Abstract
In acute myeloid leukemia (AML), internal tandem duplications (ITDs) of the juxtamembrane (JM) of FLT3 have been shown to play a crucial role in driving proliferation and survival of the leukemic clone. Here, we report the identification of FLT3_ITD mutations located in non-JM domains of the FLT3-receptor. This novel type of FLT3_ITD mutation was found in 216 of 753 (28.7%) of unselected FLT3_ITD-positive AML cases. An FLT3 receptor harbouring a prototypic non-JM ITD (FLT3_ITD627E) mediated constitutive phosphorylation of FLT3 and of STAT5, suggesting that non-JM ITDs confer constitutive activation of the receptor. FLT3_ITD627E induced transformation of hematopoietic 32D cells and led to a lethal myeloproliferative disease in a syngeneic mouse model. Our results indicate that a significant proportion of activating FLT3_ITD mutations is not confined to the JM domain of FLT3. Further studies are warranted to define the biologic and clinical characteristics of non-JM ITDs.
Introduction
Fms-like tyrosine kinase 3 (FLT3) belongs to the class III receptor tyrosine kinase (RTK) family that includes FMS, platelet-derived growth factor receptor (PDGFR), and c-KIT.1 FLT3 plays an important physiologic role in self-renewal and differentiation of hematopoietic stem and progenitor cells.2-4
In acute myeloid leukemia (AML), activating mutations in the FLT3 gene occur in 30% to 40% of adult patients and have been demonstrated to play a crucial role in driving proliferation and survival of the leukemic clone. Twenty percent to 30% of AML patients harbour an internal tandem duplication (ITD) in the juxtamembrane (JM) region of FLT3.5,6 ITDs found in FLT3 are always in frame and range from 3 base pairs (bp) to more than 400 bp.7 Ten percent of AML patients have mutations within the activation loop of the second kinase domain, predominantly substitutions of aspartate at residue 835 (D835). However, additional activating mutations in this region have also been described.8-10 Expression of FLT3_ITD receptors results in autophosphorylation of FLT3 and subsequent activation of downstream signaling.11-13 Consequently, FLT3_ITD mutations render hematopoietic cells growth factor–independent by promoting cell proliferation and inhibition of apoptosis and lead to myeloproliferative disease in a murine transplantation model.11,13-15 Generally, it is believed that the ITD insertions within FLT3 occur in the zipper or linker peptide segment of JM (JM-Z) near the JM hinge region, thereby disrupting the autoinhibitory function of the JM-domain.16 However, a detailed analysis of FLT3_ITD insertion sites has not been performed so far. Therefore, we have addressed this issue and have determined ITD insertion sites of 753 unselected FLT3_ITD-positive AML cases by direct cDNA sequencing.
Methods
Sequence analysis of FLT3_ITD from 753 unselected FLT3_ITD-positive AML cases
Mononucleated bone marrow cells from patients participating in standard diagnostic procedures and treated according to protocols of the AML Cooperative Group (AMLCG) study group or according to other intensive AML therapy protocols were obtained by Ficoll density gradient centrifugation. Some of the patients have already been described previously.7 mRNA was extracted with the MagnaPureLC mRNA Kit I (Roche Diagnostics, Mannheim, Germany). cDNA synthesis of mRNA of an equivalent of 5 to 10 × 106 cells was performed using SuperscriptII (Gibco BRL/Invitrogen, Karlsruhe, Germany) and random hexamer primers (Pharmacia, Freiburg, Germany). PCR and evaluation was performed as described previously.7 Mutation detection was done by standard agarose gel electrophoresis and in parallel by fragment analysis on a capillary sequencer as described previously.17 Direct sequencing of PCR products was performed as described7 and primers were the same as used for PCR or RT-PCR amplification, respectively.
Blood samples from AML patients were collected after informed consent was obtained in accordance with the Declaration of Helsinki. Laboratory studies on ITD variants isolated from patient material were performed with approval from the local institutional review board (Ethikkommission Mainz, no. 837.270.05 [4928]). All animal procedures were reviewed and approved by the university's supervisory animal care committee.
DNA constructs, mutation screening of FLT3_ITD, and isolation of the ITD627E allele
Genomic DNA from PB MNCs was extracted with a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) from a patient enrolled in a clinical phase 2 study (PKC412A-2104 trial) investigating the efficacy and toxicity of PKC412.18
For ITD mutation screening by PCR, the following primers were used: ITD-f: GCAATTTAGGTATGAAAGCCAGC, ITD-r: CTTTCAGCATTTTGACGGCAACC. RNA from PB was extracted with the RNeasy Mini-Kit (QIAGEN), and cDNA was generated with the SuperScript First-Strand Synthesis System (Invitrogen, Groningen, The Netherlands).
Human FLT3_WT and FLT3 JM-ITD constructs used have been described previously.10,12 The ITD allele (36 bp/12 amino acids [aa]) integrates between codons 598 and 599 in the JM domain of FLT3. Subcloning of the ITD627E allele (93 bp/31 aa) into the pAL vector was performed by amplification of the FLT3 coding sequence using cDNA generated from RNA of isolated MNCs. Primers were FLT3HindIII-f: ATAAAGCTTACCATGCCGGCGTTGGCG-CGCGAC; FLT3BamHI-r: GCGGGATCCGGCTACGAATCTTCGACCTGAGCCT. The FLT3_ITD627A mutant was generated by Medigenomix (Martinsried, Germany) by site-directed mutagenesis. All constructs were verified by sequencing.
Transfections and cell culture
Transfection and maintenance of 32D cells was performed as described previously.10 32D_ITD627E cells were generated by electroporation of 32D cells using pAL-ITD627E plasmid. 32D_ITD627A mutants were generated by electroporation of murine 32D cells with 10 μg pAL-ITD627A plasmid and selection was started 48 hours after transfection in medium without IL-3. Polyclonal cell lines were used for further experiments.
Protein extract preparation and Western blot analysis
Apoptosis assays
Colony assays
Colony assays were performed as described previously.20
In vivo tumorigenesis assay
32D syngeneic C3H/N mice were intravenously injected with 106 32D FLT3_ITD598/599 and 32D FLT3_ITD627E cells, respectively. Mice were monitored over time and killed when moribund.
Results and discussion
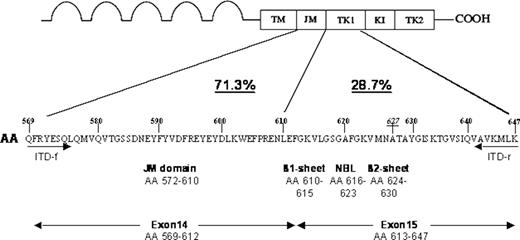
From 753 unselected FLT3_ITD-positive AML cases by using samples collected at initial diagnosis (de novo AML, secondary AML after myelodysplastic syndrome [MDS], chemotherapy-related AML) cDNA was analyzed for sites of ITD-integration by direct sequencing. Surprisingly, ITDs integrating in non-JM domains were detected in 216 (28.7%) of these patients (Figure 1): specifically, ITD integrations were found in the β1 sheet of the first kinase domain (TKD1, amino acids 610-615) in 24.6%, in the nucleotide binding loop (NBL, amino acids 616-623) in 2%, in the β2 sheet (amino acids 624-630) in 1.3% and 3′ of the β2-sheet in 0.8% of all cases (Figure 1). These results indicate that a considerable proportion of FLT3_ITDs is not confined to the JM-domain but integrates 3′ of this domain. All of the non-JM ITDs are exclusively integrating in the tyrosine kinase domain 1 of FLT3 (ie, at amino acid 610 and downstream of amino acid 610).
Analysis of FLT3_ITD integration sites from 753 unselected FLT3_ITD-positive AML cases. The localization of ITD integrations together with their respective frequencies and their position relative to functional domains of the receptor are shown for 753 ITD-positive AML patients analyzed. A significant number of ITDs (28.7%) are localized in the first tyrosine kinase domain (TK1) of FLT3. A detailed analysis showed ITD integrations in the β1-sheet of TK1, amino acids 610 to 615, in 24.6%, in the nucleotide binding loop (NBL), amino acids 616 to 623, in 2%, in the β2 sheet, amino acids 624 to 630, in 1.3% and 3′ of the β2 sheet in 0.8% of all cases. The localization of primers used to amplify, sequence, and isolate the ITD627E allele (ITD-f and ITD-r) is depicted. AA indicates amino acid; TM, transmembrane domain; JM, juxtamembrane domain; TK1, tyrosine kinase domain 1; KI, kinase insert; TK2, tyrosine kinase domain 2; and NBL, nucleotide binding loop.
Analysis of FLT3_ITD integration sites from 753 unselected FLT3_ITD-positive AML cases. The localization of ITD integrations together with their respective frequencies and their position relative to functional domains of the receptor are shown for 753 ITD-positive AML patients analyzed. A significant number of ITDs (28.7%) are localized in the first tyrosine kinase domain (TK1) of FLT3. A detailed analysis showed ITD integrations in the β1-sheet of TK1, amino acids 610 to 615, in 24.6%, in the nucleotide binding loop (NBL), amino acids 616 to 623, in 2%, in the β2 sheet, amino acids 624 to 630, in 1.3% and 3′ of the β2 sheet in 0.8% of all cases. The localization of primers used to amplify, sequence, and isolate the ITD627E allele (ITD-f and ITD-r) is depicted. AA indicates amino acid; TM, transmembrane domain; JM, juxtamembrane domain; TK1, tyrosine kinase domain 1; KI, kinase insert; TK2, tyrosine kinase domain 2; and NBL, nucleotide binding loop.
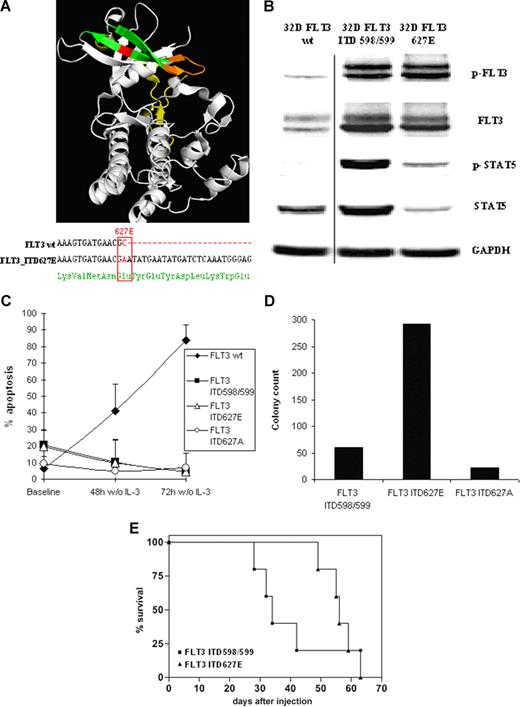
Next, we sought to assess the biologic properties of non-JM ITD receptors. Therefore, full-length cDNA of a FLT3 receptor harboring a prototypic non-JM ITD (ITD627E) not integrating into the JM domain (amino acids 572-609) but into the β2 sheet (amino acids 624-630) of TKD1 (Figure 2A top panel) was isolated and stably expressed in hematopoietic IL-3–dependent 32D cells.
Structural and functional characteristics of FLT3_ITD627E and of FLT3_ITD627A. (A) Integration site (top panel) and DNA sequence (bottom panel) of ITD_627E. The crystal structure of the cytoplasmatic part of human FLT316 (protein code: 1RJB) was integrated from the Protein Data Bank21 (PDB) and analyzed using PyMOL software (DeLano Scientific, Palo Alto, CA). The juxtamembrane (JM) domain (aa 572-609) is in yellow. The β1 sheet (aa 610-615) and β2 sheet (aa 624-630) of the FLT3 tyrosine kinase domain 1 (TKD1) are shown in green with the β sheets connected by the nucleotide binding loop (NBL, aa 616-623) in orange. The integration site of the ITD627E allele in the β2 sheet of TKD1 (aa 627) is shown in red. By PCR amplification of the FLT3 coding sequence and DNA sequencing, ITD_627E (31 amino acids) was found to integrate at nucleotide 1880 of FLT3, thereby generating a single nucleotide change from GCA to GAA. This resulted in an amino acid substitution from alanine to glutamate at amino acid position 627 (ITD627E; bottom panel). An additional mutation in FLT3_ITD627E was excluded by sequencing the entire coding sequence of the FLT3_ITD627E allele. The coding sequence of FLT3 was amplified by PCR with the following primers: FLT3mRNA-f: TGCCGCTGCTCGTTGTTTT; FLT3mRNA-r: AGAAGGCCTTGGATGCAGA. PCR products were subcloned into pCR4-TOPO vectors (Invitrogen) and plasmid DNA from single bacterial clones was isolated and FLT3 inserts were sequenced. (B) In 32D cells stably transfected with FLT3_wt receptor, a standard JM ITD598/599 integrating in the JM domain of FLT3 between codons 598 and 599 (32D_ITD598/599) and in 32D_ITD627E cells, protein expression and phosphorylation of FLT3 (Y591) and of STAT5 (Y694/Y699) was determined by immunoblotting. (C) 32D_FLT3WT, 32D_ITD598/599, 32D_ITD627E, and 32D_ITD627A cells were grown in 10% WEHI-conditioned medium and then subjected to growth factor withdrawal. The ITD mutant ITD627A is identical in length and position of integration to ITD627E (93 bp/31 aa) with the amino acid at codon 627 reverted to wild type (alanine). The percentage of cells featuring a sub-G1 DNA peak as determined by flow cytometry 48 hours and 72 hours after growth factor withdrawal are shown (mean values of 3 independent experiments ± SD). (D) Stably transfected 32D_ITD598/599, 32D_ITD627E and 32D_ITD627A cells were seeded at a fixed density (1000 cells/mL) in 35 mm dishes and grown for 7 days in RPMI 1640/methylcellulose medium (Methocult; StemCell Technologies, Vancouver, BC) supplemented with 10% FCS but in the absence of growth factors. The total number of colonies from 2 dishes of each cell line is shown. As expected, 32D_WT cells did not form colonies under these conditions (data not shown). (E) Kaplan Meier plot of survival of mice injected with 32D_ITD598/599 (n = 5) and with 32D_ITD627E cells (n = 5). The percentage of surviving mice (y-axis) is plotted with respect to time in days (x-axis). It has been previously reported that mice injected with 32D FLT3_wt cells do not develop disease within an observation period up to 3 months.12
Structural and functional characteristics of FLT3_ITD627E and of FLT3_ITD627A. (A) Integration site (top panel) and DNA sequence (bottom panel) of ITD_627E. The crystal structure of the cytoplasmatic part of human FLT316 (protein code: 1RJB) was integrated from the Protein Data Bank21 (PDB) and analyzed using PyMOL software (DeLano Scientific, Palo Alto, CA). The juxtamembrane (JM) domain (aa 572-609) is in yellow. The β1 sheet (aa 610-615) and β2 sheet (aa 624-630) of the FLT3 tyrosine kinase domain 1 (TKD1) are shown in green with the β sheets connected by the nucleotide binding loop (NBL, aa 616-623) in orange. The integration site of the ITD627E allele in the β2 sheet of TKD1 (aa 627) is shown in red. By PCR amplification of the FLT3 coding sequence and DNA sequencing, ITD_627E (31 amino acids) was found to integrate at nucleotide 1880 of FLT3, thereby generating a single nucleotide change from GCA to GAA. This resulted in an amino acid substitution from alanine to glutamate at amino acid position 627 (ITD627E; bottom panel). An additional mutation in FLT3_ITD627E was excluded by sequencing the entire coding sequence of the FLT3_ITD627E allele. The coding sequence of FLT3 was amplified by PCR with the following primers: FLT3mRNA-f: TGCCGCTGCTCGTTGTTTT; FLT3mRNA-r: AGAAGGCCTTGGATGCAGA. PCR products were subcloned into pCR4-TOPO vectors (Invitrogen) and plasmid DNA from single bacterial clones was isolated and FLT3 inserts were sequenced. (B) In 32D cells stably transfected with FLT3_wt receptor, a standard JM ITD598/599 integrating in the JM domain of FLT3 between codons 598 and 599 (32D_ITD598/599) and in 32D_ITD627E cells, protein expression and phosphorylation of FLT3 (Y591) and of STAT5 (Y694/Y699) was determined by immunoblotting. (C) 32D_FLT3WT, 32D_ITD598/599, 32D_ITD627E, and 32D_ITD627A cells were grown in 10% WEHI-conditioned medium and then subjected to growth factor withdrawal. The ITD mutant ITD627A is identical in length and position of integration to ITD627E (93 bp/31 aa) with the amino acid at codon 627 reverted to wild type (alanine). The percentage of cells featuring a sub-G1 DNA peak as determined by flow cytometry 48 hours and 72 hours after growth factor withdrawal are shown (mean values of 3 independent experiments ± SD). (D) Stably transfected 32D_ITD598/599, 32D_ITD627E and 32D_ITD627A cells were seeded at a fixed density (1000 cells/mL) in 35 mm dishes and grown for 7 days in RPMI 1640/methylcellulose medium (Methocult; StemCell Technologies, Vancouver, BC) supplemented with 10% FCS but in the absence of growth factors. The total number of colonies from 2 dishes of each cell line is shown. As expected, 32D_WT cells did not form colonies under these conditions (data not shown). (E) Kaplan Meier plot of survival of mice injected with 32D_ITD598/599 (n = 5) and with 32D_ITD627E cells (n = 5). The percentage of surviving mice (y-axis) is plotted with respect to time in days (x-axis). It has been previously reported that mice injected with 32D FLT3_wt cells do not develop disease within an observation period up to 3 months.12
Constitutive tyrosine phosphorylation of FLT3_ITD627E was detected by Western blot analysis by using a specific antiphosphotyrosine FLT3 antibody (Y591; Figure 2B). Inves-tigation of FLT3 downstream signaling in ITD627E cells revealed constitutive phosphorylation of STAT5 (Figure 2B), indicating that FLT3_ITD627E is an activating FLT3 mutation. By using fluorescence-activated cell sorting (FACS) analysis, surface expression of FLT3 in 32D_ITD627E cells in comparison to 32D_ITD598/599 cells was found to be comparable (data not shown).
Next, we addressed the question whether constitutive activity of FLT3_ITD627E is solely dependent on the ITD integration site at this particular position of the tyrosine kinase domain or whether the amino acid exchange from alanine to glutamate induced by ITD integration at position 627 is involved. Therefore, the amino acid exchange at position 627 was reverted to alanine by site directed mutagenesis. 32D cells were stably transfected with this mutant (32D_ITD627A) and both cell lines (32D_ITD627E and 32D_ITD627A) were analyzed in comparison to 32D cells stably expressing an FLT3 wt receptor (32D FLT3_wt) or a standard JM ITD (32D_ITD598/599 cells). In cell-culture experiments, FLT3_ITD627E cells and FLT3_ITD627A cells showed protection from apoptotic cell death upon growth factor withdrawal (Figure 2C) and revealed capability of clonal growth in methylcellulose medium (Figure 2D). Together, these results indicate that FLT3_ITD627E and FLT3_ITD627A are activating mutations of FLT3 leading to transformation of hematopoietic 32D cells. In addition, these results show that the point mutation A627E is not essential for the transforming phenotype of FLT3_ITD627E.
To investigate the biologic consequences of FLT3_ITD627E on transformation of 32D cells in vivo, a syngeneic mouse model was used. C3H/N mice were injected with 32D_ITD627E cells or with 32D_ITD598/599 cells. In this model, both cell lines induced a lethal hematopoietic disease with a median latency of 33 (ITD598/599) and 56 days (ITD627E; Figure 2E). This indicates that the activating mutation FLT3_ITD627E has similar in vivo characteristics compared with a typical JM ITD mutation.
The intramolecular mechanism of non-JM ITDs leading to constitutive activation of the FLT3 receptor is currently unknown. However, our results using FLT3_ITD627E/A show that ITD insertions in the β2 sheet result in activating FLT3 mutations. This may be mediated by a secondary change in the structural conformation of the JM domain, thereby again leading to disruption of the autoinhibitory function of this domain. So far, the relevance of the pronounced variability in FLT3_ITDs insertion sites for the biology of non-JM ITD mutations is unknown. Thus, further studies are warranted to define the biologic and clinical characteristics of non-JM ITDs.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Deutsche Krebshilfe Foundation and the Wilhelm Sander-Stiftung to T.F.
Authorship
Contribution: F.B. designed experiments, performed research, analyzed data, and participated in writing the manuscript; S.S. and B.M. designed experiments, performed research, and analyzed data; R.G. designed and performed mice experiments and analyzed data; B.C. and A.B. performed research; J.D. designed and supervised mice experiments and analyzed data; T.H. designed and supervised experimental work; C.H. analyzed data and contributed to interpretation of experimental data; and T.F. designed and supervised experimental work, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Fischer, Johannes Gutenberg University, Langenbeckstr 1, Mainz, Germany 55101; t.fischer@3-med.klinik.uni-mainz.de.