LPS or IL-25 or both were analyzed by the Kaplan-Meier method, and Wilcoxon statistics were generated to test the homogeneity between treatment groups.
Results
IL-25R is highly expressed by human CD14+ cells
To identify cell targets of IL-25, we initially assessed the expression of IL-25R on human PBMCs by flow cytometry. Seventy-five percent of CD14+ cells expressed IL-25R (Figure 1A). IL-25R was detected in less than 25% of CD3+, CD19+, and CD56+ cells (Figure 1A). We also analyzed IL-25R expression in whole blood, and, again, showed that it was preferentially expressed by CD14+ cells (not shown), excluding the possibility that the cell separation affected IL-25R expression. Blood CD14+ cells that express high levels of CD16 can produce large amounts of inflammatory cytokines12 ; nearly all CD14+CD16+ cells expressed IL-25R (Figure 1B). There was no difference in the mean fluorescence intensity of IL-25R expression between CD14+CD16− cells and CD14+CD16+ cells (Figure 1C right inset). In line with the above findings, IL-25R RNA transcripts were seen in human blood CD14+ cells as well as in THP-1 cells, a human monocytic cell line (Figure 1C,D).
IL-25R is expressed by human blood CD14+ cells. (A) Representative dot-plots showing IL-25R in human PBMCs isolated from healthy volunteers and simultaneously analyzed for the expression of IL-25R, CD3, CD14, CD19, and CD56. Numbers in the selected areas indicate the percentages of CD3, CD14, CD19, and CD56 cells positive for IL-25R. One of 6 representative experiments in which similar results were obtained is shown. (B) CD16+ cells express IL-25R. CD14+ cells were purified from PBMCs and stained for IL-25R and CD16. One of 3 representative experiments is shown. Right inset shows the mean fluorescence intensity (MFI) of IL-25R expression in CD14+ cells either positive or negative for CD16. Data indicate mean (± SD) of 3 separate experiments. (C,D) Real-time PCR data for the membrane-bound IL-25R isoform RNA transcripts in blood CD14+ (CD14) cells (C) and THP-1 (D). Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. CD14+ and THP-1 experiments were performed 6 and 3 times, respectively.
IL-25R is expressed by human blood CD14+ cells. (A) Representative dot-plots showing IL-25R in human PBMCs isolated from healthy volunteers and simultaneously analyzed for the expression of IL-25R, CD3, CD14, CD19, and CD56. Numbers in the selected areas indicate the percentages of CD3, CD14, CD19, and CD56 cells positive for IL-25R. One of 6 representative experiments in which similar results were obtained is shown. (B) CD16+ cells express IL-25R. CD14+ cells were purified from PBMCs and stained for IL-25R and CD16. One of 3 representative experiments is shown. Right inset shows the mean fluorescence intensity (MFI) of IL-25R expression in CD14+ cells either positive or negative for CD16. Data indicate mean (± SD) of 3 separate experiments. (C,D) Real-time PCR data for the membrane-bound IL-25R isoform RNA transcripts in blood CD14+ (CD14) cells (C) and THP-1 (D). Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. CD14+ and THP-1 experiments were performed 6 and 3 times, respectively.
IL-25 inhibits the cytokine response of CD14+ cells after stimulation with TLR ligands and inflammatory cytokines
Because monocytes make a vigorous cytokine response to TLR ligands, we next assessed whether IL-25 regulated such a response. In initial time-course studies CD14+ cells were preincubated with 10, 50, and 100 ng/mL IL-25 for 30 minutes and then stimulated with LPS (10-1000 ng/mL) or PGN (5-100 μg/mL) for 4 to 10 hours. Analysis of TNF-α RNA transcripts showed that LPS and PGN dose-dependently enhanced TNF-α RNA expression and that a maximal effect was seen with 100 ng/mL LPS and 10 μg/mL PGN at 6 hours (not shown). At this time point, IL-25 inhibited LPS- and PGN-induced TNF-α expression in a dose-dependent fashion, and the maximal inhibition was seen with 50 ng/mL IL-25 (not shown). Therefore, these doses were used in the subsequent experiments. Next, we excluded that the isolation procedure used to obtain CD14+ cells activated cells and induced RNA expression associated with TLR-4 ligation. TNF-α RNA expression in blood monocytes isolated by positive selection was the same as in cells obtained by negative selection (not shown). Moreover, the isolation procedure did not affect the response of monocytes to LPS in terms of TNF-α RNA induction (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Figure 2 shows that IL-25 pretreatment markedly inhibited the expression of various proinflammatory cytokines, but not IL-10, after cell activation with LPS and PGN. Time-course studies with various doses of LPS and PGN showed that the stimulus used was not supramaximal for IL-10 RNA induction (not shown). Analysis of cytokines in the cell-culture supernatants confirmed that IL-25 significantly reduced the secretion of IL-12p40, IL-1β, TNF-α, and IL-6 after 48-hour stimulation with LPS or PGN (Figure 3).
