Abstract
Interferon-γ (IFN-γ) inhibits graft-versus-host disease (GVHD) in lethally irradiated mice receiving allogeneic hematopoietic cell transplantation (allo-HCT) but promotes lethality in unirradiated and sublethally irradiated recipients. We investigated the role of IFN-γ in GVHD in sublethally irradiated B6D2F1 recipients of B6 allo-HCT. B6D2F1 mice receiving wild-type B6 splenocytes alone died rapidly, whereas those receiving wild-type B6 splenocytes plus marrow survived long-term. Mice in both groups showed rapid elimination of host hematopoietic cells but minimal parenchymal tissue injury. However, mice receiving allo-HCT from IFN-γ–deficient donors died rapidly regardless of whether donor marrow was given, and they exhibited severe parenchymal injury but prolonged survival of host hematopoietic cells. IFN-γ plays a similar role in another model involving delayed B6 donor leukocyte infusion (DLI) to established mixed allogeneic (B6→BALB/c) chimeras. IFN-γ promotes DLI-mediated conversion from mixed to full donor chimerism while attenuating GVHD. Importantly, IFN-γ enhances graft-versus-leukemia (GVL) effects in both models. Our data indicate that previously reported IFN-γ–induced early mortality in allo-HCT recipients is due to augmentation of lymphohematopoietic graft-versus-host reaction (LGVHR) and can be avoided by providing an adequate source of donor hematopoietic stem/progenitor cells. Furthermore, the magnitude of GVL is correlated with the strength of LGVHR, and IFN-γ reduces the potential of this alloreactivity to cause epithelial tissue GVHD.
Introduction
Graft-versus-host disease (GVHD) remains the major complication of allogeneic hematopoietic cell transplantation (allo-HCT), the only curative therapy for many hematopoietic malignancies.1 Interferon-γ (IFN-γ) plays important roles in both innate and adaptive immune responses.2-4 IFN-γ activates macrophages and stimulates the activity of NK cells and cytotoxic CD8 T cells, and has been considered a pathogenic factor in acute GVHD. However, we and others have shown that IFN-γ is not required for and can inhibit the development of GVHD in lethally irradiated allo-HCT recipients.5-9 This cytokine has also been shown to mediate the protective effect against GVHD of interleukin-12 (IL-12) and IL-18, 2 potent cytokines that stimulate Th1 responses.5,10,11 Although the mechanisms are not fully understood, IFN-γ was found to negatively regulate alloreactive T cells by inhibiting cell division and promoting cell death,9 and to prevent tissue damage through direct interaction with recipient parenchymal cells.12
However, the inhibitory effect of IFN-γ on GVHD has only been observed in allo-HCT recipients treated with lethal total body irradiation (TBI), which is eliminated from reduced-intensity conditioning regimens. Although the pathogenic mechanism is unknown, IFN-γ has been reported to accelerate death in nonirradiated13 and sublethally irradiated14 mice receiving allogeneic lymph node and spleen cells or purified CD4+ cells. Because reduced-intensity conditioning is being used increasingly in clinical protocols, these studies argue that inhibition of IFN-γ might be beneficial in this setting.
In this study, we explored the role of IFN-γ in the induction of GVHD and graft-versus-leukemia (GVL) effects in 2 allo-HCT models. In the first model, recipient mice were treated with a sublethal dose of TBI one day before allo-HCT. The second model involves delayed donor leukocyte infusion (DLI) in preestablished mixed allogeneic chimeras, an approach that has been applied to achieve GVL effects in the clinic.15,16 In both allo-HCT models, IFN-γ was found to inhibit GVHD while promoting lymphohematopoietic graft-versus-host reactions (LGVHR) and GVL effects.
Methods
Animals
Female IFN-γ knockout (GKO) mice on the C57BL/6 (H-2b) background were purchased from The Jackson Laboratory (Bar Harbor, ME). Wild-type (WT) C57BL/6 (H-2b), BALB/c (H-2d), and B6D2F1 (H-2bxd) mice were purchased from The Jackson Laboratory or Frederick Cancer Research Facility (National Institutes of Health, Frederick, MD). Mice were used in experiments at 8 to 12 weeks of age and housed in a specific pathogen–free microisolator environment. All animal procedures were approved by the institutional review board at Massachusetts General Hospital.
Allo-HCT in sublethally irradiated recipients
Recipient B6D2F1 mice were sublethally (6 Gy) irradiated, followed the next day by intravenous injection of 2 to 3 × 107 splenocytes alone or along with 107 bone marrow cells (BMCs) from WT or GKO B6 mice. Sublethally irradiated B6D2F1 mice without cell injection were used as non-HCT controls. In some groups, P815 cells (a DBA/2-derived mastocytoma cell line) were injected intravenously at day −2 or day 0 with respect to HCT. Mice in different groups were randomized between cages to avoid cage-related bias. Chimerism in white blood cells (WBCs) and bone marrow was assessed at various times after transplantation using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA), in which fluorescein isothiocyanate (FITC)–conjugated antirecipient (H-2Dd) mAb 34–2-12 (BD Biosciences PharMingen, San Diego, CA) was used to distinguish between donor and recipient T cells and nonreactive mouse IgG2a (HOPC-1)-FITC was used as an isotype control.
Preparation of mixed allogeneic chimeras and administration of delayed DLI
Mixed chimeras were prepared by injection of a mixture of 0.5 × 107 T cell-depleted (TCD) syngeneic BALB/c and 1.5 × 107 TCD allogeneic WT or GKO B6 BMCs into lethally irradiated (8 Gy) BALB/c mice. TCD BMCs were prepared by depleting CD4+ and CD8+ cells with anti-CD4 (L3T4) and CD8a (Ly-2) microbeads using the magnetic-activated cell sorter separation system (Miltenyi Biotec, Auburn, CA). T-cell depletion was analyzed by flow cytometry, and completeness of depletion (< 0.35% cells of the depleted phenotype remaining) was verified in each experiment. DLI was performed using spleen cells (1.5 × 107) from WT or GKO B6 donors at day 56 after initial TCD BMC injection. In some experiments, 3 × 105 A20 cells (a BALB/c-derived B-cell leukemia/lymphoma cell line) were administered intravenously one week after DLI for evaluating antitumor responses. Animals were randomized between cages to avoid cage-related bias. Levels of donor chimerism in WBCs were followed up by flow cytometry before and after DLI, in which FITC-conjugated anti–H-2Db mAb KH95 (BD Biosciences Pharmingen) was used to detect donor cells, and HOPC-1-FITC was used as the isotype control.
Necropsy and histologic analysis
Carcasses were saved in 10% formalin after death for autopsy. Necropsies were performed by observers who were unaware of the assignment of mice to which treatment group. Tissues (liver and lung) from randomly chosen samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin, and sections were evaluated blindly by a pathologist. Slides were observed using an Olympus BX40 light microscope (Olympus, Melville, NY) with 20×/0.5 numeric aperture (NA) objectives, and photographed using a Hitachi HV-C20 color camera (Hitachi, Nashua, NH).
Statistical analysis
Survival data are presented as Kaplan-Meier survival curves, and differences between groups were analyzed by the log-rank test using GraphPad Prism version 4 (GraphPad Software, San Diego, CA). Differences between group means were tested using Student t test by Microsoft Excel software (Microsoft, Redmond, WA). A probit transformation was applied to the percentage of donor chimerism using the inverse function of a standard normal cumulative distribution, whereas body weight was analyzed using a square root transform. A linear mixed effects model was used to fit an overall trend in time to each transformed outcome, with a random intercept and slope assumed for each mouse. The model assumed an unstructured covariance matrix for the chimerism data and an autoregressive structure of order 1 for the body weight within animals. The comparisons between experimental groups were based on hypothesis tests of the fixed-effect contrasts between groups, using the F test for inference. The statistical analysis was conducted using SAS version 9.1 (SAS Institute, Cary, NC). A P value less than .05 was considered to be significant.
Results
Donor-derived IFN-γ promotes the destruction of recipient hematopoietic cells while inhibiting parenchymal tissue GVHD in sublethally irradiated allogeneic recipients
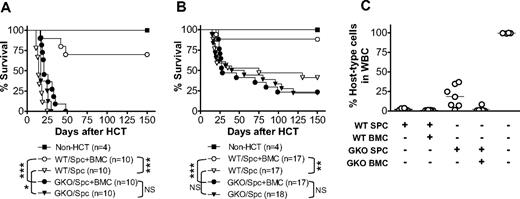
We hypothesized that IFN-γ promotes LGVHR but inhibits destruction of parenchymal tissues, thereby mediating GVL effects while inhibiting GVHD in allo-HCT recipients. We therefore compared the survival of sublethally (6 Gy)-irradiated B6D2F1 mice that received allogeneic splenocytes (3 × 107/mouse) alone or along with BMCs (107/mouse) from WT or GKO B6 donors (Figure 1A). All sublethally irradiated B6D2F1 mice that received WT B6 splenocytes alone (without BMCs, so that the inoculum contained minimal numbers of donor hematopoietic stem/progenitor cells) died between 11 and 25 days after allo-HCT. However, most sublethally irradiated B6D2F1 mice that received a similar number of WT B6 splenocytes plus BMCs, in which hematopoietic failure was prevented by provision of donor BMCs, survived long-term. This indicates that the death of B6D2F1 mice receiving WT B6 splenocytes alone was predominantly caused by destruction of the recipient hematopoietic system. Injection of a similar number of GKO B6 splenocytes alone also resulted in 100% mortality between 17 and 30 days. However, in contrast to the recipients of WT allo-HCT, addition of donor BMCs to splenocytes failed to extend the survival of the recipients of GKO allo-HCT. All sublethally irradiated B6D2F1 mice that received GKO B6 splenocytes plus BMCs died between 17 and 48 days after allo-HCT (not significant, compared with those receiving only GKO splenocytes). We have previously shown that both WT and GKO B6 BMCs, when given at a dose of 5 × 106 or more per mouse, are sufficient to rescue lethally irradiated B6D2F1 mice.9 Furthermore, GKO splenic T cells induced more severe GVHD than WT splenic T cells regardless of whether WT or GKO TCD BMCs were given (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, the failure to improve survival by giving donor BMCs in the recipients of GKO allo-HCT suggests that parenchymal tissue injury, but not hematopoietic failure, is the primary cause of death in these mice.
IFN-γ inhibits GVHD while accelerating the destruction of recipient hematopoietic cells in sublethally irradiated allogeneic recipients. B6D2F1 mice received 6 Gy TBI one day before administration of 3 × 107 (A) or 2 × 107 (B,C) allogeneic splenocytes alone or along with 107 BMCs from WT or GKO B6 donors. Non-HCT controls were sublethally irradiated B6D2F1 mice receiving no transplantation. (A,B) Survival of non-HCT B6D2F1 controls and mice receiving WT or GKO B6 splenocytes alone (Spc) or splenocytes plus BMCs (Spc + BMC). *P < .05; **P < .01; ***P < .005. NS indicates not significant. (C) Levels of host-type (H-2d+) cells in WBCs prepared at day 50 from surviving mice in panel B. Each symbol represents an individual animal.
IFN-γ inhibits GVHD while accelerating the destruction of recipient hematopoietic cells in sublethally irradiated allogeneic recipients. B6D2F1 mice received 6 Gy TBI one day before administration of 3 × 107 (A) or 2 × 107 (B,C) allogeneic splenocytes alone or along with 107 BMCs from WT or GKO B6 donors. Non-HCT controls were sublethally irradiated B6D2F1 mice receiving no transplantation. (A,B) Survival of non-HCT B6D2F1 controls and mice receiving WT or GKO B6 splenocytes alone (Spc) or splenocytes plus BMCs (Spc + BMC). *P < .05; **P < .01; ***P < .005. NS indicates not significant. (C) Levels of host-type (H-2d+) cells in WBCs prepared at day 50 from surviving mice in panel B. Each symbol represents an individual animal.
Similar results were obtained from another series of experiments in which the recipients were injected with a reduced number (2 × 107) of allogeneic donor splenocytes. As shown in Figure 1B, the recipients of WT B6 splenocytes plus BMCs showed significantly improved survival compared with those receiving WT splenocytes alone (P = .004), whereas most recipients of GKO allo-HCT died of GVHD regardless of whether BMCs were given at the same time (not significant, comparing recipients of GKO splenocytes with and without BMCs). At day 50 after HCT, blood was collected from surviving allo-HCT recipients to measure chimerism. All recipients of WT allo-HCT that received splenocytes alone or along with BMCs showed complete or nearly complete elimination of recipient-type (H-2d+) hematopoietic cells (Figure 1C). In contrast, recipient-type cells were detected in 6 of 7 mice receiving GKO B6 splenocytes alone (range, 7%-37%) and in one of 6 mice receiving GKO B6 splenocytes plus BMCs (8.3%). The less complete elimination of recipient hematopoietic cells by GKO B6 splenocytes compared with WT B6 splenocytes (Figure 2) further indicates that donor-derived IFN-γ promotes LGVHR. In addition, the development of full donor chimerism in the recipients of WT donor splenocytes alone that survived long-term suggests that the small number of hematopoietic stem/progenitor cells in the spleen17 may take over hematopoiesis in allo-HCT recipients if there is sufficient time for them to expand, ie, if the mice do not die of rapid GVHD.
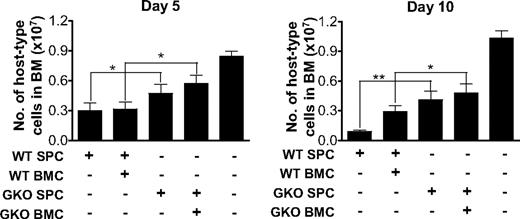
Reduced destruction of recipient bone marrow cells in B6D2F1 mice receiving GKO allo-HCT. B6D2F1 mice received 6 Gy TBI one day before administration of 2 × 107 allogeneic splenocytes alone or along with 107 BMCs from WT or GKO B6 donors (n = 6 per group). Non-HCT controls were sublethally irradiated B6D2F1 mice receiving no transplantation (n = 3). BMCs were prepared at days 5 and 10, and percentages of recipient-type (H-2d+) cells were determined by flow cytometry. The total number of recipient-type BMCs was calculated as the product of the percentage of host-type cells and the total bone marrow cellularity. Shown are numbers (mean ± SD) of host-type nucleated cells in bone marrow. *P < .05; **P < .01.
Reduced destruction of recipient bone marrow cells in B6D2F1 mice receiving GKO allo-HCT. B6D2F1 mice received 6 Gy TBI one day before administration of 2 × 107 allogeneic splenocytes alone or along with 107 BMCs from WT or GKO B6 donors (n = 6 per group). Non-HCT controls were sublethally irradiated B6D2F1 mice receiving no transplantation (n = 3). BMCs were prepared at days 5 and 10, and percentages of recipient-type (H-2d+) cells were determined by flow cytometry. The total number of recipient-type BMCs was calculated as the product of the percentage of host-type cells and the total bone marrow cellularity. Shown are numbers (mean ± SD) of host-type nucleated cells in bone marrow. *P < .05; **P < .01.
We also compared the levels of recipient-type (H-2d+) hematopoietic cells in bone marrow between recipients of WT and GKO allo-HCT at early times (days 5 and 10) after transplantation. Although a reduction in host-type BMCs was seen in B6D2F1 recipients of both WT and GKO B6 splenocytes compared with sublethally irradiated non-HCT controls, the reduction was more marked in the recipients of WT B6 splenocytes than in those receiving GKO B6 splenocytes (Figure 2). These data provide further evidence that WT allo-HCT mediates more rapid and vigorous LGVHR than GKO allo-HCT.
Donor-derived IFN-γ attenuates tissue GVHD in sublethally irradiated allo-HCT recipients
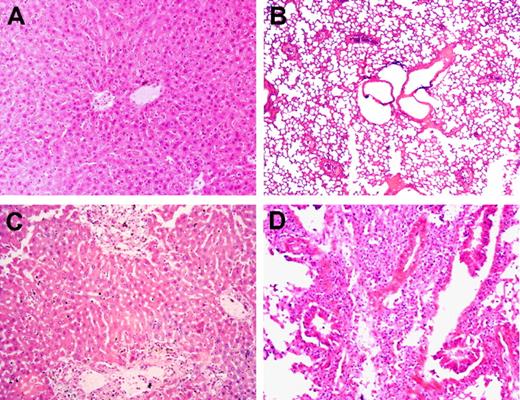
We next examined the pathologic changes in liver and lungs from randomly selected recipient mice that died between 13 and 48 days after allo-HCT. In mice that received WT B6 splenocytes alone or WT B6 splenocytes plus BMCs, minimal to moderate inflammatory infiltrates were detected in the liver, but no pathologic GVHD was seen in lungs (Table 1; Figure 3). This further supports the possibility that the recipients of WT B6 splenocytes alone died of hematopoietic failure, but not GVHD. In contrast, more severe inflammatory infiltrates and the associated tissue lesions were detected in all of these tissues from mice that received GKO B6 splenocytes, regardless of whether they were given donor BMCs. Taken together, our results demonstrate that WT T cell–mediated GVHR predominantly targets host hematopoietic cells and does not cause death if adequate donor hematopoietic stem/progenitor cells are given to the recipients. In contrast, GVHR mediated by GKO T cells is less efficient in eliminating host hematopoietic cells but more potent in causing epithelial tissue injury, which cannot be prevented by providing donor hematopoietic stem/progenitor cells.
Pathologic GVHD
| . | WT Allo-HCT . | GKO Allo-HCT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spc + BMC . | Spc . | Spc + BMC . | Spc . | ||||||||
| DOD | 16 | 48 | 13 | 14 | 14 | 21 | 21 | 26 | 17 | 17 | 17 |
| Liver | − | + | + | + | ++ | + | + | ++ | ++ | +++ | + |
| Lung | − | − | − | − | − | + | ++ | +++ | +++ | +++ | ++ |
| . | WT Allo-HCT . | GKO Allo-HCT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spc + BMC . | Spc . | Spc + BMC . | Spc . | ||||||||
| DOD | 16 | 48 | 13 | 14 | 14 | 21 | 21 | 26 | 17 | 17 | 17 |
| Liver | − | + | + | + | ++ | + | + | ++ | ++ | +++ | + |
| Lung | − | − | − | − | − | + | ++ | +++ | +++ | +++ | ++ |
Recipient mice received 6 Gy TBI followed by administration of 3 × 107 splenocytes alone (Spc) or along with 107 bone marrow cells (Spc + BMC) from WT or GKO B6 donors. Carcasses were saved in 10% formalin after death. Tissue sections were examined blindly by a pathologist and graded as follows: − indicates no detectable lesions; +, minimal lesions; ++, moderate lesions; +++, severe lesions.
DOD indicates date of death (days after HCT).
Lack of donor IFN-γ results in increased pathologic GVHD in sublethally irradiated allo-HCT recipients. B6D2F1 mice received 6 Gy TBI followed the next day by administration of 3 × 107 splenocytes alone or along with 107 BMCs from WT or GKO B6 donors. Carcasses were preserved in formalin after death, and GVHD target tissues were prepared for pathologic examination (findings are summarized in Table 1). Liver (A) and lung (B) sections (hematoxylin and eosin; original magnification ×200) from a recipient of WT B6 splenocytes alone that died at day 14. Liver (C) and lung (D) sections (hematoxylin and eosin; original magnification ×200) from a recipient of GKO B6 splenocytes alone that died at day 17.
Lack of donor IFN-γ results in increased pathologic GVHD in sublethally irradiated allo-HCT recipients. B6D2F1 mice received 6 Gy TBI followed the next day by administration of 3 × 107 splenocytes alone or along with 107 BMCs from WT or GKO B6 donors. Carcasses were preserved in formalin after death, and GVHD target tissues were prepared for pathologic examination (findings are summarized in Table 1). Liver (A) and lung (B) sections (hematoxylin and eosin; original magnification ×200) from a recipient of WT B6 splenocytes alone that died at day 14. Liver (C) and lung (D) sections (hematoxylin and eosin; original magnification ×200) from a recipient of GKO B6 splenocytes alone that died at day 17.
Donor-derived IFN-γ is required for the optimal induction of GVL effects in sublethally irradiated allo-HCT recipients
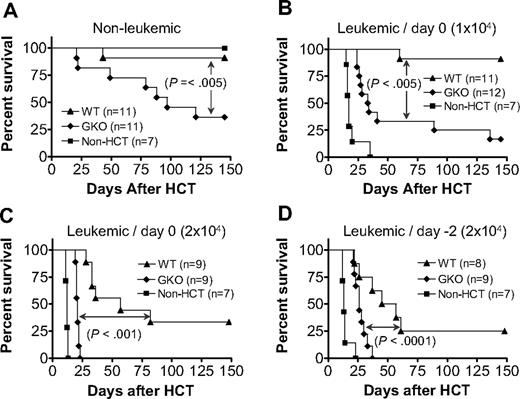
LGVHR has been shown to selectively eliminate host lymphohematopoietic cells, including lymphoma cells, without severe GVHD.18-21 Furthermore, IFN-γ mediates the GVL effect of donor CD8 T cells while inhibiting their GVHD-inducing activity in lethally irradiated recipients of CD4-depleted allo-HCT from fully major histocompatibility complex-mismatched allogeneic donors.22 Here we investigated whether IFN-γ can also mediate GVL effects while inhibiting GVHD in sublethally irradiated allo-HCT recipients, in which both donor CD4 and CD8 T cells are administered. In these experiments, B6D2F1 mice were injected with 2 × 107 spleen cells and 107 BMCs from WT or GKO B6 donors 1 day after 6 Gy TBI. Leukemic groups received DBA2 mouse-derived P815 mastocytoma cells intravenously either at the same time as allo-HCT or 2 days before allo-HCT (ie, 1 day before TBI) at a dose of 104 or 2 × 104 per mouse. Again, GKO allo-HCT induced more severe GVHD than WT allo-HCT (Figure 4A). However, the stronger GVHD-inducing activity of GKO allo-HCT was paradoxically associated with a reduced GVL effect compared with WT allo-HCT (Figure 4B-D). Although improved survival was seen in both WT and GKO allo-HCT groups compared with mice that received tumor cells without allo-HCT, the leukemic recipients of GKO allo-HCT died significantly earlier and had a much higher mortality rate than those receiving WT allo-HCT. Because most nonleukemic recipients of GKO allo-HCT were surviving by the time when the leukemic recipients of GKO allo-HCT had died, leukemia, but not GVHD, was the presumed cause of death in the leukemic group. This was further confirmed by necropsy, in which tumors were found in all dead (or killed because of hind limb paralysis) leukemic recipients of GKO allo-HCT (Table 2). In contrast, only a few leukemic recipients of WT allo-HCT showed evidence for tumor at autopsy. Of note, many of the mice receiving 2 × 104 tumor cells, especially those that survived more than 3 weeks (most of these mice were in WT allo-HCT groups), developed hind limb paralysis, a sign of tumor cell infiltration in the spine/nervous system (Table 2). Together, these results indicate that donor cell-derived IFN-γ is required for the optimal induction of GVL effects, while inhibiting GVHD in sublethally irradiated mice after allo-HCT.
Donor IFN-γ is required for optimal GVL effects in sublethally irradiated allo-HCT recipients. B6D2F1 mice received 6 Gy TBI followed the next day by administration of 2 × 107 splenocytes and 107 BMCs from WT (▲) or GKO (♦) B6 donors (n = 8-12 per group). B6D2F1 mice that received sublethal TBI without HCT were used as controls (■; n = 7 per group). Leukemic recipients were additionally injected with 104 (B) or 2 × 104 (C,D) P815 tumor cells, either on day 0 (B,C) or day −2 (D) with respect to allo-HCT. Survivals of nonleukemic (A) and leukemic (B-D) recipients are shown.
Donor IFN-γ is required for optimal GVL effects in sublethally irradiated allo-HCT recipients. B6D2F1 mice received 6 Gy TBI followed the next day by administration of 2 × 107 splenocytes and 107 BMCs from WT (▲) or GKO (♦) B6 donors (n = 8-12 per group). B6D2F1 mice that received sublethal TBI without HCT were used as controls (■; n = 7 per group). Leukemic recipients were additionally injected with 104 (B) or 2 × 104 (C,D) P815 tumor cells, either on day 0 (B,C) or day −2 (D) with respect to allo-HCT. Survivals of nonleukemic (A) and leukemic (B-D) recipients are shown.
Reduced antitumor responses in recipients of GKO allo-HCT
| Experiment . | Treatment . | N . | Death (n) . | Tumor at autopsy, n* . | Paralysis, n† . | Tumor-free, n‡ . | |
|---|---|---|---|---|---|---|---|
| P815 . | Allo-HCT . | ||||||
| A | Day 0 (104) | WT | 11 | 1 | 1 | 0 | 10 |
| GKO | 12 | 10 | 10 | 0 | 2 | ||
| None | 7 | 7 | 7 | 0 | 0 | ||
| B | Day 0 (2 × 104) | WT | 9 | 6 | 0 | 6 | 3 |
| GKO | 9 | 9 | 9 | 1 | 0 | ||
| None | 7 | 7 | 7 | 0 | 0 | ||
| C | Day −2 (2 × 104) | WT | 8 | 6 | 2 | 5 | 2 |
| GKO | 9 | 9 | 9 | 4 | 0 | ||
| None | 7 | 7 | 7 | 1 | 0 | ||
| Experiment . | Treatment . | N . | Death (n) . | Tumor at autopsy, n* . | Paralysis, n† . | Tumor-free, n‡ . | |
|---|---|---|---|---|---|---|---|
| P815 . | Allo-HCT . | ||||||
| A | Day 0 (104) | WT | 11 | 1 | 1 | 0 | 10 |
| GKO | 12 | 10 | 10 | 0 | 2 | ||
| None | 7 | 7 | 7 | 0 | 0 | ||
| B | Day 0 (2 × 104) | WT | 9 | 6 | 0 | 6 | 3 |
| GKO | 9 | 9 | 9 | 1 | 0 | ||
| None | 7 | 7 | 7 | 0 | 0 | ||
| C | Day −2 (2 × 104) | WT | 8 | 6 | 2 | 5 | 2 |
| GKO | 9 | 9 | 9 | 4 | 0 | ||
| None | 7 | 7 | 7 | 1 | 0 | ||
Number of mice with tumor at autopsy.
Number of mice that were killed because of the development of hind limb paralysis.
Number of long-term surviving mice without tumor or paralysis.
Role of donor-derived IFN-γ in the induction of GVHD and GVL effects in established mixed allogeneic chimeras after delayed DLI
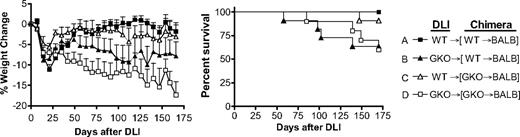
In these experiments, mixed allogeneic chimeras were prepared by injecting mixed TCD BMCs from BALB/c plus WT or GKO B6 mice into lethally irradiated BALB/c mice. Eight weeks later, mixed chimeras that were initially injected with WT or GKO B6 BMCs received DLI (ie, donor splenocytes) from WT or GKO B6 donors without additional conditioning. All chimeras that received GKO DLI (groups B and D) exhibited continued loss of body weight throughout the experiments and some died of GVHD (Figure 5). Although statistical significance was not attained, GKO DLI caused more severe body weight loss in the chimeras that received GKO B6 BMCs initially (group D) than in those receiving WT B6 BMCs (group B), indicating that IFN-γ produced by donor BMCs or BMC-derived cells may attenuate GVHD. However, IFN-γ produced by DLI cells is more effective in inhibiting GVHD than that from donor BMC-derived cells, as the chimeras receiving WT DLI, regardless of whether WT or GKO B6 BMCs were given initially, showed significantly improved body weight recovery (P < .005 for group D vs group A or C) and better survival compared with those receiving GKO DLI (Figure 5).
DLI from GKO allogeneic donors induces more severe GVHD than WT DLI in preestablished mixed allogeneic chimeras. Lethally (8 Gy) irradiated BALB/c mice were reconstituted with a mixture of TCD BALB/c plus WT (■, n = 8; and ▲, n = 11) or GKO (△, n = 11; and □, n = 10) B6 BMCs 8 weeks before DLI (ie, injection of 1.5 × 107 splenocytes) from WT (■ and △) or GKO (▲ and □) B6 mice. Recipient mice were followed for body weight changes (left) and survival (right). Combined data from 2 similar experiments are shown.
DLI from GKO allogeneic donors induces more severe GVHD than WT DLI in preestablished mixed allogeneic chimeras. Lethally (8 Gy) irradiated BALB/c mice were reconstituted with a mixture of TCD BALB/c plus WT (■, n = 8; and ▲, n = 11) or GKO (△, n = 11; and □, n = 10) B6 BMCs 8 weeks before DLI (ie, injection of 1.5 × 107 splenocytes) from WT (■ and △) or GKO (▲ and □) B6 mice. Recipient mice were followed for body weight changes (left) and survival (right). Combined data from 2 similar experiments are shown.
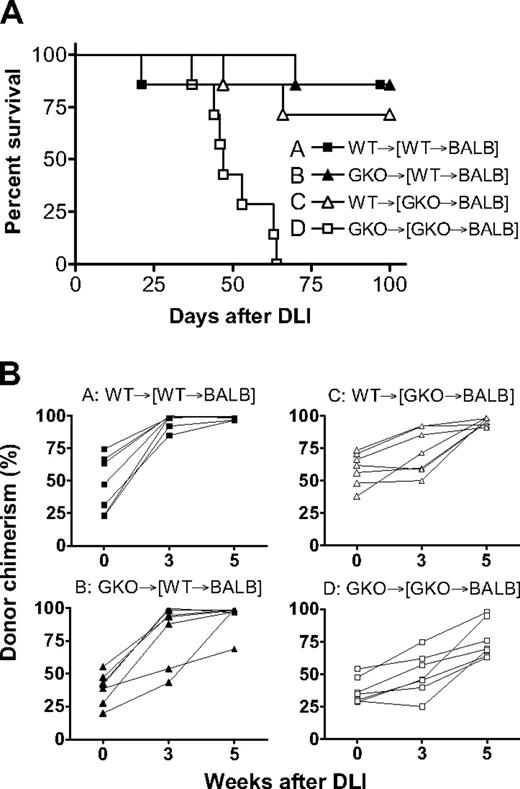
We also determined the role of donor-derived IFN-γ in the induction of GVL effects by delayed DLI in mixed chimeras, in which BALB/c-derived B lymphoma A20 cells were administered. As shown in Figure 6A, GKO DLI failed to mediate significant GVL effects in mixed chimeras that received GKO B6 BMCs initially (group D), and all mice died by 57 days after A20 cell injection. Leukemia was the cause of death in these mice because: (1) most of the similar chimeras that received GKO DLI without A20 cells survived long-term (Figure 5); and (2) gross evidence for tumor was detected by autopsy in all of these mice (Table 3). In contrast, most of the chimeras that received WT BMCs plus WT DLI (group A), WT BMCs plus GKO DLI (group B), or GKO BMCs plus WT DLI (group C) survived long-term (Figure 6A; P < .005 for group D vs group A, B, or C) and were free of tumor at autopsy (Table 3). All chimeras that received WT DLI showed significantly more efficient conversion from mixed to full donor chimerism compared with those receiving GKO BMCs plus GKO DLI (Figure 6B; P < .001 for group D vs group A or C). Although statistical significance was not attained, mixed chimeras that received WT BMCs initially seemed to show more efficient conversion than those that received GKO BMCs initially after WT (group A vs C) or GKO (group B vs D) DLI (Figure 6B). Taken together, these results indicate that IFN-γ produced by both DLI cells and donor BMC-derived cells can promote GVL effects and LGVHR.
Donor-derived IFN-γ facilitates GVL effects and promotes conversion to full donor chimerism in mixed chimeras after delayed DLI. Lethally (8 Gy) irradiated BALB/c mice were reconstituted with a mixture of TCD BALB/c plus WT (■ and ▲, n = 7 per group) or GKO (△ and □, n = 7 per group) B6 BMCs 8 weeks before DLI (ie, injection of 1.5 × 107 splenocytes) from WT (■ and △) or GKO (▲ and □) B6 mice. All mice received 3 × 105 A20 leukemia cells one week after DLI. Survival (A) and kinetics of donor chimerism in peripheral blood (B) from a representative experiment are shown.
Donor-derived IFN-γ facilitates GVL effects and promotes conversion to full donor chimerism in mixed chimeras after delayed DLI. Lethally (8 Gy) irradiated BALB/c mice were reconstituted with a mixture of TCD BALB/c plus WT (■ and ▲, n = 7 per group) or GKO (△ and □, n = 7 per group) B6 BMCs 8 weeks before DLI (ie, injection of 1.5 × 107 splenocytes) from WT (■ and △) or GKO (▲ and □) B6 mice. All mice received 3 × 105 A20 leukemia cells one week after DLI. Survival (A) and kinetics of donor chimerism in peripheral blood (B) from a representative experiment are shown.
Gross evidence for tumor at autopsy in mixed chimeric recipients of DLI
| Group (n) . | Chimera . | LI donor . | Death, n . | Tumor at autopsy, n* . | Tumor-free, n† . |
|---|---|---|---|---|---|
| A (7) | WT B6→BALB/c | WT B6 | 1 | 0 | 6 |
| B (7) | WT B6→BALB/c | GKO B6 | 1 | 1 | 6 |
| C (7) | GKO B6→BALB/c | WT B6 | 2 | 2 | 5 |
| D (7) | GKO B6→BALB/c | GKO B6 | 7 | 7 | 0 |
| Group (n) . | Chimera . | LI donor . | Death, n . | Tumor at autopsy, n* . | Tumor-free, n† . |
|---|---|---|---|---|---|
| A (7) | WT B6→BALB/c | WT B6 | 1 | 0 | 6 |
| B (7) | WT B6→BALB/c | GKO B6 | 1 | 1 | 6 |
| C (7) | GKO B6→BALB/c | WT B6 | 2 | 2 | 5 |
| D (7) | GKO B6→BALB/c | GKO B6 | 7 | 7 | 0 |
LI indicates leukocyte infusion.
Number of mice with tumor at autopsy.
Number of long-term surviving mice without tumor at autopsy.
Discussion
IFN-γ is produced by activated immune cells, including T cells, NK cells, and antigen-presenting cells, and its production is often seen in allo-HCT recipients.23,24 Previous studies have shown that elimination of IFN-γ production magnifies GVHD in lethally irradiated murine allo-HCT recipients.5-9,12 However, IFN-γ paradoxically accelerates death in nonirradiated and sublethally irradiated mice receiving allogeneic donor lymph node and spleen cells or purified T cells.13,14 Because the mice in these studies did not receive donor bone marrow, either hematopoietic failure or GVHD could be the primary cause of death. To address this question, we first compared the development of GVHD in sublethally irradiated B6D2F1 mice that received allogeneic splenocytes alone or along with BMCs from B6 parental donors. We found that IFN-γ promotes alloresponses that predominantly target the recipient hematopoietic cells, while inhibiting injury of epithelial GVHD target tissues. Mice that received splenocytes plus BMCs from WT B6 donors survived long-term, but those receiving WT B6 splenocytes without BMCs died rapidly because of hematopoietic failure. However, recipient mice developed lethal GVHD after receiving GKO B6 splenocytes, regardless of whether or not donor BMCs were given at the same time. Unlike the recipients of WT allo-HCT, these mice showed markedly prolonged survival of host hematopoietic cells but severe epithelial tissue GVHD-associated damage. These results demonstrate that IFN-γ can inhibit GVHD even in sublethally irradiated mice and that the acceleration of death by IFN-γ in mice receiving allogeneic T cells without BMCs was the result of the destruction of host hematopoietic cells but not epithelial GVHD. IFN-γ not only augments the LGVHR of CD4 T cells14 but also promotes the destruction of host hematopoietic cells by donor CD8 T cells (Figure S2). Importantly, similar to previous observations in lethally irradiated models, IFN-γ is required for the development of antitumor responses that have been shown to be mediated by both donor CD4 and CD8 T cells.25 The GVL effect was markedly diminished in the sublethally irradiated recipients of GKO allo-HCT compared with those receiving WT allo-HCT.
IFN-γ was also found to enhance LGVHR and GVL effects while inhibiting GVHD in mixed allogeneic chimeras after delayed administration of DLI. Consistent with previous studies,18,19,21 DLI from WT allogeneic donors mediated strong GVL effects and rapid conversion from mixed chimerism to full donor chimerism, without causing severe GVHD in preestablished mixed chimeras. However, DLI showed markedly reduced GVL effects but increased ability to cause GVHD in mixed chimeras in the absence of donor-derived IFN-γ. DLI from GKO donors failed to prevent death from leukemia but resulted in more severe GVHD in mixed chimeras prepared with GKO allogeneic BMCs. Interestingly, GKO DLI-induced GVL effects in the chimeras established with WT B6 BMCs were comparable with those induced by WT DLI in the chimeras established with GKO B6 BMCs, indicating that IFN-γ produced by both DLI cells and donor BMC-derived cells may enhance GVL effects. Our preliminary studies using Rag2 KO and Rag2/γc double KO mice suggest that IFN-γ produced by allogeneic donor BMC-derived T cells is probably essential for restoration of the antitumor responses of GKO DLI in preestablished mixed allogeneic chimeras (W.A., H.W., Y.-G.Y., unpublished data, October 2005). IFN-γ produced by DLI cells and donor BM-derived cells was also found to promote the conversion to full donor chimerism. Importantly, IFN-γ produced by the 2 sources of cells was protective against GVHD, with DLI cell-derived IFN-γ appearing more effective.
IFN-γ plays an important role in the generation and function of CD4 regulatory T (Treg) cells,26 which have been shown to protect against GVHD.27,28 However, the role of IFN-γ in Treg function is probably not the major mechanism for its inhibition of GVHD, as IFN-γ mediates similar protection in mice receiving CD4-depleted allo-HCT.9,29 We and others have shown that IFN-γ induces apoptosis of activated T cells,30-33 including alloreactive T cells,9 and suppresses T-cell proliferation.9 A recent study demonstrated that IFN-γ can mediate antihematopoietic activity through its direct effect on hematopoietic cells, presumably by killing and inhibition of proliferation of hematopoietic cells.34 The present study suggests a model wherein IFN-γ inhibits alloreactive T cell–induced epithelial tissue injury but promotes destruction of the recipient hematopoietic cells, including leukemic cells. However, our study does not explain the increased epithelial GVHD observed in the absence of IFN-γ. Either enhanced trafficking of activated donor T cells to epithelial tissues, increased donor T-cell expansion (because of reduced apoptosis and/or increased proliferation) in the epithelial tissues, or a combination of both might be responsible, and we are currently exploring these possibilities. A recent study using IFN-γ receptor-deficient mice showed that the direct interaction of IFN-γ with the recipient pulmonary parenchyma, but not with the donor cells, prevents idiopathic pneumonia syndrome in lethally irradiated mice after allo-HCT.12 Although this mechanism cannot fully explain the distinct effects of IFN-γ on LGVHR/GVL effects and GVHD, it is consistent with our observation of an inhibitory effect of IFN-γ on alloresponses in parenchymal GVHD target tissues. In addition, GVHD target tissue injury in allo-HCT recipients is largely mediated by inflammatory responses triggered by tissue-infiltrating alloreactive T cells, but alloreactive T cell–mediated cytotoxicity plays a critical role in eliminating host leukemia and normal hematopoietic cells. IFN-γ mediates potent inhibition of the development of highly inflammatory Th17 cells.35,36 Although the role of Th17 cells in GVHD remains controversial,37-39 these cells have been shown to mediate lethal acute GVHD in mice.38,39 A recent report showed that the absence of IFN-γ promotes expansion of IL-17A–producing CD4+ cells and IL-17A production, and that administration of anti-IL-17A-depleting antibodies ameliorates GVHD in mice receiving GKO allo-HCT.40 Further understanding of the role of IFN-γ in the development of these immune responses may provide insight into the mechanisms of IFN-γ–mediated separation of GVHD and GVL effects.
IFN-γ has been shown to mediate antitumor effects by directly inhibiting tumor cell growth and inducing T-cell–mediated antitumor responses.41-44 IFN-γ has also been shown to augment the sensitivity of tumor cells to cytotoxic T lymphocytes by up-regulating surface expression of Fas and major histocompatibility complex on tumor cells.45,46 We have previously shown that IFN-γ is required for the optimal induction of donor CD8 T cell–mediated GVL effects against EL4 (B6 lymphoma) cells in lethally irradiated allo-HCT recipients.29 In vitro studies showed that IFN-γ does not significantly inhibit EL4 cell proliferation but may sensitize EL4 cells to the cytotoxicity of alloreactive cytotoxic T lymphocytes.29 We have recently established a leukemia model using T lymphoma cells that do not express a functional IFN-γ receptor. Preliminary data showed that WT allo-HCT also mediates significantly stronger GVL effects than GKO allo-HCT against IFN-γ–unresponsive leukemia cells, indicating that IFN-γ may mediate GVL effects independently of its direct effect on the leukemic cells (H.W., Y.-G.Y., unpublished data, September 2008). Taken together, these studies provide direct evidence that IFN-γ plays an important role in regulating the alloreactivity of donor immune cells and that the absence of donor-derived IFN-γ may promote GVHD but reduce antitumor responses in reduced intensity conditioning allo-HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Christene Huang and Giovanna Andreola for critical review of this manuscript, Mr Orlando Moreno for outstanding animal husbandry, and Ms Kelly Walsh for expert assistance with the manuscript.
This work was supported by grants from National Institutes of Health (Bethesda, MD; 5P01CA111519-020002) and American Cancer Society (Atlanta, GA; RSG-03-227-01-LIB).
National Institutes of Health
Authorship
Contribution: H.W. designed and performed experiments, analyzed data, and wrote the paper; W.A. designed and performed experiments; B.Y.Y. performed statistical analysis; M.-G.W. performed pathologic analysis; S.W. performed experiments; M.S. contributed to the development of the project and wrote the paper; and Y.-G.Y. conceived the research project, designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Guang Yang, Transplantation Biology Research Center, Massachusetts General Hospital, Harvard Medical School, MGH-East, Bldg 149-5102, 13th St, Boston, MA 02129; e-mail: yongguang.yang@tbrc.mgh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal