Abstract
Delta-like 4 (DLL4) is one of the Notch ligands and plays an important role in vascular development. DLL4 blockade inhibits tumor growth by promoting nonproductive angiogenesis, which is characterized by an increase in vascular density and decrease in tissue perfusion. However, a detailed mechanism remains unclear. In this study, newly developed neutralizing antibodies against mouse and human DLL4 were used to investigate the possible involvement of VEGF-DLL4-ephrinB2 cascade in nonproductive angiogenesis caused by DLL4 blockade. DLL4 blockade and soluble ephrinB2 treatment suppressed tumor growth and induced nonproductive angiogenesis. DLL4 was expressed in subcutaneous tumors, and DLL4 blockade suppressed ephrinB2 expression in the tumors. DLL4 blockade significantly promoted human umbilical vein endothelial cell (HUVEC) proliferation in vitro, and the effect was additive to that of VEGF. Both DLL4 blockade and VEGF significantly increased cord length and branch points in a tubular formation assay. Expression of ephrinB2 in HUVECs was enhanced by VEGF alone, and the enhancement was inhibited by DLL4 blockade. Moreover, when we studied the effect of ephrinB2 RNA interference on HUVEC tubular formation, knockdown of ephrinB2 mimicked the effect of DLL4. These results suggest that ephrinB2 plays a crucial role in nonproductive angiogenesis caused by DLL4 blockade.
Introduction
Tumor angiogenesis is an important process in solid tumor growth.1 Growing tumors stimulate neovascularization through the secretion of proangiogenic growth factors, in particular, basic fibroblast growth factor and vascular endothelial growth factor (VEGF).2 Based on accumulated studies concerning VEGF in tumor angiogenesis, anti-VEGF inhibitors such as bevacizumab, sunitinib, and sorafenib have been approved for clinical treatment of solid tumors in humans.3
Notch signals are evolutionarily conserved signaling mechanisms for intercellular communication and cell fate decision.4 Four Notch receptors (Notch1, 2, 3, 4) and 5 ligands (Jagged 1 and 2, and Delta-like 1, 3, and 4) have been identified in mammals.5,6 Once the ligand binds to the Notch receptor, it triggers the proteolytic release of the Notch intracellular domain, which translocates into the nucleus to form a nuclear complex with the transcription factor CSL (CBF1/RBP-j/Su(H)/Lag-1) and activates transcription of the downstream target gene. The hairy/enhancer of Split (HES1, 5, and 7) and the HES-related proteins (HEY1 and 2 and HEYL) are the main target genes of the Notch signaling in mammals.7-9
Several observations indicate that Notch signaling plays a critical role in vascular development.10-12 Notch1 and Notch4 genes are expressed in endothelial cells within the embryonic vasculature, and Notch1 alone– or Notch1 plus Notch4–deficient mice display a severe defect in angiogenic vascular remodeling.13-15 Overexpression of Notch4 also causes vascular abnormalities.16 These observations suggest that an optimal range of Notch signaling is required for vascular development.
Delta-like 4 (DLL4) is the most recently identified Notch ligand and was found to interact with Notch1 and Notch4.17,18 Haploinsufficiency of DLL4 produces severe vascular abnormalities that result in embryonic lethality.19,20 Although many genes are involved in the developing vasculature, except for the VEGF gene, DLL4 is the only gene whose haploinsufficiency leads to major vascular defects and embryonic lethality.21
Notch signaling regulates not only embryonic vasculature but also tumor angiogenesis. Notch1, Notch4, Jagged1, and DLL4 are reported to participate in tumor angiogenesis.22 Especially, DLL4 is highly expressed in human clear-cell renal cell carcinomas, bladder cancers, and breast cancers, and its interaction with VEGF is reported.22-26 Therefore, DLL4 is expected to be a potentially important target of antiangiogenic therapy.
Eph receptor tyrosine kinases and their membrane-bound ephrin ligands mediate various cell-to-cell communications by bidirectional signaling and are involved in numerous developmental processes.27 Eph receptors have been divided in 2 subclasses, classes A and B, depending on the type of interaction with the ephrin ligands.28,29 In general, Eph class A receptors (EphA) bind to GPI-anchored ephrin ligands (ephrinA), whereas Eph class B receptors (EphB) bind to ephrin ligands containing transmembrane domain (ephrinB).28,29 In vascular development, ephrinB2 and its cognate receptor EphB have attracted the most interest, because their homozygous mutation results in embryonic lethality.30,31 EphrinB2 is expressed mainly on arterial endothelial cells, whereas venous endothelial cells express EphB4 as its main receptor.32 Recently, ephrinB2 was reported to be involved in the maturation of tumor blood vessels.26,33-35
It has been described that VEGF induces the expression of DLL4 in endothelial cells and DLL4 up-regulates ephrinB2 expression in both physiological and pathological conditions.26,36-40 This VEGF-DLL4-ephrinB2 cascade is reported in transgenic mice developing hepatocarcinoma.26 Recently, several groups have reported that the DLL4 blockade inhibits tumor growth by inducing nonproductive angiogenesis manifested by an increased tumor vascular density but a decreased tissue perfusion. However, the precise mechanism has not been elucidated.41-44
In this study, whether the VEGF-DLL4-ephrinB2 cascade plays an important role in the antitumor effect of DLL4 blockade and the nonproductive angiogenesis in vivo and in vitro was investigated.
Methods
Reagents
An anti–mouse DLL4 mAb (HMD4-2, hamster IgG) was generated by immunizing an Armenian hamster with recombinant mouse DLL4 (R&D Systems, Minneapolis, MN) and screening mAbs that blocked rat Notch1-Fc (R&D Systems) binding to mouse DLL4-expressing CHO cells (provided by Dr S. Chiba at Tokyo University, Tokyo, Japan). The anti–human DLL4 mAb (MHD4-46, mouse IgG1) was generated by immunizing a BALB/c mouse with recombinant human DLL4 (R&D Systems) and screening mAbs that blocked human Notch1-Fc (R&D Systems) binding to human DLL4-expressing CHO cells (provided by Dr S. Chiba). The anti–human Notch1 mAb (MHN1-519, mouse IgG1) was generated by immunizing a BALB/c mouse with recombinant human Notch1-Fc (R&D Systems). The following antibodies were used for Western blotting or immunohistochemistry: anti-factor VIII–related antigen antibody (DAKO, Carpinteria, CA), rabbit antiephrinB2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for Western blotting, goat antiephrinB2 antibody (R&D Systems) for immunohistochemistry, rabbit anti-EphB4 antibody (Santa Cruz Biotechnology), anti–β-actin antibody (R&D Systems), and hypoxyprobe-1 (Chemicon, Temecula, CA). Recombinant human VEGF and recombinant mouse ephrinB2-Fc were purchased from R&D Systems.
Cell cultures
A lung squamous cell carcinoma cell line KLN205 was obtained from Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University, and a Lewis lung carcinoma cell line (LLC) was purchased from ATCC (Rockville, MD). KLN205 cells were cultured in MEM (Life Technologies, Gaithersburg, MD) supplemented with 10% FBS, 1% NEAA, and antibiotics. LLC cells were cultured in DMEM (Life Technologies) supplemented with 10% FBS and antibiotics. Human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells (HMVECs) were purchased from Kurabo (Osaka, Japan). HUVECs were cultured in HuMedia-EG2 (Kurabo) containing 2% FCS. HMVECs were cultured in HuMedia-MvG (Kurabo) containing 5% FCS. Both HUVECs and HMVECs were used at passage 4 or below.
Mouse tumor models
KLN205 cells (5 × 105/animal) were implanted subcutaneously into the flank of male 6-week-old BDF1 mice on day 0. LLC cells (3 × 105 cells/animal) were implanted into the male 6-week-old C57BL/6 mice in the same way. Mice that received an implant of KLN205 cells were randomly allocated into 4 groups. Two groups received an intraperitoneal injection of HMD4-2 (0.25 mg/day per animal) or control hamster IgG (0.25 mg/day per animal) every 3 days from day 6, another 2 groups received intratumoral injection of recombinant mouse ephrinB2-Fc (0.1 mg/day per animal) or control human IgG (0.1 mg/day per animal) every other day from day 20. Mice that received an implant of LLC cells were randomly allocated into 3 groups. Each group received an intraperitoneal injection of high-dose HMD4-2 (1.0 mg/day per animal), low-dose HMD4-2 (0.25 mg/day per animal), or control hamster IgG (0.25 mg/day per animal) every 3 days from day 6. Half of the mice in each group were killed at the intended day, that is, when the diameter of the tumor became approximately 1 cm for HMD4-2 experiments and approximately 1.5 cm for soluble epheinB2 experiments, and then the tumors were removed and immediately frozen with liquid nitrogen or fixed in 10% neutral buffered formalin. The rest of mice were killed at day 36 in mice that received an implant of KLN205 cells, and at day 25 in mice that received an implant of LLC cells. All animal studies were performed with the permission of the Regional Council of Tohoku University and conformed to the Guide for the Care and Use of Laboratory Animals.45
RNA isolation and reverse-transcription–PCR
Total RNA was extracted from the subcutaneous tumors, and from cultured HUVECs stimulated with or without VEGF, KLN205 cells, and LLC cells using Iso-gene (Nippongene, Osaka, Japan) according to the manufacturer's instructions. First-strand cDNA was synthesized from RNA (1 μg) using a One-Step RNA PCR Kit (Takara, Shiga, Japan). The following primers were used for polymerase chain reaction (PCR): human DLL4 forward (5′-GACCACTTCGGCCACTATGT-3′), reverse (5′-CCTGTCCACTTTCTTCTCG-3′); murine DLL4 forward (5′-AGCTGGAAGTGGACTGTGGT-3′), reverse (5-TAGAGTCCCTGGGAGAGCAA-3′); human Notch1 forward (5′-CAGGCAATCCGAGGACTATG-3′), reverse (5′-CAGGCGTGTTGTTCTCACAG-3′); human Notch4 forward (5′-CACTGAGCCAAGGCATAGAC-3′), reverse (5′-ATCTCCACCTCACACCACTG-3′); human β-actin forward (5′-CATCACCATTGGCAATGAGC-3′), reverse (5′-CGATCCACACGGAGTACTTG-3′); and murine GAPDH forward (5′-CACCACCATGGAGAAGGCCGGG-3′), reverse (5′-GTGTAGCCCAAGATGCCCTTCA-3′).
Western blot analysis
HUVECs (5 × 105) and HMVECs (8 × 105) were plated onto 10-mm collagen-coated culture dishes in HuMedia-EBM (Kurabo) containing 0.1% FCS for 16 hours, and stimulated with 100 ng/mL VEGF for 6 hours. Then the medium was exchanged, and the HUVECs and HMVECs were treated with MHD4-46 or control mouse IgG (50 μg/mL) for 12 hours. The cells were lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer (0.5 mL). Minced tumors (0.2 mg) were lysed in ice-cold RIPA buffer (2 mL), homogenized with 5 passes of a homogenizer, and solubilized for 30 minutes at 4°C with shaking. After centrifugation at 12 000g for 30 minutes, protein concentrations of supernatants were measured by the BCA protein assay kit (Pierce, Rockford, IL). The supernatants were mixed in Laemmli buffer and boiled. The cell lysates were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membrane was immunoblotted with antibodies to β-actin, DLL4, ephrinB2, or EphB4. The membranes were developed with the enhanced chemiluminescence (ECL) Advance Western Blotting Detection System Plus (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. Quantification of signals was performed with ImageJ (National Institutes of Health [NIH], Bethesda, MD).
Immunohistochemistry
Immunohistologic staining was performed as described previously.46 When the diameter of the tumor became approximately 1 cm for HMD4-2 experiments and approximately 1.5 cm for soluble epheinB2 experiments, tumor tissues were fixed in 10% formalin, and embedded in paraffin. Paraffin-embedded sections were cut at 5-μm thickness, deparaffinized, and rehydrated. Endogenous peroxidase activity was blocked with hydrogen peroxide/methanol, and antigen retrieval was performed in a pH 6.0 citrate buffer by autoclave for 5 minutes. After the washing and blocking steps, the resultant tissue sections were incubated overnight with primary antibodies, followed by biotinylated secondary antibodies for 30 minutes at room temperature. Then, immunoreactivity was visualized with an ABC kit (Vector, Burlingame, CA), and detected by 3-amino-9-ethylcarbazole (Vector). The following primary antibodies were used: polyclonal anti–human factor VIII–related antigen antibody (1:200; DAKO), hamster anti–mouse DLL4 antibody (1:5; HMD4-2), and goat anti–mouse ephrinB2 antibody (1;300; R&D Systems). For secondary antibodies, biotinylated goat anti–mouse IgG antibody (1:200; Vector), goat anti–hamster IgG antibody (1:100; Santa Cruz Biotechnology), and rabbit anti–goat IgG antibody (1:800; Vector) were used.
To measure the hypoxic area, pimonidazole hydrochloride (hypoxyprobe-1; Chemicon) was injected intraperitoneally at a dose of 60 mg/kg 30 minutes before killing, and the vasculature was perfused transcardially with 5 mL of 10% formalin prior to the killing. The distribution of hypoxyprobe-1 was detected using an antihypoxyprobe antibody according to the recommended protocol. The sections were counterstained with hematoxylin.
The intratumoral microvessel area and hypoxic area were quantified. In brief, the intratumoral endothelial cells were stained immunohistochemically with antihuman factor VIII–related antigen antibody, and the hypoxic area was stained with antihypoxyprobe antibody. The images that contained the highest number of endothelial cells were chosen for each section by an initial scan at ×100 magnification. Then the areas of factor VIII–positive cells and those stained with antihypoxyprobe antibody were measured in the selected image at ×100 magnification. At least 4 fields were measured for each section, and the highest count was taken. Two independent investigators evaluated the areas. The sections were photographed with digital camera (Olympus, Melville, NY) attached to an inverted microscope (Nikon, Tokyo, Japan).
Flow cytometry
HUVECs and HMVECs were cultured, treated with trypsin-EDTA, and suspended in PBS. The cells were first incubated with PBS containing 0.1% BSA to block nonspecific binding. After washing, the cells were incubated on ice with a PE-labeled control hamster IgG, PE-labeled control mouse IgG, biotinylated MHD4-46, or biotinylated MHN1-519. The cells were then incubated with PE-conjugated streptavidin (eBioscience, San Diego, CA). After washing again, the cells were subjected to flow cytometry on a FACScan (BD Biosciences, San Jose, CA), and the data were analyzed with CellQuest software (BD Biosciences). For all samples, dead cells were excluded from the analysis by propidium iodide staining.
Cell proliferation assay
The cell proliferation assay was performed as previously described.46 Briefly, HUVECs (4 × 103/well) or HMVECs (5 × 103/well) were plated onto 96-well plates containing the medium supplemented with MHD4-46 (50 μg/mL), VEGF (100 ng/mL), or control IgG. HUVECs were cultured for 4 days, and the medium was exchanged on day 2. The cell number was determined by water-soluble tetrazolium (WST) assay using a cell counting kit (Dojindo, Kumamoto, Japan).
In vitro angiogenesis (tubular formation) assay
HUVECs and HMVECs were cultured in each culture medium until they were approximately 80% confluent. Before each experiment, the culture medium was replaced with serum-free HuMedia-EB2 (Kurabo) for 16 hours. The cells were then trypsinized, counted, and resuspended at a concentration of 8 × 104 cells/mL in HuMedia-EB2 containing 0.5% FCS and supplemented with rhVEGF (100 ng/mL), MHD4-46 (50 μg/mL), control IgG, or a combination of these. The wells of 24-well tissue culture plates were coated with 0.5 mL/well growth factor–reduced Matrigel (Becton Dickinson Labware, Bedford, MA), which was allowed to solidify at 37.8°C for 30 minutes, according to the manufacturer's instructions. The cell suspensions were then plated (0.5 mL/well) onto the surface of the Matrigel and incubated at 37.8°C. The cells were observed using an inverted phase contrast microscope (Nikon Eclipse TE300) after 12 hours. Images were captured with a laser scanning confocal imaging system (Bio-Rad, Hercules, CA). The length of the cordlike structure and the branch point number were measured in randomly selected fields located within 5 mm of the center of the well using the NIH image program.
In vitro invasion assay
Cell invasion assays were performed using a 96-well BME Cell Invasion Assay (R&D Systems) according to the manufacturer's instructions. This kit was constructed mainly of the 96-well Boyden chamber, 8.0-μm polyester membrane, and the basement membrane extract (BME). In brief, the upper wells of the cell invasion device were coated with the BME, and BMEs were polymerized prior to the addition of cells to the upper wells. HUVECs were starved for 16 hours before the experiment, and seeded onto the upper wells (5 × 104 cells/well) in HuMedia-EB2 without FCS. Lower wells were given HuMedia-EB2 supplemented with VEGF (100 ng/mL), MHD4-46 (50 μg/mL), control IgG, or a combination of these. Twenty-four hours later, HUVECs that had invaded through the ECM to the underside of the membrane were detached from the membranes with the Cell Dissociation Solution, and stained using a Calcein AM (Invitrogen, Tokyo, Japan). The fluorescence was measured by the Fluoroskan Ascent (Labsystems, Arlington, MA). The number of invaded cells was calculated from standard curves.
RNA interference
RNA interference was used to down-regulate the expression of ephrinB2 in HUVECs. Small interference RNA (siRNA) for ephrinB2 (5′-GGAAUAAAGAUCCAACAAG-3′ or 5′-GGACUGGUACUAUACCCAC-3′) and nonspecific control siRNA (negative control no. 1) were designed and synthesized by Silencer Validated siRNA Design from Ambion (Austin, TX). For gene knockdown experiments, HUVECs were plated in a 10-cm dish, and were transfected with siRNAs (10 nM, control siRNA, siRNA to ephrinB2) using transfection reagent (siPORT NeoFX Transfection Agent; Ambion) according to the manufacturer's instructions.
Data analysis
Statistical analysis of the results was performed using the unpaired Student t test for the comparison between 2 sample groups and ANOVA with Fisher least significant difference test for the multiple comparisons. A value of P less than .05 was considered significant.
Results
Properties of neutralizing antibodies against mouse and human DLL4
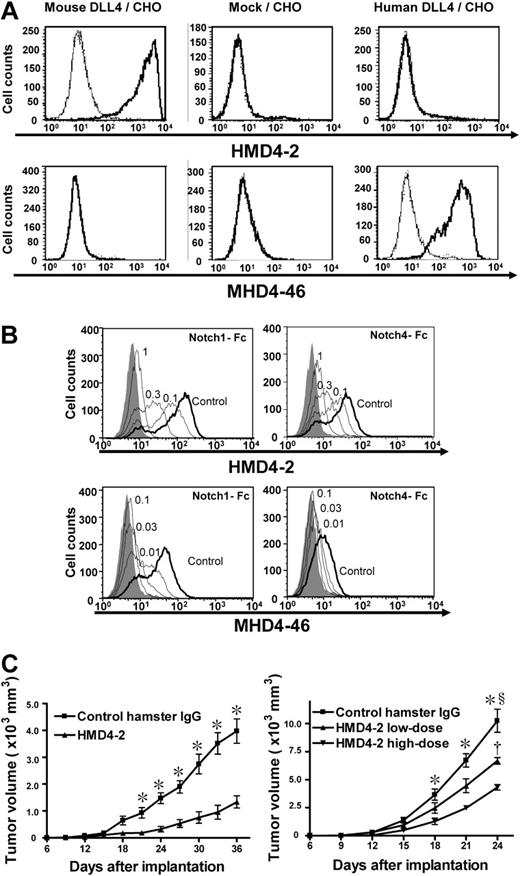
The selective binding of HMD4-2 and MHD4-46 to CHO cells expressing mouse or human DLL4 was confirmed by flow cytometry (Figure 1A). HMD4-2 selectively bound to mouse DLL4, and MHD4-46 selectively bound to human DLL4. We also confirmed the ability of these antibodies to block Notch1-Fc and Notch4-Fc binding to DLL4-expressing CHO cells in a dose-dependent manner (Figure 1B). HMD4-2 blocked the Notch1-Fc and Notch4-Fc binding to mouse DLL4, and MHD4-46 blocked the Notch1-Fc and Notch4-Fc binding to human DLL4. Furthermore, we confirmed that the HMD4-2 did not bind to CHO cells expressing mouse DLL1, Jagged1, or Jagged2 by flow cytometry.47 Similarly, we confirmed that the MHD4-46 did not bind to CHO cells expressing human DLL1, Jagged1, or Jagged2 by flow cytometry (N.Y. and H.Y., unpublished observation).
Characterization of HMD4-2 and MHD4-46 and effect of HMD4-2 on tumor growth. (A) Flow cytometric analysis showed the specific bindings of HMD4-2 to mouse DLL4 and MHD4-46 to human DLL4. Bold histogram shows the staining with biotinylated HMD4-2 or MHD4-46 followed by PE-labeled streptavidin. Thin histogram shows the staining with biotinylated control hamster IgG or control mouse IgG1 followed by PE-labeled streptavidin. (B) The antibodies blocked Notch1-Fc and Notch4-Fc binding to DLL4. Binding of mouse Notch1-Fc (top left) and mouse Notch4-Fc (top right) to mouse Dll4/CHO cells was blocked by the indicated doses (μg) of HMD4-2. Binding of human Notch1-Fc (bottom left) and mouse Notch4-Fc (bottom right) to human Dll4/CHO cells was blocked by the indicated doses (μg) of MHD4-46. The shaded histograms indicate the background staining without Notch-Fc. (C) HMD4-2 inhibited subcutaneous tumor growth in mouse tumor models with KLN205 (left) and LLC (right). The vertical axis showed the tumor volume and horizontal axis showed the time after implantation. The values represent the mean plus or minus SE (n = 7-10 per group). *P < .05 versus control, §P < .05 versus HMD4-2 low dose, †P < .05 versus HMD4-2 high dose, by Student unpaired t test or 1-way ANOVA with Fisher least-significant-difference test at each time point.
Characterization of HMD4-2 and MHD4-46 and effect of HMD4-2 on tumor growth. (A) Flow cytometric analysis showed the specific bindings of HMD4-2 to mouse DLL4 and MHD4-46 to human DLL4. Bold histogram shows the staining with biotinylated HMD4-2 or MHD4-46 followed by PE-labeled streptavidin. Thin histogram shows the staining with biotinylated control hamster IgG or control mouse IgG1 followed by PE-labeled streptavidin. (B) The antibodies blocked Notch1-Fc and Notch4-Fc binding to DLL4. Binding of mouse Notch1-Fc (top left) and mouse Notch4-Fc (top right) to mouse Dll4/CHO cells was blocked by the indicated doses (μg) of HMD4-2. Binding of human Notch1-Fc (bottom left) and mouse Notch4-Fc (bottom right) to human Dll4/CHO cells was blocked by the indicated doses (μg) of MHD4-46. The shaded histograms indicate the background staining without Notch-Fc. (C) HMD4-2 inhibited subcutaneous tumor growth in mouse tumor models with KLN205 (left) and LLC (right). The vertical axis showed the tumor volume and horizontal axis showed the time after implantation. The values represent the mean plus or minus SE (n = 7-10 per group). *P < .05 versus control, §P < .05 versus HMD4-2 low dose, †P < .05 versus HMD4-2 high dose, by Student unpaired t test or 1-way ANOVA with Fisher least-significant-difference test at each time point.
DLL4 blockade suppressed tumor growth by inducing nonproductive angiogenesis
To examine the effect of DLL4 blockade on solid tumor growth in vivo, KLN205 cells and LLC cells were implanted subcutaneously in BDF1 or C57BL/6 mice, respectively, and the mice were treated with HMD4-2 or control hamster IgG, as described in “Mouse tumor models.” KLN205 tumors showed similar growth rates until day 18 (Figure 1C left). After day 21, the KLN205 tumors treated with HMD4-2 (0.25 mg) were significantly smaller than those treated with control IgG. In the C57BL/6 mice that received an implant of LLC cells, we treated mice with 2 different doses of HMD4-2 (Figure 1C right). Tumors of the high-dose HMD4-2 (1 mg) group were significantly smaller than those of the control group after day 18. Tumors of the low-dose HMD4-2 (0.25 mg) group were significantly smaller than those of the control group and were significantly larger than tumors of the high-dose HMD4-2 group at day 24.
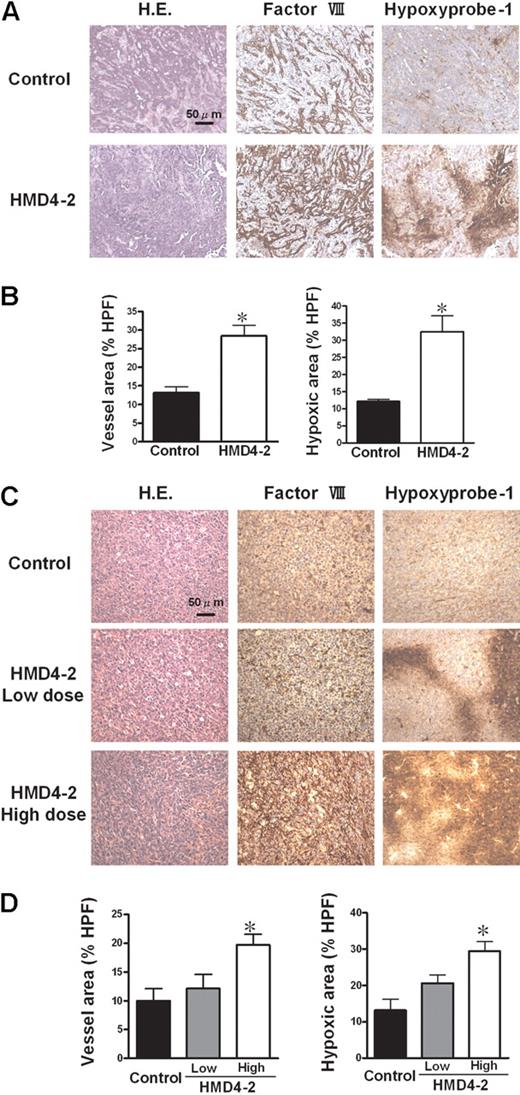
To evaluate angiogenesis and hypoxic lesions in these tumors, we immunostained the sections for factor VIII, which is expressed on endothelial cells, and used a hypoxyprobe-1 kit to detect hypoxic lesions. The immunostaining showed a significant increase of factor VIII–positive endothelial cells in tumors treated with HMD4-2 (Figure 2A-D). However, hypoxic lesions were also significantly increased in HMD4-2–treated mice (Figure 2A-D). Moreover, these effects were dose dependent in LLC groups (Figure 2C,D). These results suggested that the suppressive effect of HMD4-2 on tumor growth was caused by the increased nonfunctional angiogenesis resulting in a reduced blood supply represented as an increase of hypoxic area in tumors.
Effect of HMD4-2 on tumor angiogenesis and hypoxia. (A,C) Hematoxylin and eosin (H.E.) staining and immunohistologic staining for factor VIII–related antigen and for hypoxia (hypoxyprobe-1 kit) of KLN205 tumors (A) and LLC tumors (C). The hypoxic area was stained brown. (B,D) The quantitative analyses of histologic data of KLN205 tumors (B) and LLC tumors (D). The factor VIII–positive vessel area and hypoxic area were measured, and the percentages of the area in HPF (×100) were compared in each mouse tumor model. Data are mean plus or minus SE (n = 7 per group). *P < .05 versus control by Student unpaired t test or 1-way ANOVA with Fisher least-significant-difference test.
Effect of HMD4-2 on tumor angiogenesis and hypoxia. (A,C) Hematoxylin and eosin (H.E.) staining and immunohistologic staining for factor VIII–related antigen and for hypoxia (hypoxyprobe-1 kit) of KLN205 tumors (A) and LLC tumors (C). The hypoxic area was stained brown. (B,D) The quantitative analyses of histologic data of KLN205 tumors (B) and LLC tumors (D). The factor VIII–positive vessel area and hypoxic area were measured, and the percentages of the area in HPF (×100) were compared in each mouse tumor model. Data are mean plus or minus SE (n = 7 per group). *P < .05 versus control by Student unpaired t test or 1-way ANOVA with Fisher least-significant-difference test.
DLL4 was expressed in KLN205 tumors and DLL4 blockade suppressed ephrinB2 expression
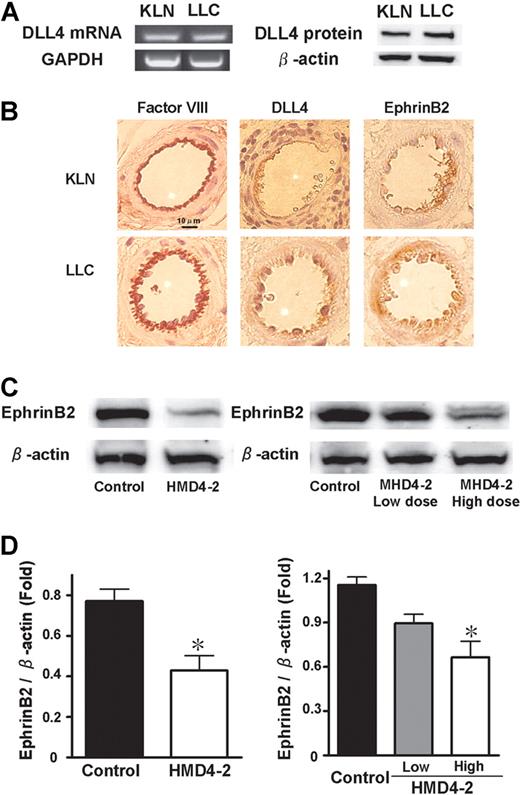
In vitro cultured KLN205 cells and LLC cells did not express DLL4 as estimated by reverse-transcription (RT)–PCR, Western blotting, or flow cytometry (data not shown). Therefore, whether DLL4 was expressed in the KLN205 and LLC tumor tissues was investigated. KLN205 and LLC tumors were isolated, and homogenized to extract mRNAs and proteins. In both KLN205 and LLC tumors, DLL4 was detected at comparable levels by both Western blotting and RT-PCR (Figure 3A). Immunohistochemical staining of KLN205 and LLC tumors showed that both DLL4 and ephrinB2 were expressed in the vascular endothelium in relatively larger vessels where cells were positively stained by factor VIII–related antigen antibody (Figure 3B). We further investigated ephrinB2 expression in HMD4-2–treated tumors. Western blotting showed that the expression of ephrinB2 was significantly decreased in the HMD4-2–treated tumors in comparison with those treated with control IgG in both KLN205 and LLC tumors, and these effects were dose dependent (Figure 3C,D). EphB4 expression in these tumors was not influenced by HMD4-2 treatments (data not shown).
Expression of DLL4 and ephrinB2 in KLN205 and LLC tumors. (A) Expression of DLL4 in subcutaneous KLN205 and LLC tumors was detected by RT-PCR (left) and Western blotting (right). (B) Representative immunohistochemical staining of factor VIII–related antigen, DLL4, and ephrinB2. Data were obtained from serial sections of the same area in both KLN205 and LLC tumors. (C) Western blotting of ephrinB2 in KLN205 (left) and LLC (right) tumors. (D) The relative expression of ephrinB2 was measured by densitometric analysis in KLN205 tumors (left) and LLC tumors (right). The results are expressed as the ratio of the amount of ephrinB2 protein to the amount of β-actin. Data are mean plus or minus SE (n = 7 per group). *P < .05 versus control by Student unpaired t test (left) or 1-way ANOVA with Fisher least-significant-difference test (right).
Expression of DLL4 and ephrinB2 in KLN205 and LLC tumors. (A) Expression of DLL4 in subcutaneous KLN205 and LLC tumors was detected by RT-PCR (left) and Western blotting (right). (B) Representative immunohistochemical staining of factor VIII–related antigen, DLL4, and ephrinB2. Data were obtained from serial sections of the same area in both KLN205 and LLC tumors. (C) Western blotting of ephrinB2 in KLN205 (left) and LLC (right) tumors. (D) The relative expression of ephrinB2 was measured by densitometric analysis in KLN205 tumors (left) and LLC tumors (right). The results are expressed as the ratio of the amount of ephrinB2 protein to the amount of β-actin. Data are mean plus or minus SE (n = 7 per group). *P < .05 versus control by Student unpaired t test (left) or 1-way ANOVA with Fisher least-significant-difference test (right).
DLL4 blockade promoted proliferation of endothelial cells
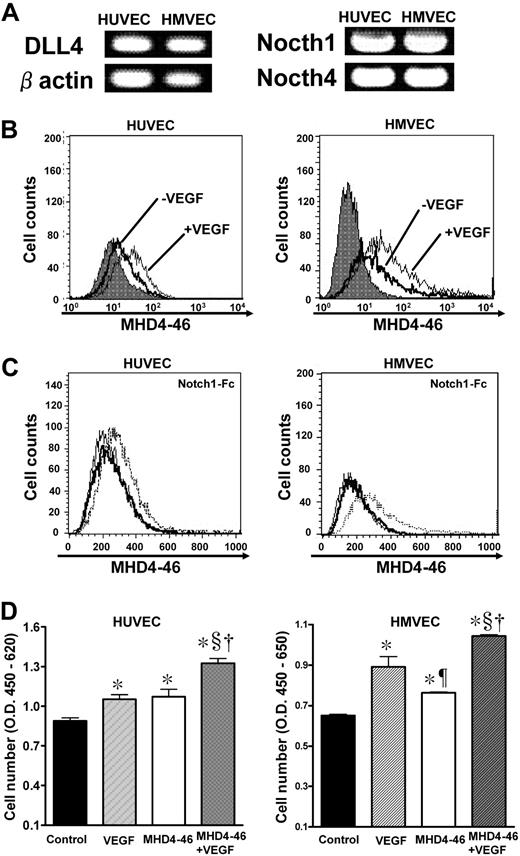
We found that HUVECs and HMVECs expressed DLL4, Notch1, and Notch4 as estimated by RT-PCR and flow cytometry for DLL4 with MHD4-46 (Figure 4A,B). VEGF (100 ng/mL) stimulation increased the DLL4 expression as estimated by flow cytometry (Figure 4B bottom). We confirmed the ability of MHD4-46 to block Notch1-Fc binding to DLL4 on HUVECs and HMVECs (Figure 4C). Then, the effect of DLL4 blockade on proliferation of these cell lines using MHD4-46 was investigated. MHD4-46 significantly promoted proliferation of these cell lines, and this proliferative effect was comparable with that of VEGF in HUVECs (Figure 4D). Notably, the proliferative effects of MHD4-46 and VEGF were additive.
DLL4 blockade facilitated proliferation of HUVECs in vitro. (A) Expression of DLL4, Notch1, and Notch4 in HUVECs and HMVECs was examined by RT-PCR. (B) The change of DLL4 expression was examined before or after VEGF stimulation by flow cytometry. The gray histogram shows the background staining with control mouse IgG1. (C) The dotted histogram shows the binding of Notch1-Fc after preincubation of the indicated cells with control IgG. The bold histogram shows the binding of Notch1-Fc after preincubation of the indicated cells with MHD4-46. Thin histogram shows the background staining. (D) HUVECs and HMVECs were treated with VEGF, MHD4-46, or both, and proliferation was determined by WST assay. The values represent mean plus or minus SE of triplicate wells repeated twice. *P < .05 versus control, ¶ and §P < .05 versus VEGF, †P < .05 versus MHD4-46 by 1-way ANOVA with Fisher least-significant-difference test.
DLL4 blockade facilitated proliferation of HUVECs in vitro. (A) Expression of DLL4, Notch1, and Notch4 in HUVECs and HMVECs was examined by RT-PCR. (B) The change of DLL4 expression was examined before or after VEGF stimulation by flow cytometry. The gray histogram shows the background staining with control mouse IgG1. (C) The dotted histogram shows the binding of Notch1-Fc after preincubation of the indicated cells with control IgG. The bold histogram shows the binding of Notch1-Fc after preincubation of the indicated cells with MHD4-46. Thin histogram shows the background staining. (D) HUVECs and HMVECs were treated with VEGF, MHD4-46, or both, and proliferation was determined by WST assay. The values represent mean plus or minus SE of triplicate wells repeated twice. *P < .05 versus control, ¶ and §P < .05 versus VEGF, †P < .05 versus MHD4-46 by 1-way ANOVA with Fisher least-significant-difference test.
Effect of DLL4 blockade on in vitro tubular formation and invasion
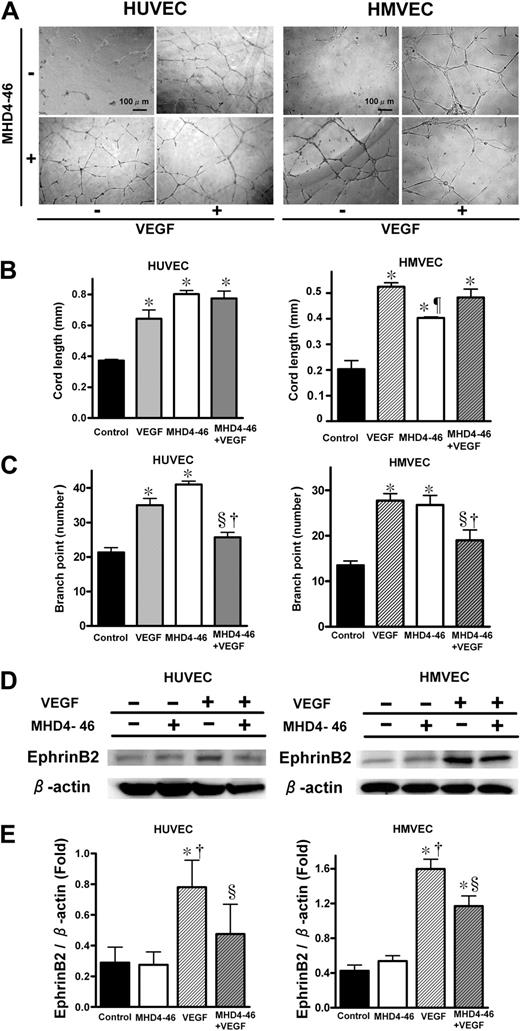
Tubular formation and invasion assays were performed to investigate the interaction between MHD4-46 and VEGF in in vitro angiogenesis. Tubular formations of HUVECs and HMVECs were affected by the addition of VEGF and/or MHD4-46 (Figure 5A). As shown in Figure 5B, either VEGF or MHD4-46 significantly increased cord length in the tubular formation assay. However, the combination of MHD4-46 and VEGF did not show an additive effect in cord length (Figure 5B). As shown in Figure 5C, both MHD4-46 and VEGF significantly increased the branch point number. In contrast, the branch point number in the combined application of MHD4-46 and VEGF was the same as the control and significantly lower than VEGF or MHD4-46 alone, suggesting that the effects of VEGF and MHD4-46 could counteract each other (Figure 5C). We also performed a cell invasion assay using the Boyden chamber. MHD4-46 itself did not induce the cell invasion. In addition, MHD4-46 had no apparent effect on VEGF-induced invasion of these cell lines (data not shown).
DLL4 blockade increased cord length, but decreased branch point number and suppressed VEGF-induced ephrinB2 expression. (A) Effect of MHD4-46 on HUVEC and HMEVC tubular formation. Representative microphotographs at ×100 magnification from triplicate wells of experiments repeated twice are shown. (B,C) Quantitative data of cord length (B) and brunch point number (C). (D) Protein expression of ephrinB2 and β-action in HUVECs and HMVECs. (E) The relative expression of ephrinB2 to β-action was measured by densitometric analysis. Data are shown as mean plus or minus SE of triplicate wells from the representative result of 3 independent experiments. *P < .05 versus control, §P < .05 versus VEGF, †P < .05 versus MHD4-46 by 1-way ANOVA with Fisher least-significant-difference test.
DLL4 blockade increased cord length, but decreased branch point number and suppressed VEGF-induced ephrinB2 expression. (A) Effect of MHD4-46 on HUVEC and HMEVC tubular formation. Representative microphotographs at ×100 magnification from triplicate wells of experiments repeated twice are shown. (B,C) Quantitative data of cord length (B) and brunch point number (C). (D) Protein expression of ephrinB2 and β-action in HUVECs and HMVECs. (E) The relative expression of ephrinB2 to β-action was measured by densitometric analysis. Data are shown as mean plus or minus SE of triplicate wells from the representative result of 3 independent experiments. *P < .05 versus control, §P < .05 versus VEGF, †P < .05 versus MHD4-46 by 1-way ANOVA with Fisher least-significant-difference test.
DLL4 blockade suppressed VEGF-induced ephrinB2 expression in endothelial cells
Since we found that the ephrinB2 expression in both KLN205 and LLC tumors was reduced by the DLL4 blockade in vivo, we hypothesized that this reduction occurred in the endothelial cells in tumor blood vessels. Therefore, we investigated the effect of DLL4 blockade on VEGF-induced ephrinB2 expression in HUVECs and HMVECs (Figure 5D,E). VEGF alone significantly enhanced the ephrinB2 expression in these endothelial cell lines, whereas MHD4-46 alone did not. The VEGF-induced enhancement of ephrinB2 was significantly inhibited by the addition of MHD4-46 (Figure 5E), suggesting that VEGF-induced ephrinB2 expression was mediated by DLL4 in endothelial cells.
Knockdown of ephrinB2 mimicked the effect of DLL4 blockade
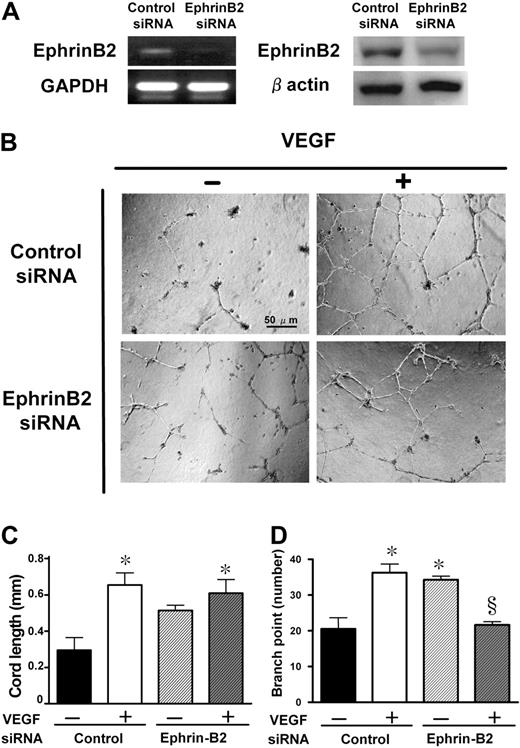
To investigate whether the DLL4-induced nonproductive angiogenesis resulted from suppression of ephrinB2, we performed an in vitro tubular formation assay after knockdown of ephrinB2 by RNA interference. Following 24 hours of transfection, a significant reduction of both mRNA and protein levels was achieved (Figure 6A). These HUVECs were plated on matrigel-coated wells, and stimulated with VEGF for 12 hours (Figure 6B). Although the knockdown of ephrinB2 alone did not significantly increase the cord length, it significantly increased the branch point number (Figure 6D). In the presence of VEGF in the tubular formation, knockdown of ephrinB2 significantly inhibited VEGF-induced enhancement of the branch point number but not the cord length (Figure 6C,D). We confirmed this effect using 2 different siRNAs as described in “RNA interference.”
Knockdown of ephrinB2 with siRNA and its effects on in vitro tubular formation. (A) Knockdown of ephrinB2 in HUVECs was evaluated both at mRNA and protein levels. The reduction rates in fold expression (ephrinB2 siRNA/control siRNA) of mRNA and protein levels were approximately 27% and 60%, respectively. (B) Effect of ephrinB2 knockdown on HUVEC tubular formation. Representative microphotographs at ×100 magnification from triplicate wells of 2 repeated experiments are shown. Quantitative data of cord length (C) and branch point number (D). Data are shown as mean plus or minus SE of triplicate wells in 2 experiments. *P < .05 versus control siRNA without VEGF and §P < .05 versus control siRNA with VEGF by 1-way ANOVA with Fisher least-significant-difference test.
Knockdown of ephrinB2 with siRNA and its effects on in vitro tubular formation. (A) Knockdown of ephrinB2 in HUVECs was evaluated both at mRNA and protein levels. The reduction rates in fold expression (ephrinB2 siRNA/control siRNA) of mRNA and protein levels were approximately 27% and 60%, respectively. (B) Effect of ephrinB2 knockdown on HUVEC tubular formation. Representative microphotographs at ×100 magnification from triplicate wells of 2 repeated experiments are shown. Quantitative data of cord length (C) and branch point number (D). Data are shown as mean plus or minus SE of triplicate wells in 2 experiments. *P < .05 versus control siRNA without VEGF and §P < .05 versus control siRNA with VEGF by 1-way ANOVA with Fisher least-significant-difference test.
Soluble form of ephrinB2 induced nonproductive angiogenesis and suppressed tumor growth
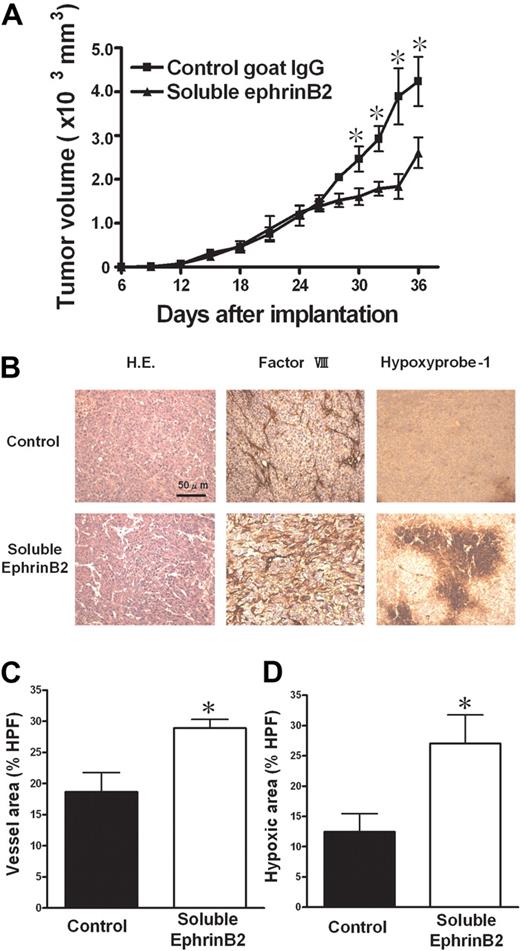
We investigated the effect of ephrinB2 interference on in vivo solid tumor growth using a soluble form of ephrinB2, recombinant mouse ephrinB2-Fc, as described in “Mouse tumor models.” Intratumoral injection of ephrinB2-Fc significantly suppressed KLN205 tumor growth compared with control IgG (Figure 7A). Histologic analysis revealed a significant increase in factor VIII–related antigen-positive vessels and hypoxic area when treated with ephrinB2-Fc, indicating angiogenesis (Figure 7B-D). These results suggested that ephrinB2/EphB signaling plays an important role in nonproductive angiogenesis.
Effect of soluble ephrinB2 on tumor growth. (A) A soluble form of ephrinB2 (ephrinB2-Fc) inhibited subcutaneous tumor growth in a mouse tumor models with KLN205. (B) Hematoxylin and eosin (H.E.) staining and immunohistologic staining for factor VIII–related antigen and for hypoxia (hypoxyprobe-1 kit). (C,D) The quantitative analyses of histologic data. The factor VIII–positive vessel area (C) and hypoxic area (D) were measured, and the percentages of the area in HPF (×100) were compared. Data are mean plus or minus SE (n = 7 per group). *P < .05 versus control by Student unpaired t test.
Effect of soluble ephrinB2 on tumor growth. (A) A soluble form of ephrinB2 (ephrinB2-Fc) inhibited subcutaneous tumor growth in a mouse tumor models with KLN205. (B) Hematoxylin and eosin (H.E.) staining and immunohistologic staining for factor VIII–related antigen and for hypoxia (hypoxyprobe-1 kit). (C,D) The quantitative analyses of histologic data. The factor VIII–positive vessel area (C) and hypoxic area (D) were measured, and the percentages of the area in HPF (×100) were compared. Data are mean plus or minus SE (n = 7 per group). *P < .05 versus control by Student unpaired t test.
Discussion
In this study, we showed that the blockade of DLL4 by HMD4-2 inhibited tumor growth accompanied by nonproductive angiogenesis and decreased ephrinB2 expression in tumor tissues. In addition, knockdown of ephrinB2 mimicked the effect of DLL4 on the HUVEC tubular formation. Moreover, we found that the interference of ephrinB2/Eph signaling induced nonproductive angiogenesis in tumors. These results suggest the decrease in ephrinB2 by DLL4 blockade was involved in the nonproductive angiogenesis.
A similar antitumor effect of DLL4 blockade was recently reported and characterized by nonproductive angiogenesis.41,43 In this abnormal angiogenesis, thin, dense, and nonperfusing vessels with few pericytes were observed.42 In our study, we also observed the same phenomena. It was also previously reported that VEGF induced the expression of DLL4 in vivo and in vitro38,39 and that DLL4 stimulation markedly induced ephrinB2 on endothelial cells in vitro.37 Another group reported the VEGF-DLL4-ephrinB2 cascade played a key role in the remodeling of tumor vessels.26 However, it has not been elucidated whether this cascade was involved in the nonproductive angiogenesis.
Up-regulation of ephrinB2 signaling enlarges vessel size and decreases vessel density in mouse tumor models.33-35 These effects of ephrinB2 signaling seemed to be opposite to those of DLL4 blockade. Moreover, ephrinB2 and its receptor EphB4 were critical for recruitment of mural cells and assembly of the vessel wall.32,48,49 Therefore, we investigated the ephrinB2 expression in this nonproductive angiogenesis, and confirmed the decrease in ephrinB2 expression in the tumors treated with HMD4-2. In vitro, the blockade of DLL4 with MHD4-46 promoted HUVEC proliferation and increased cord length and branch point number in the in vitro tubular formation assay. These data may explain the in vivo data such as the increased number and dense network of endothelial cells in the tumors. However, the combination of VEGF and MHD4-46 had no additive effect on cord length and diminished the facilitating effect on branch point number, suggesting the existence of cross-talk in the intracellular signaling pathways of vascular differentiations between VEGF and DLL4 in endothelial cells. The differences in vivo and in vitro may be explained by differences in the environment around endothelial cells, since tumor angiogenesis was performed not only by endothelial cells but also by various surrounding cells such as cancer cells, mural cells, pericytes, and macrophages.
We also investigated the relationship between DLL4 blockade and ephrinB2 expression in HUVECs in vitro. HUVECs have weak basal ephrinB2 expression, and this expression was enhanced by the addition of VEGF (Figure 5). MHD4-46 blocked this cascade and inhibited the VEGF-induced ephrinB2 expression. Although the other pathways were also implicated, these results suggest that VEGF-induced ephrinB2 expression was mediated, at least in a part, by DLL4. Knockdown of ephrinB2 resulted in an increase in branch point number, but did not affect cord length in the presence of VEGF. Since knockdown of ephrinB2 mimicked the effect of MHD4-46 on VEGF-induced endothelial differentiation, this suggests that DLL4 blockade induced the abnormal angiogenesis through the ephrinB2 suppression.
The soluble form of ephrinB2 is known to perturb ephrinB2/Eph signaling.50-52 In HUVECs, soluble ephrinB2 suppressed VEGF-induced ERK1/2 phosphorylation and Ras activation, resulting in inhibition of VEGF-induced endothelium proliferation, sprouting, and migration.53 In HMVECs, soluble ephrinB2 inhibited VEGF-induced migration by direct activation of the kinase activity of EphB4.50 In a mouse model of proliferative retinopathy, soluble ephrinB2 reduced retinal neovascularization.51 In our in vivo experiments, soluble ephrinB2 inhibited tumor growth and induced nonproductive angiogenesis (Figure 7). Moreover, we found that DLL4 blockade inhibited ephrinB2 in a dose-dependent manner, and induced nonproductive angiogenesis also in a dose-dependent manner in LLC tumors (Figures 1C, 2, and 3C,D). Taken together, involvement of ephrinB2 is implied as an underlying mechanism of nonproductive angiogenesis induced by DLL4 blockade.
Eph/ephrin pathways appear to be essential for morphogenesis of various organs in the embryo, because they regulate many of the dynamic changes that occur during this embryonic stage, including cell migration, axon guidance, and angiogenesis.27 Mutant animals lacking ephrinB2 die before day 11 of embryonic development, displaying defects in angiogenic remodeling.30,31 Eph and ephrin molecules are up-regulated not only in the embryonic stage but also in cancers and may affect tumor growth and neovascularization.26,33-35
In regard to ephrinB2, recent studies showed that EphB4/ephrinB2 interaction inhibited endothelial sprouting and promoted circumferential growth of vessels, and another group reported reverse signaling via ephrinB2 induced low microvascular density but large vessel diameter resulting in tumor progression.33-35 The ephrinB2 function in tumor angiogenesis seems to be quite contrary to that of DLL4 blockade. Here, we demonstrated DLL4 blockade induced nonproductive angiogenesis, at least in part, through ephrinB2 suppression. We do not consider ephrinB2 as the only target of DLL4, because DLL4 or VEGF is the gene whose haploinsufficiency led to embryonic lethality, whereas ephrinB2 is not.21 Our data suggest that a decrease in ephrinB2 expression played, at least in part, an important role in the nonproductive angiogenesis induced by DLL4 blockade.
Solid tumors require blood vessels for growth. Therefore, antiangiogenic therapies have emerged as an important option for treating several types of cancers.1 It was considered that antiangiogenic therapy killed cancer cells by depriving oxygen and nutrients, so that it could reduce drug delivery and induce hypoxia-related resistance to chemotherapy and radiotherapy.52 However, the combination of anti-VEGF agents with cytotoxic drugs or irradiation showed promising results in many clinical and basic studies.3,52 To resolve this paradox, it was proposed that an antiangiogenic agent could transiently “normalize” the abnormal tumor vasculature, resulting in more efficient delivery of oxygen and drugs, and many reports support this hypothesis.49 However, the nonproductive angiogenesis induced by DLL4 blockade showed little perfusion of blood and hypoxia.41-44 It was opposed to “normalization,” but significantly inhibited tumor growth. Therefore, DLL4 blockade might cause more severe and sustained hypoxia than anti-VEGF therapy.
DLL4 blockade could be effective for tumors resistant to anti-VEGF therapy.41 It is conceivable that the target of DLL4 blockade in tumor angiogenesis was to inhibit tumor vascular remodeling or maturation, whereas that of anti-VEGF therapy was to switch off the trigger of tumor angiogenesis. Therefore, DLL4 blockade would be effective for tumors that do not respond to anti-VEGF therapy.
In our experiments, we showed the antitumor effect of DLL4 blockade using newly developed neutralizing antibodies against DLL4, and demonstrated the VEGF-DLL4-ephrinB2 cascade could be involved in the nonproductive angiogenesis induced by DLL4 blockade. The effect of DLL4 blockade on tumor angiogenesis seems to be different from that of anti-VEGF therapy, and this difference could provide us a new option for the therapeutic strategy for cancer treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by a Grant-In-Aid for Scientific Research from the Ministry of Education, Science and Culture of the Japanese government to M.A. (no. 19790553) and to S.E. (nos. 18014004, 19590688).
Authorship
Contribution: S.Y. and S.E. designed and performed research, analyzed data, and wrote the paper; M.A., T.O., and T.E. performed research and analyzed data; K.N. performed research; A.K. and N.Y. contributed vital new reagents and analyzed data; H.Y. organized analysis, contributed vital new reagents, and wrote the paper; and H.A. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satoru Ebihara, Department of Geriatrics and Gerontology, Institute of Development, Aging and Cancer, Tohoku University, Seiryo-machi 4-1, Aoba-ku, Sendai, 980-8575, Japan; e-mail: sebihara@idac.tohoku.ac.jp.