IL-25 inhibits the expression of inflammatory cytokines induced by LPS and PGN in human blood CD14+ cells. CD14+ cells were isolated from 5 healthy volunteers and preincubated with medium or IL-25 (50 ng/mL) for 30 minutes then either left unstimulated (M = medium) or stimulated with LPS (100 ng/mL) or PGN (10 μg/mL) for a further 6 hours. RNA was extracted and amplified by real-time PCR. Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. LPS/PGN-treated cells versus LPS/PGN + IL-25–treated cells: *P = .02; **P = .04.
IL-25 inhibits the expression of inflammatory cytokines induced by LPS and PGN in human blood CD14+ cells. CD14+ cells were isolated from 5 healthy volunteers and preincubated with medium or IL-25 (50 ng/mL) for 30 minutes then either left unstimulated (M = medium) or stimulated with LPS (100 ng/mL) or PGN (10 μg/mL) for a further 6 hours. RNA was extracted and amplified by real-time PCR. Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. LPS/PGN-treated cells versus LPS/PGN + IL-25–treated cells: *P = .02; **P = .04.
IL-25 inhibits the LPS- and PGN-induced secretion of inflammatory cytokines. Blood CD14+ cells were isolated from 5 healthy volunteers and preincubated with medium or IL-25 (50 ng/mL) for 30 minutes then either left unstimulated (M = medium) or stimulated with LPS (100 ng/mL) or PGN (10 μg/mL) for a further 48 hours. Cytokine production was assessed by ELISA. LPS/PGN-treated cells versus LPS/PGN + IL-25–treated cells: *P < .01; **P = .03.
IL-25 inhibits the LPS- and PGN-induced secretion of inflammatory cytokines. Blood CD14+ cells were isolated from 5 healthy volunteers and preincubated with medium or IL-25 (50 ng/mL) for 30 minutes then either left unstimulated (M = medium) or stimulated with LPS (100 ng/mL) or PGN (10 μg/mL) for a further 48 hours. Cytokine production was assessed by ELISA. LPS/PGN-treated cells versus LPS/PGN + IL-25–treated cells: *P < .01; **P = .03.
Treatment of CD14+ cells with IL-25 did not affect either the percentage of TLR-2–positive cells or TLR-4–positive cells, or the average median fluorescence intensity of TLR expression, at early (ie, 6 hours; Figure S2) and late (ie, 48 hours, not shown) time points. In addition, IL-25 did not alter the fraction of AV/PI-positive cells (not shown).
Treatment of blood CD14+ cells with IL-25 also reduced IL-6, IL-8, and TNF-α transcripts after stimulation with TNF-α and IFN-γ (Figure S3), thus indicating that IL-25 renders CD14+ cells resistant to multiple inflammatory signals.
Inhibition of CD14+ cell cytokine response by IL-25 requires Socs-3
Socs-1 and Socs-3 are negative regulators of both TLR and cytokine-induced JAK-Stat signaling pathways.13 Socs-1 RNA expression was induced by LPS at 2 hours, and this effect was significantly enhanced by IL-25 (Figure S4). Socs-1 RNA expression was also enhanced by PGN at 1 and 2 hours, but no further increase was seen in cells stimulated with PGN and IL-25 (Figure S4). Stimulation of CD14+ cells with LPS also significantly increased Socs-3 RNA, and this effect was markedly augmented by IL-25 at 0.5 and 2 hours (Figure 4A). Socs-3 RNA expression was also induced at 0.5 hour after the addition of PGN, peaked at 1 hour, and diminished at 2 hours. The combination of IL-25 and PGN exerted a greater effect on the induction of Socs-3 RNA at 0.5 hour than in cells stimulated with PGN alone (Figure 4A). On the basis of these data, we considered that Socs-3 rather than Socs-1 could be involved in the IL-25–negative regulation of CD14+ cell responses to LPS and PGN. We thus assessed whether knocking down Socs-3 with siRNA could abolish the IL-25–mediated inhibitory effects on CD14+ cell cytokine response. To assess the transfection efficiency and viability of transfected cells, we initially transfected CD14+ cells with a fluorescent siRNA, and the percentages of both transfected and PI-positive cells were evaluated at different time points. The representative experiment shown in Figure 4B indicates that greater than 40% of cells were transfected at 48 hours. Notably, only a small fraction of transfected cells were PI-positive after siRNA transfection. Next, CD14+ cells were transfected with human Socs-3 or control siRNA. After 48 hours, cells were extensively washed, cultured in the presence or absence of IL-25 for 30 minutes, and then stimulated with LPS or PGN for a further 2 to 6 hours. Treatment of cells with Socs-3 siRNA led to a marked inhibition of Socs-3 RNA and protein (Figure 4C,D). Importantly, expression of TNF-α and of IL-6 induced by LPS and PGN was not decreased by IL-25 in Socs-3–deficient CD14+ cells (Figure 4E), thus indicating that Socs-3 is necessary for the IL-25–induced suppression of inflammatory cytokines.
The inhibitory effect of IL-25 on CD14+ cell cytokine response requires Socs-3. (A) Socs-3 RNA levels in response to IL-25 stimulation in blood CD14+ cells induced by LPS and PGN. CD14+ cells were preincubated with or without IL-25 (50 ng/mL) for 30 minutes then stimulated with LPS or PGN for the indicated time points. Data indicate the mean ± SD of 4 separate experiments. Untreated versus LPS-treated cells, §P = .04; untreated versus PGN-treated cells, §P = .03, §§P = .02; LPS-treated versus LPS + IL-25–treated cells, *P = .001; PGN-treated versus PGN + IL-25–treated cells, **P = .03. (B) Representative dot plots showing the percentages of PI-positive and fluorescent (FITC)–labeled siRNA-transfected CD14+ cells. Blood CD14+ cells were transfected with a fluorescent siRNA as indicated in “Methods,” and the percentages of PI-positive and FITC-labeled cells were then evaluated at the indicated time points by flow cytometry. One of 3 representative experiments is shown. (C,D) Silencing of Socs-3 expression in human blood CD14+ cells. Cells were cultured with control or Socs-3 siRNA. After 2 days, cells were washed and cultured with IL-25 for 30 minutes followed by stimulation with LPS for 2 hours. Socs-3 RNA expression was evaluated by real-time PCR (C). Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. *P = .001. (D) Representative Western blots showing Socs-3 and β-actin protein in extracts of cells cultured as indicated in panel C. One of 3 experiments in which similar results were obtained is shown. (E) IL-25 fails to inhibit the cytokine expression induced by LPS and PGN in Socs-3–deficient cells. Cells were transfected with control or Socs-3 siRNA. After 2 days, cells were washed and then stimulated with IL-25 for 30 minutes followed by LPS/PGN for a further 6 hours. Data indicate mean (± SD) of 5 separate experiments in which cells purified from 5 healthy donors were used. LPS/PGN-treated versus LPS/PGN + IL-25–treated cells, *P < .04.
The inhibitory effect of IL-25 on CD14+ cell cytokine response requires Socs-3. (A) Socs-3 RNA levels in response to IL-25 stimulation in blood CD14+ cells induced by LPS and PGN. CD14+ cells were preincubated with or without IL-25 (50 ng/mL) for 30 minutes then stimulated with LPS or PGN for the indicated time points. Data indicate the mean ± SD of 4 separate experiments. Untreated versus LPS-treated cells, §P = .04; untreated versus PGN-treated cells, §P = .03, §§P = .02; LPS-treated versus LPS + IL-25–treated cells, *P = .001; PGN-treated versus PGN + IL-25–treated cells, **P = .03. (B) Representative dot plots showing the percentages of PI-positive and fluorescent (FITC)–labeled siRNA-transfected CD14+ cells. Blood CD14+ cells were transfected with a fluorescent siRNA as indicated in “Methods,” and the percentages of PI-positive and FITC-labeled cells were then evaluated at the indicated time points by flow cytometry. One of 3 representative experiments is shown. (C,D) Silencing of Socs-3 expression in human blood CD14+ cells. Cells were cultured with control or Socs-3 siRNA. After 2 days, cells were washed and cultured with IL-25 for 30 minutes followed by stimulation with LPS for 2 hours. Socs-3 RNA expression was evaluated by real-time PCR (C). Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. *P = .001. (D) Representative Western blots showing Socs-3 and β-actin protein in extracts of cells cultured as indicated in panel C. One of 3 experiments in which similar results were obtained is shown. (E) IL-25 fails to inhibit the cytokine expression induced by LPS and PGN in Socs-3–deficient cells. Cells were transfected with control or Socs-3 siRNA. After 2 days, cells were washed and then stimulated with IL-25 for 30 minutes followed by LPS/PGN for a further 6 hours. Data indicate mean (± SD) of 5 separate experiments in which cells purified from 5 healthy donors were used. LPS/PGN-treated versus LPS/PGN + IL-25–treated cells, *P < .04.
Induction of Socs-3 by IL-25 depends on p38 MAPK
Socs-3 expression can be regulated by Map kinases in many different cell types.14,15 Because IL-25 can activate Map kinases,16,17 we investigated their involvement in the IL-25–mediated Socs-3 induction. Pretreatment of CD14+ cells with SB202190, an inhibitor of p38, abrogated the inductive effect of LPS and IL-25 on Socs-3 RNA expression (Figure 5A). By contrast, PD98059, an inhibitor of ERK, and 420116, an inhibitor of JNK, had no effect (Figure S5A). At the concentrations used, these inhibitors were able to selectively reduce the phosphorylation/activation status of ERK and JNK in cells treated with LPS and IL-25 (Figure S5B). Specificity of SB202190 was confirmed by showing that this compound blocked p38 but neither JNK nor ERK1/2 phosphorylation in LPS + IL-25–treated cells (Figure S5C). In line with the above data, SB202190, but neither PD98059 nor 420116, abrogated the inhibitory effect of IL-25 on Socs-3 RNA after PGN stimulation (Figure S6A,B).
Induction of Socs-3 by IL-25 relies on p38 Map kinase activity. (A) CD14+ cells were preincubated with SB202190 (a p38 inhibitor) or vehicle (DMSO) and then treated with IL-25, LPS, or both as indicated in “Methods.” *P = .001. (B) CD14+ cells were cultured with or without IL-25 for the indicated time points, and both p-p38 and total p38 were then evaluated by Western blotting of total extracts. (C) CD14+ cells were cultured with or without IL-25 for 30 minutes and then either left unstimulated or stimulated with LPS for the indicated times. Lower insets in both panels B and C show the quantitative analysis of p-p38/total p38 protein ratio, as measured by densitometry scanning of Western blots. Values are expressed in arbitrary units (a.u.). (D) Representative Western blots showing p-Ser-MKP-1 and β-actin in CD14+ cells cultured in the presence or absence of IL-25 (50 ng/mL) for the indicated time points. (E) Preincubation of cells with SB202190 prevents the negative regulation of IL-25 on the cytokine response induced by LPS. Cells were cultured as indicated in “Methods,” and RNA was then extracted and analyzed for the indicated cytokines by real-time PCR. *P < .01.
Induction of Socs-3 by IL-25 relies on p38 Map kinase activity. (A) CD14+ cells were preincubated with SB202190 (a p38 inhibitor) or vehicle (DMSO) and then treated with IL-25, LPS, or both as indicated in “Methods.” *P = .001. (B) CD14+ cells were cultured with or without IL-25 for the indicated time points, and both p-p38 and total p38 were then evaluated by Western blotting of total extracts. (C) CD14+ cells were cultured with or without IL-25 for 30 minutes and then either left unstimulated or stimulated with LPS for the indicated times. Lower insets in both panels B and C show the quantitative analysis of p-p38/total p38 protein ratio, as measured by densitometry scanning of Western blots. Values are expressed in arbitrary units (a.u.). (D) Representative Western blots showing p-Ser-MKP-1 and β-actin in CD14+ cells cultured in the presence or absence of IL-25 (50 ng/mL) for the indicated time points. (E) Preincubation of cells with SB202190 prevents the negative regulation of IL-25 on the cytokine response induced by LPS. Cells were cultured as indicated in “Methods,” and RNA was then extracted and analyzed for the indicated cytokines by real-time PCR. *P < .01.
IL-25 enhanced the levels of p-p38 (Figure 5B). Moreover, preincubation of CD14+ cells with IL-25 augmented the expression of p-p38 in LPS-treated (Figure 5C) and PGN-treated (not shown) cells. Consistently, IL-25 inhibited the serine phosphorylation of MKP-1 in a time-dependent fashion (Figure 5D). SB202190 did not alter cytokine transcripts in unstimulated cells, but it slightly augmented LPS-induced RNA expression of TNF-α and IL-6 (Figure 5E). Moreover, SB202190 abrogated the negative effect of IL-25 on the cytokine expression induced by LPS (Figure 5E) and PGN (not shown). Finally, we showed that SB202190 did not alter the rate of CD14+ cell apoptosis (3.3% vs 3.6% in cells cultured with DMSO) and did not affect the expression of HLA-DR and CD80 on CD14+ cells stimulated with IL-25 or LPS or both (not shown), thus excluding toxic effects. To confirm the role of p38 in the IL-25–induced inflammatory cytokine suppression, we silenced p38 in CD14+ cells (Figure 6A) and evaluated their response to IL-25. Notably, IL-25 was not able to inhibit LPS- and PGN-induced cytokine expression in p38-deficient cells (Figure 6B,C).
Silencing of p38 expression in human blood CD14+ cells. (A) Cells were cultured with control or p38 siRNA. After 2 days, total extracts were prepared and analyzed for p38 and β-actin by Western blotting. One of 3 separate experiments is shown. (B,C) IL-25 fails to inhibit the cytokine expression induced by LPS and PGN in p38-deficient cells. Cells were treated with control or p38 siRNA, then stimulated with IL-25 for 30 minutes followed by LPS/PGN for 6 hours. Data indicate mean (± SD) of 5 separate experiments in which cells purified from 5 healthy donors were used.
Silencing of p38 expression in human blood CD14+ cells. (A) Cells were cultured with control or p38 siRNA. After 2 days, total extracts were prepared and analyzed for p38 and β-actin by Western blotting. One of 3 separate experiments is shown. (B,C) IL-25 fails to inhibit the cytokine expression induced by LPS and PGN in p38-deficient cells. Cells were treated with control or p38 siRNA, then stimulated with IL-25 for 30 minutes followed by LPS/PGN for 6 hours. Data indicate mean (± SD) of 5 separate experiments in which cells purified from 5 healthy donors were used.
IL-25–treated mice show decreased in vivo cytokine response
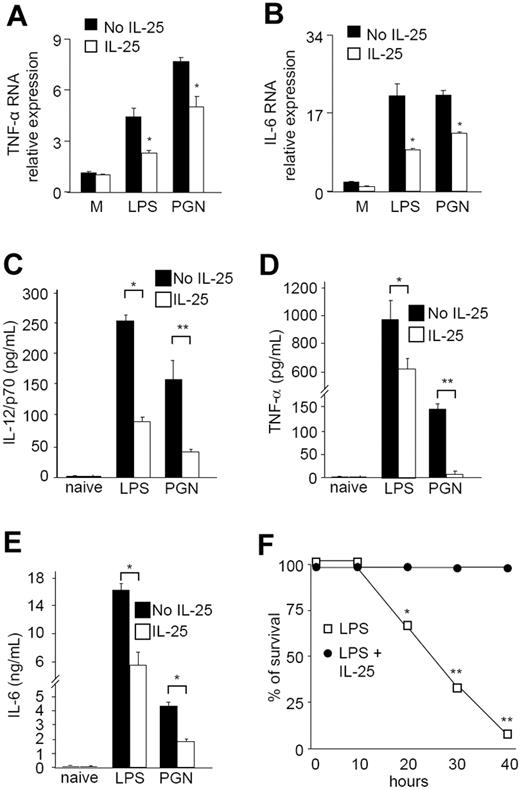
The in vitro studies described earlier provided considerable evidence that IL-25 negatively regulates multiple inflammatory pathways in human monocytes. To translate these results into mice, we examined whether IL-25 modulated the LPS/PGN responses of CD11c+ cells isolated from mouse spleen. Preincubation of cells with murine IL-25 for 30 minutes significantly inhibited the expression of TNF-α and IL-6 induced by LPS and PGN (Figure 7A,B). To confirm this result in vivo, we determined whether IL-25 inhibited the cytokine response induced by systemic administration of LPS and PGN. Mice were treated with recombinant murine IL-25 (10 μg/mouse) intraperitoneally 1 hour before LPS or PGN injection. This dose of IL-25 was selected on the basis of previous studies showing that IL-25 is biologically active in vivo when administered at concentrations ranging from 1 to 100 μg/day.5,18 Serum samples were collected after 5 hours and analyzed for cytokine production. The serum levels of IL-12/p70, TNF-α, and IL-6 were markedly increased by systemic administration of LPS or PGN (Figure 7C-E). Mice pretreated with IL-25 exhibited diminished production of all cytokines (Figure 7C-E). By contrast, IL-4 and IL-17A were undetectable regardless of whether mice were injected or not with IL-25. IL-25 also increased the survival of LPS-treated mice (Figure 7F).
IL-25 inhibits the expression of TNF-α and IL-6 induced by LPS and PGN in CD11c+ cells isolated from the spleen of Balb/c mice. IL-25 inhibited the expression of TNF-α (A) and IL-6 (B). CD11c+ cells were preincubated with medium (no IL-25) or IL-25 for 30 minutes, then either left unstimulated (M = medium) or stimulated with LPS or PGN for a further 6 hours. RNA was then extracted and amplified by real-time PCR. Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. *P = .01. IL-25–treated mice have decreased serum levels of IL-12p70 (C), TNF-α (D), and IL-6 (E) after systemic administration of LPS or PGN. Groups of 5 Balb/c mice were injected intraperitoneally with PBS (no IL-25) or PBS containing murine IL-25 (10 μg/mouse). One hour later animals received PBS (naive), LPS (300 μg/mouse), or PGN (300 μg/mouse). Serum samples were collected at 5 hours and analyzed for cytokine levels by ELISA. Data indicate the mean (± SD) of all experiments. *P = .03; **P < .02. (F) IL-25 protects mice from death. Mice were injected with or without IL-25 and then treated with LPS as indicated above. Similar results were obtained in 3 separate experiments. *P = .02; **P = .001.
IL-25 inhibits the expression of TNF-α and IL-6 induced by LPS and PGN in CD11c+ cells isolated from the spleen of Balb/c mice. IL-25 inhibited the expression of TNF-α (A) and IL-6 (B). CD11c+ cells were preincubated with medium (no IL-25) or IL-25 for 30 minutes, then either left unstimulated (M = medium) or stimulated with LPS or PGN for a further 6 hours. RNA was then extracted and amplified by real-time PCR. Levels are normalized to β-actin and indicate the mean (± SD) of all experiments. *P = .01. IL-25–treated mice have decreased serum levels of IL-12p70 (C), TNF-α (D), and IL-6 (E) after systemic administration of LPS or PGN. Groups of 5 Balb/c mice were injected intraperitoneally with PBS (no IL-25) or PBS containing murine IL-25 (10 μg/mouse). One hour later animals received PBS (naive), LPS (300 μg/mouse), or PGN (300 μg/mouse). Serum samples were collected at 5 hours and analyzed for cytokine levels by ELISA. Data indicate the mean (± SD) of all experiments. *P = .03; **P < .02. (F) IL-25 protects mice from death. Mice were injected with or without IL-25 and then treated with LPS as indicated above. Similar results were obtained in 3 separate experiments. *P = .02; **P = .001.
Discussion
In this study we show that human monocytes express high levels of a functional IL-25R and that IL-25 negatively regulates monocyte inflammatory cytokine expression driven by various inflammatory stimuli, including TLR ligands. Our findings conflict with results of a recently published report by Wang et al,10 showing that human monocytes, blood T, and natural killer T (NKT) cells do not express transcripts for IL-25R. It is likely that this discrepancy may simply reflect differences in the sensitivity of the PCR method used in these studies, because we were also able to confirm IL-25R expression on monocytes by flow cytometry and real-time PCR and to show functional effects of IL-25 on these cells.
Analysis of molecular mechanisms underlying the inhibition of the cytokine response showed that IL-25 does not alter the expression of both TLR-2 and TLR-4. Because IL-25 has no effect on the expression of IL-10 induced by LPS/PGN and does not affect the fraction of AV/PI-positive cells, it is unlikely that inhibition of TLR responses by IL-25 is due to induction of either exhaustion of cytokine production or cell death.
The expression of a previously identified inhibitory molecule of TLR and cytokine receptor signaling, namely Socs-3,14,15 was enhanced by IL-25 in monocytes after stimulation with LPS or PGN. Subsequent studies strongly suggested that Socs-3 was the mediator of IL-25–induced inhibition of monocyte cytokine response. Indeed, silencing of Socs-3 gene expression by specific siRNA abolished the inhibitory effect of IL-25. Overall, these data are consistent with previous studies showing that Socs-3 negatively regulates cytokine production by monocytes/macrophages after LPS stimulation.19
Another novel and interesting observation uncovered by our study is that induction of Socs-3 by IL-25 relies on the activity of p38 Map kinase. Confirming previous results,16,17 we found that IL-25 by itself is sufficient to trigger p38 phosphorylation and to cooperate with both LPS and PGN in enhancing the level of active p38 in monocytes. Moreover, it was also shown that preincubation of monocytes with a specific inhibitor of p38, but not with inhibitors of ERK or JNK, completely abolished IL-25–mediated Socs-3 induction. These results collectively suggest the possibility that IL-25 sustains LPS-induced activation of p38; therefore, IL-25–driven Socs-3 induction could be part of a feedback mechanism that limits LPS signaling.
It has been previously reported that Socs-3 can be induced in a cell type–specific manner by a large number of stimuli via the activation of multiple intracellular pathways, including p38 Map kinase. For instance, Socs-3 expression can be enhanced by activation of p38 in B cells after stimulation with IL-4, and in macrophages after stimulation with TLR ligands, and TNF-α, or infection with Mycobacterium avium.14,15,20,21 There is also evidence that in hepatocytes, IL-6–induced Socs-3 expression occurs via the p38 Map kinase pathway.22 The molecular basis underlying the regulation of the IL-25–driven Socs-3 induction by p38 Map kinase is still obscure. A possibility is that IL-25 regulates Socs-3 at the transcriptional level. Indeed, previous studies have shown that the Socs-3 promoter contains numerous potential regulatory elements and that activation of the p38 signaling cascade in HepG2 cells enhances basal activity and IL-6–induced transcriptional activation of a Socs-3 promoter reporter construct.22 Transcriptional activation of the Socs-3 promoter by IL-6 also requires specificity protein 3, a ubiquitously expressed transcription factor that can be activated by p38.14,23 Another hypothesis is that IL-25 may enhance Socs-3 mRNA stability by activating p38. In this context, note that p38 has been shown to be necessary for TNF-α–mediated stabilization of Socs-3 mRNA in macrophages and that both fibroblasts and macrophages deficient for Map kinase-activated protein kinase 2, a downstream target of p38, have reduced levels of Socs-3, in part because of a diminished Socs-3 mRNA stability.24 Studies are now in progress to address these issues. Further experimentation needs to be performed to examine the molecular mechanisms upstream of p38 Map kinase activation in IL-25–treated monocytes. Map kinases are inactivated by a family of dual-specificity protein phosphatases that differ in their substrate specificity, tissue distribution, inducibility by extracellular stimuli, and cellular localization.25 Among these, MKP-1 is primarily localized in the nucleus and has been reported to play a major role in the control of p38 activation.25 Both expression and activity of MKP-1 are tightly regulated. For example, MKP-1 can be transcriptionally induced by various stimuli, including ERK1/2, p53, and Jak2. MKP-1 can also be phosphorylated at Ser359 and Ser364 in its carboxy-terminal region. This modification inhibits MKP-1 degradation through the ubiquitin pathway, with the downstream consequence of attenuating p38 activation. In line with this, we showed that IL-25 is able to dephosphorylate MKP-1 at serine 359, raising the possibility that the ability of IL-25 to sustain p38 activation is secondary to the inhibition of MKP-1.
Activation of p38 has been traditionally considered as a crucial mediator of the functional response of immune cells to bacterial products/components, and numerous studies have shown that such a pathway is an attractive target for suppressing bacteria-driven inflammatory responses.26,27 Nonetheless, it was also shown that p38 inhibitors can enhance rather than inhibit the production of inflammatory mediators in monocytes/macrophages after LPS stimulation. For instance, Marriott et al28 showed that pharmacologic inhibitors of p38 (eg, SB203580 and SB212090) enhanced LPS-induced IL-12 expression in cultures of human PBMCs and mouse whole blood. In line with this, Kim et al29 showed that inhibition of p38 potentiates LPS-induced production of reactive oxygen species, IL-12, and TNF-α by mouse macrophages, and Utsugi et al30 showed that SB203580 enhances the LPS-induced IL-23/p19 production in human monocytic THP-1 cells. Therefore, these later data well fit with our demonstration that silencing of p38 in purified CD14+ cells augments the LPS-induced RNA expression of IL-6 and TNF-α.
We further strengthened the importance of IL-25 in the negative control of cytokine response by monocytes in a well-established model of endotoxemia. Administration of IL-25 to mice markedly inhibited LPS- and PGN-driven production of IL-12, IL-6, and TNF-α. These cytokines play a pivotal role in LPS-induced endotoxic shock, and inhibition of the LPS-induced cascade of proinflammatory cytokines is the primary mechanism through which anti-inflammatory molecules confer protection against the lethal effects of LPS administration.31-33 In line with this, we showed that IL-25 pretreatment protected animals from the LPS-induced death. Importantly, the therapeutic effect of IL-25 in this model was associated with no significant change in the production of Th2- and Th17-related cytokines (ie, IL-4 and IL-17A, respectively), thus arguing against the hypothesis that some of the negative effects of IL-25 on the course of LPS-mediated endotoxemia are indeed due to its ability to either promote Th2- or inhibit Th17-associated cell responses.4,5,8
Taken together, our data indicate that IL-25 negatively regulates the production of monocyte-derived inflammatory cytokines and suggest that IL-25 may be an attractive candidate for the treatment of monocyte-mediated immune and tumoral disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Fondazione Umberto di Mario, Rome, the Broad Medical Research Program Foundation (Nr: IBD-0242), and Giuliani SpA, Milan, Italy.
Authorship
Contribution: R.C. performed cultures with human and mouse cells, analyzed cytokines expression in cells stimulated with IL-25, and conducted experiments in mice with LPS-endotoxemia; C.S. and M.S. performed flow cytometry staining and contributed to isolate mucosal mononuclear cells; A.R. and M.C.F. performed Western blotting experiments and ELISA; F.P. and T.T.M. contributed to supervise parts of the project and to write the paper; and G.M. designed the research, supervised the project, and wrote the manuscript.
Conflict-of-interest disclosure: G.M. has filed a patent entitled “A treatment for inflammatory diseases.” The other authors declare no competing financial interests.
Correspondence: Giovanni Monteleone, Cattedra di Gastroenterologia, Dipartimento di Medicina Interna, Università Tor Vergata, Via Montpellier, 1, 00133 Rome, Italy; e-mail: gi.monteleone@med.uniroma2.it.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal