Abstract
We conducted a phase 1/2 trial combining lenalidomide (R) with adriamycin (A) and dexamethasone (D) for relapsed and relapsed-refractory myeloma to determine tolerability and efficacy of this novel regimen, RAD, delivered for six 28-day cycles. A total of 69 intensively pretreated patients with a median age of 65 years (range, 46-77 years) were enrolled. Using pegfilgrastim (G), the maximum tolerated dose (MTD) was formally not reached at the highest dose level (R, 25 mg on days 1-21; A, 9 mg/m2 intravenously on days 1-4; and D, 40 mg on days 1-4 and 17-20; dose level 5+G), which was then used to determine efficacy. Grades 3/4 neutropenia and thrombocytopenia were seen in 48% and 38% of patients, respectively. Thromboembolic events occurred in 4.5% and severe infections in 10.5% of patients. On an intent-to treat analysis, overall response rate (ORR) was 73% for the whole study and 77% including 74% complete response (CR) plus very good partial response (VGPR) for dose level 5+G. Response rates and progression-free survival did not differ between relapsed and relapsed-refractory patients. Deletion of chromosome 17p and elevated β2-microglobulin were associated with significantly inferior response and shortened time to progression. In conclusion, RAD induces substantial and durable remission with an acceptable toxicity profile in patients with relapsed and relapsed-refractory myeloma. This trial was registered at www.ClinicalTrials.gov as no. NCT00306813.
Introduction
Despite therapeutic milestones achieved, multiple myeloma (MM), a malignancy arising from the antibody-secreting plasma cell, remains an incurable disease.1 In the past few years, different novel classes of drugs targeting both the myeloma plasma cell and its microenvironment showed efficacy in chemoresistant disease. The proteasome inhibitor bortezomib (Velcade; Millennium Pharmaceuticals, Cambridge, MA) prolongs time to progression and improves overall survival in patients with relapsed and refractory MM.2,3 Thalidomide has been known to be effective in myeloma since the 1990s inducing response rates between 30% (single-agent) and 50% (combined with dexamethasone).4-6 Lenalidomide (Revlimid; Celgene, Summit, NJ) is a derivative of thalidomide and showed significant antimyeloma activity in pretreated patients in a phase 1 trial without harboring the profound toxicity profile of thalidomide.7 Lenalidomide mainly induces apoptosis of myeloma cells, inhibits angiogenesis, stimulates a T- and natural killer (NK) cell–mediated immune response, regulates cytokine expression, and overcomes bone marrow stromal cell–mediated drug resistance.7,8 The compound has been approved for relapsed MM both by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) based on the results of 2 randomized phase 3 trials. Patients were to receive either high-dose dexamethasone alone or in combination with lenalidomide. The combination arm was superior with respect to response, time to disease progression, and overall survival.9,10 To further improve therapeutic efficacy, we evaluated lenalidomide in conjunction with adriamycin and dexamethasone (RAD) for the treatment of patients with refractory or relapsed MM in a phase 1/2 trial based on the observation that VAD (vincristin, adriamycin, dexamethasone) with adriamycin continuous infusion was effective in cases resistant to alkylators.11 Because high-dose dexamethasone (ie, three 4-day courses with a daily dose of 40 mg) is associated with significant toxicity, we omitted one 4-day dexamethasone course per cycle according to a modified VAD (m-VAD) protocol to enhance tolerability.12
Methods
Eligibility
Patients at least 18 years of age with histologically confirmed relapsed or relapsed-refractory MM were eligible after no more than 3 previous lines of antineoplastic therapy. Relapse was defined as evidence of progressive disease (PD) after at least a partial remission (PR) achieved by any preceding therapy, whereas relapsed-refractory disease was defined as progression during administration of any therapeutic regimen or within 60 days after its completion. Patients had to have measurable disease parameters and were required to have an 0 to 2 Eastern Cooperative Oncology Group (ECOG) performance status, a left ventricular ejection fraction of 55% or greater on a 2-dimensional echocardiogram, a life expectancy of at least 3 months, and (if applicable) a negative serum pregnancy test. At baseline evaluation, the following laboratory values had to be met: absolute neutrophil count (ANC) of 1.5 × 109/L or greater; platelet count (PLT) of 100 × 109/L or greater; serum creatinine below 221 μM (2.5 mg/dL); and aspartate and alanine aminotransferase levels less than or equal to 3 times the upper limit of normal (ULN). Previous treatment with lenalidomide was not permitted. Further exclusion criteria were any antimyeloma drug therapy 4 weeks or less before study entry and anthracycline-containing regimens 3 months or less before study entry, any thromboembolic events in the patient's history, congestive heart failure more than or equal to New York Heart Association (NYHA) class II, sensory and/or motor neuropathy of grade 2 or higher; cumulative dose of doxorubicin greater than 300 mg/m2 or equivalent, and active, uncontrolled infection. Review boards at participating institutions approved the study, which was conducted according to the International Conference on Harmonization and the Guidelines for Good Clinical Practice, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Study design
This study was designed as a noncomparative, open-label multicenter phase 1/2 study. Within the phase 1 part, the primary objective was to assess the maximum tolerated dose (MTD) of lenalidomide when given in conjunction with adriamycin and dexamethasone as a VAD-like regimen. The MTD was defined as the highest dose level with an incidence of dose-limiting toxicity (DLT) of less than 33%. DLT and adverse events were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0 (NCI CTCAE; Bethesda, MD). DLT was defined as grade 4 hematologic toxicity; febrile neutropenia; or a grade 3 toxicity in any other organ system except alopecia, nausea and vomiting, a treatment delay of 2 weeks or more, and the inability to administer the full scheduled dose of RAD in cycle 1 due to toxicity. For dose escalation according to a modified Fibonacci schema,13 3 patients had to have completed the first cycle without DLT. Upon occurrence of a DLT during cycle 1 of a given patient, an additional 3 patients had to receive 1 cycle without a DLT to be observed. In this case, further dose escalation was permitted. If 2 of the initial 3 patients or 2 of 6 patients experienced a DLT during the first 4-week treatment cycle, then the MTD was the dose from the next lower dose level. The primary endpoint in the phase 2 part of the study was response to therapy (complete response [CR] + very good partial response [VGPR] + partial response [PR]), and secondary endpoints were time to progression (TTP), progression-free survival (PFS), and disease control rate (more than or equal to stable disease [SD]). TTP was defined as the interval from first dose of study drug to disease progression, overall survival (OS) as the interval from first dose of study drug to death from any cause; and PFS as the interval from first dose of study drug to any sign of progression or death, whichever occurred first.
Drug administration
Patients received oral lenalidomide on days 1 through 21; intravenous adriamycin as a continuous infusion over 96 hours starting on day 1 of the cycle; and oral dexamethasone on days 1 through 4 and 17 through 20. Cycles were repeated every 4 weeks for a maximum of 6 cycles. The initial dose (dose level 1 [DL 1]) of lenalidomide (R) was 10 mg, that of adriamycin (doxorubicin [A]) was 4 mg/m2 per day, and that of dexamethasone was 40 mg per day on all dose levels (Table 1). The foreseen dose escalation steps were as follows: DL2, lenalidomide 10 mg, doxorubicin 6 mg/m2; DL3, lenalidomide 10 mg, doxorubicin 9 mg/m2; and DL4, lenalidomide 15 mg, doxorubicin 9 mg/m2. The protocol was subsequently amended for granulocyte colony-stimulating factor (G-CSF) support: DL 4+G consisted of lenalidomide 15 mg, doxorubicin 9 mg/m2 along with pegfilgrastim (Amgen, Thousand Oaks, CA) at a fixed dose of 6 mg on day 6. A further increase of lenalidomide to 25 mg daily for 21 days in conjunction with doxorubicin 9 mg/m2 and pegfilgrastim was scheduled for DL 5+G (Table 2). A total of 6 cycles without any form of maintenance treatment was planned on all DLs. For thromboprophylaxis, throughout all treatment cycles patients were to receive either aspirin (100 mg once daily) or low-molecular-weight heparin if they had an implanted venous port at a standard dose (ie, enoxaparin 40 mg or dalteparin 5000 U once daily each) before starting RAD therapy.
Lenalidomide, adriamycin, and dexamethasone (RAD) dose levels explored in the phase 1 part
| Dose level . | Lenalidomide, mg (d) . | Adriamycin, mg/m2 (d) . | Dexamethasone, mg (d) . | Pegfilgrastim, mg (d) . |
|---|---|---|---|---|
| 1 | 10 (1-21) | 4 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 2 | 10 (1-21) | 6 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 3 | 10 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 4 | 15 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 4 + G | 15 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | 6 (6) |
| 5 + G | 25 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | 6 (6) |
| Dose level . | Lenalidomide, mg (d) . | Adriamycin, mg/m2 (d) . | Dexamethasone, mg (d) . | Pegfilgrastim, mg (d) . |
|---|---|---|---|---|
| 1 | 10 (1-21) | 4 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 2 | 10 (1-21) | 6 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 3 | 10 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 4 | 15 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | |
| 4 + G | 15 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | 6 (6) |
| 5 + G | 25 (1-21) | 9 (1-4, cont.) | 40 (1-4 and 17-20) | 6 (6) |
Cont. indicates continuous infusion.
Patient characteristics
| Patient number | 69 |
| Median age (range), y | 65 (45-77) |
| Median time since diagnosis (range), mo | 49 (1-167) |
| Relapsed-refractory myeloma, n (%) | 22 (32) |
| Sex, n (%) | |
| Male | 46 (67) |
| Female | 23 (33) |
| Myeloma type, n (%) | |
| IgG | 34 (49) |
| IgA | 13 (19) |
| IgD | 3 (6) |
| Light chain | 16 (23) |
| Oligosecretory | 3 (6) |
| Number of previous therapies (range) | |
| 1 | 16 (23) |
| 2 or more | 53 (77) |
| Autologous stem cell transplantation, n (%) | 50 (72) |
| Autologous and allogeneic stem cell transplantation, n (%) | 8 (12) |
| Only conventional pretreatment | 11 (16) |
| Bortezomib, n (%) | 39 (57) |
| Thalidomide, n (%) | 14 (20) |
| Anthracyclines, n (%) | 58 (84) |
| Cumulative doxorubicin dose > 50 mg/m2 (or equivalent) during RAD exceeded, n (%) | 2 (3) |
| WHO performance status 0 or 1, n (%) | 56 (81) |
| Bone disease present, n (%) | 52 (75) |
| Evaluable for β2 microglobulin, n (%) | 59 (86) |
| Normal β2 microglobulin (< 3.5 mg/L) | 42 (71) |
| Elevated (≥ 3.5 mg/L) β2 microglobulin | 17 (29) |
| Evaluable for cytogenetics, n (%) | 37 (54) |
| Del (13q) | 15 (41) |
| t (4;14) | 4 (11) |
| del (17p) | 5 (14) |
| Patient number | 69 |
| Median age (range), y | 65 (45-77) |
| Median time since diagnosis (range), mo | 49 (1-167) |
| Relapsed-refractory myeloma, n (%) | 22 (32) |
| Sex, n (%) | |
| Male | 46 (67) |
| Female | 23 (33) |
| Myeloma type, n (%) | |
| IgG | 34 (49) |
| IgA | 13 (19) |
| IgD | 3 (6) |
| Light chain | 16 (23) |
| Oligosecretory | 3 (6) |
| Number of previous therapies (range) | |
| 1 | 16 (23) |
| 2 or more | 53 (77) |
| Autologous stem cell transplantation, n (%) | 50 (72) |
| Autologous and allogeneic stem cell transplantation, n (%) | 8 (12) |
| Only conventional pretreatment | 11 (16) |
| Bortezomib, n (%) | 39 (57) |
| Thalidomide, n (%) | 14 (20) |
| Anthracyclines, n (%) | 58 (84) |
| Cumulative doxorubicin dose > 50 mg/m2 (or equivalent) during RAD exceeded, n (%) | 2 (3) |
| WHO performance status 0 or 1, n (%) | 56 (81) |
| Bone disease present, n (%) | 52 (75) |
| Evaluable for β2 microglobulin, n (%) | 59 (86) |
| Normal β2 microglobulin (< 3.5 mg/L) | 42 (71) |
| Elevated (≥ 3.5 mg/L) β2 microglobulin | 17 (29) |
| Evaluable for cytogenetics, n (%) | 37 (54) |
| Del (13q) | 15 (41) |
| t (4;14) | 4 (11) |
| del (17p) | 5 (14) |
Efficacy and safety assessments
At baseline evaluation, patients were assessed for current disease activity of MM using bone marrow aspiration, trephine biopsy, molecular cytogenetic analysis, serum and 24-hour urine samples for determination of the monoclonal component, skeletal survey, and previous treatments for myeloma. Upon suspicion of extramedullary disease sites, an appropriate imaging procedure was applied. During treatment, a complete blood count (CBC) was required once weekly on treatment days 8, 15, and 22. Similarly, repeat echocardiographic measurement of left-ventricular function (LVEF) as well as 12-lead electrocardiographs were obtained before each subsequent cycle as well as after termination of the last treatment cycle. Response was assessed on day 1 of every cycle (ie, every 4 weeks) by serum and urine studies. Response was defined by the stringent European Bone Marrow Transplantation (EBMT) criteria, published by Bladé et al,14 requiring confirmation of PR or better by a complete work-up within 2 weeks after response was maintained for more than 6 weeks. A PR subcategory was added (VGPR) according to the novel uniform response criteria.15 For CR, negative immunofixation in serum and urine was required along with disappearance of the M-component, extramedullary lesions, and a plasma cell count of less than 5% in bone marrow biopsy. A VGPR required an at least 90% reduction of the M-component in serum or urine paraprotein levels less than 100 mg per 24 hours. A PR required at least a 50% reduction of M-component in serum and at least a 90% decrease in urine. SD was noted for responses that did not meet criteria for CR, VGPR, PR, or PD (defined as an increase of 25% or more in M-component).
Statistical analysis
TTP, PFS, and OS analysis was performed using the Kaplan-Meier estimation and calculated from the first day of study drug. The 95% confidence intervals (CIs) were generated by the Greenwood formula, and comparisons for survival functions were performed using the log-rank test. Associations between categoric variables and response rates were analyzed using the χ2 and Fisher exact tests. A retrospective multivariate analysis was performed using a Cox proportional hazard regression model to assess the independent influence of patient- and disease-specific variables on TTP. Several factors that had been associated with TTP were examined.9,10 All P values were 2-sided, and statistical significance was considered at the .05 alpha level.
Results
Patient characteristics
Between February 2005 and June 2007, at total of 69 patients were enrolled from 5 German centers, 66 (95.6%) of whom were evaluable for adverse effects and for efficacy. Baseline characteristics are listed in Table 2. Median age was 65 (range, 45-77) years. A total of 53 patients (77%) received RAD as third line, 31 of whom (45%) as fourth line (45%) treatment. A total of 58 subjects (84%) had received autologous (50 patients, 72%) or autologous followed by allogeneic stem cell transplantation (SCT; 8 patients; 12%); 39 patients (57%) had previously received bortezomib, and 14 (20%) thalidomide. Most patients (84%) had previously received anthracycline-based regimens. Median time from diagnosis of multiple myeloma to RAD exposure was 49 months (range, 1-167 months). A total of 22 (32%) patients had relapsed-refractory disease. Fluorescence in situ hybridization (FISH) analysis of bone marrow was performed in 37 (54%) of the 69 patients as previously described:16 deletion 13q14 (13q−) was detected in 15 patients (41%), translocation t(4;14) in 4 (11%) patients, and deletion 17p13 (17p−) in 5 (14%) patients.
Adverse events and toxicities
At DL 1 through DL 3, no DLT was observed. At DL 4, 2 of 6 patients experienced a brief grade 4 nonfebrile neutropenia. No DLT was observed at dose level 4 with growth factor support (pegfilgrastim 6 mg; DL 4+G). We therefore performed a further dose increase: lenalidomide 25 mg plus G-CSF (DL 5+G). Because no DLT was observed, MTD was not formally reached and dose level 5+G was considered safe. Efficacy of the RAD regimen was assessed using DL 5+G (phase 2 part). In this study, no death was reported as a consequence of adverse advents. Most frequent grade 3/4 toxicities were neutropenia (48%), thrombocytopenia (38%), and anemia (16.5%). A total of 7 patients (10.5%) experienced grade 3 or 4 infections: 3 patients each had pneumonia and septicemia, requiring intravenous use of antibiotics, and 1 additional patient had septicemia accompanied by hypotension. Further grade 3 events were thrombosis of basilar artery (1 patient) and pain (1 patient). The lenalidomide and doxorubicin doses had to be reduced in 14 patients for hematologic toxicity, whereas the dexamethasone dose was changed in 4 patients. Drugs were permanently discontinued in 3 patients (see next sentence for reasons; Table 4). A total of 3 patients in DL 1 to DL 4+G and 11 patients in DL 5+G were affected from dose reductions, 7 of which were necessary from cycle 2 onward. Regarding DL 5+G, 89% of the targeted lenalidomide and doxorubicin dose was delivered to the patients. A total of 3 patients prematurely discontinued study drug treatment for grade 3 toxicities: in addition to the patient with thrombosis of basilar artery, 1 patient each had septicemia due to port-a-cath and severe pneumonia. In all, 66 (96%) of 69 patients completed the assigned treatment and received all 6 cycles of the RAD regimen. No cardiotoxic side effects (LVEF of less than 55%; cardiac arrhythmias) were observed. However, a cumulative doxorubicin dose of 550 mg/m2 was exceeded in only 2 (3%) of 58 patients pretreated with anthracycline-based combinations while on this trial. Grade 2 venous thromboembolic events were reported from 2 patients, while 1 patient experienced thrombosis of basilar artery (grade 3) for an overall incidence of 4.5%. Neither neurotoxicity nor constitutional symptoms of grade 3/4 was observed, while mild tingling or numbness was reported for 26 patients and fatigue was reported for 35 patients. Lack of newly developing neuropathies may have been due to relatively strict inclusion criteria that prevented enrollment of patients with clear neurologic impairment. The incidences of severe (grade 3/4) hematotoxicity in different age groups (younger than 65 years vs 65 years and older) were analyzed. Only neutropenia (25% vs 50%) occurred more often in elderly patients (P = .036).
Efficacy
A total of 48 of 66 patients responded to treatment for an overall response rate (ORR; CR+VGPR+PR) of 73% (95% CI, 60%-83%). In all, 10 patients (15%; 95% CI, 8%-26%) achieved CR, and an additional 30 patients (45%; 95% CI, 33%-58%) achieved VGPR. Only 5 patients (7.5%; 95% CI, 3%-17%) had PD. Response occurred early after initiation of treatment, with a median time to response (ie, paraprotein reduction of at least 50% [serum] or 90% [urine]) of 4 weeks (range, 3-28 weeks; ie, 1 treatment cycle).
Considering all patients who received treatment on DL 1 to DL 4+G versus DL 5+G, a highly significant difference in depth of response was noted: none (0%; DL 1-4+G; 95% CI, 0%-17%) versus 10 patients (22%; 95% CI, 11%-36%) for DL 5+G achieved CR (P = .01), and VGPR occurred at a rate of 25% (95% CI, 9%-49%) versus 54% (95% CI, 61%-87%; P < .001 for CR+VGPR between the groups; Fisher exact test). Thus, the ORR at DL 5+G was 77% (95% CI, 64%-89%), including 74% CR+VGPR (95% CI, 59%-87%; Table 3). Of note, evaluable patients in DL 1 to 4+G were not less intensively pretreated compared with those in DL 5+G: significantly more patients in the latter (72% vs 30%; P = .002) had been exposed to prior bortezomib, while proportions for absolute numbers of previous therapies, prior thalidomide, and prior transplantation were comparable.
Best response to lenalidomide, adriamycin, and dexamethasone (RAD) in relation to dose levels: intention-to-treat analysis
| Best response (according to EBMT criteria, Bladé et al14 ) . | Patients, n (%) per study dose level . | ||
|---|---|---|---|
| 1 to 4+ G: 22 (32) . | 5+ G: 47 (68) . | Total 69 (100) . | |
| Complete response | 0 (0) | 10 (21) | 10 (14.5) |
| Very good partial response | 5 (23) | 25 (53) | 30 (43) |
| Partial response | 7 (32) | 1 (2) | 8 (11.5) |
| Stable disease | 6 (27) | 7 (15) | 13 (19) |
| Progression | 2 (9) | 3 (6) | 5 (7) |
| Not evaluable | 2 (9) | 1 (2) | 3 (4) |
| Best response (according to EBMT criteria, Bladé et al14 ) . | Patients, n (%) per study dose level . | ||
|---|---|---|---|
| 1 to 4+ G: 22 (32) . | 5+ G: 47 (68) . | Total 69 (100) . | |
| Complete response | 0 (0) | 10 (21) | 10 (14.5) |
| Very good partial response | 5 (23) | 25 (53) | 30 (43) |
| Partial response | 7 (32) | 1 (2) | 8 (11.5) |
| Stable disease | 6 (27) | 7 (15) | 13 (19) |
| Progression | 2 (9) | 3 (6) | 5 (7) |
| Not evaluable | 2 (9) | 1 (2) | 3 (4) |
Adverse events associated with RAD treatment
| Adverse event . | WHO grade, n (%) . | N total (%) . | |||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||
| Hematotoxicity | |||||
| Anemia | 16 (24) | 31 (47) | 6 (9) | 5 (7.5) | 59 (89) |
| Leukopenia | 11 (17) | 25 (38) | 19 (29) | 5 (7.5) | 60 (91) |
| Neutropenia | 3 (4.5) | 11 (17) | 16 (24) | 16 (24) | 46 (70) |
| Thrombocytopenia | 12 (18) | 10 (15) | 15 (23) | 10 (15) | 47 (71) |
| Nonhematologic toxicity | |||||
| Nausea | 8 | 4 | 0 (0) | 0 (0) | 12 (18) |
| Vomiting | 3 (4.5) | 2 (3) | 0 (0) | 0 (0) | 5 (7.5) |
| Diarrhea | 7 (11) | 4 (6) | 0 (0) | 0 (0) | 11 (17) |
| Infection | 23 (35) | 8 (12) | 6 (9) | 1 (1.5) | 38 (58) |
| Fever | 7 (11) | 2 (3) | 0 (0) | 0 (0) | 9 (14) |
| Fatigue | 26 (39) | 9 (14) | 0 (0) | 0 (0) | 35 (53) |
| Neurotoxicity | 24 (36) | 2 (3) | 0 (0) | 0 (0) | 26 (39) |
| Pain | 15 (23) | 4 (6) | 1 (1.5) | 0 (0) | 20 (30.5) |
| Cardiac events | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Thromboembolism | n.a. | 2 (3) | 1 (1.5) | 0 | 3 (4.5) |
| AEs leading to discontinuation | 0 (0) | (0) | 3 (4.5) | 0 (0) | 3 (4.5) |
| Adverse event . | WHO grade, n (%) . | N total (%) . | |||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||
| Hematotoxicity | |||||
| Anemia | 16 (24) | 31 (47) | 6 (9) | 5 (7.5) | 59 (89) |
| Leukopenia | 11 (17) | 25 (38) | 19 (29) | 5 (7.5) | 60 (91) |
| Neutropenia | 3 (4.5) | 11 (17) | 16 (24) | 16 (24) | 46 (70) |
| Thrombocytopenia | 12 (18) | 10 (15) | 15 (23) | 10 (15) | 47 (71) |
| Nonhematologic toxicity | |||||
| Nausea | 8 | 4 | 0 (0) | 0 (0) | 12 (18) |
| Vomiting | 3 (4.5) | 2 (3) | 0 (0) | 0 (0) | 5 (7.5) |
| Diarrhea | 7 (11) | 4 (6) | 0 (0) | 0 (0) | 11 (17) |
| Infection | 23 (35) | 8 (12) | 6 (9) | 1 (1.5) | 38 (58) |
| Fever | 7 (11) | 2 (3) | 0 (0) | 0 (0) | 9 (14) |
| Fatigue | 26 (39) | 9 (14) | 0 (0) | 0 (0) | 35 (53) |
| Neurotoxicity | 24 (36) | 2 (3) | 0 (0) | 0 (0) | 26 (39) |
| Pain | 15 (23) | 4 (6) | 1 (1.5) | 0 (0) | 20 (30.5) |
| Cardiac events | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Thromboembolism | n.a. | 2 (3) | 1 (1.5) | 0 | 3 (4.5) |
| AEs leading to discontinuation | 0 (0) | (0) | 3 (4.5) | 0 (0) | 3 (4.5) |
n.a. indicates not applicable according to Common Terminology Criteria for Adverse Events v3.0.
Considering disease status on enrollment, RAD was evenly effective in relapsed (ORR on an intent-to-treat basis, 75%) and in relapsed-refractory (ORR, 71%; P = .91) patients.
TTP, PFS, and OS
At the date of analysis, median duration of follow-up from study entry was 14.6 months (range, 7-35 months). Progression, relapse, or death had occurred in 49 of 66 patients.
Median TTP was 45 weeks (95% CI, 39.3-50.7 weeks) and 1-year survival probability was 88% (Figure 1A,B). Regarding depth of response, no significant difference in TTP was noted between CR (51.8 weeks [95% CI, 46.8-53.9 weeks]); VGPR (46.2 weeks [95% CI, 40.2-48.7 weeks]); and PR (45.4 weeks [95% CI, 41.0-47.9 weeks]).
Time to progression and overall survival. (A) Kaplan-Meier plot for time to progression (TTP). The vertical axis represents the proportion of patients who are alive and progression-free from first dose of study drug. The horizontal axis represents time in weeks. (B) Kaplan-Meier plot for overall survival (OS). The vertical axis represents the proportion of patients who are alive. The horizontal axis represents the time in weeks from first dose of study drug.
Time to progression and overall survival. (A) Kaplan-Meier plot for time to progression (TTP). The vertical axis represents the proportion of patients who are alive and progression-free from first dose of study drug. The horizontal axis represents time in weeks. (B) Kaplan-Meier plot for overall survival (OS). The vertical axis represents the proportion of patients who are alive. The horizontal axis represents the time in weeks from first dose of study drug.
Median PFS was 40 weeks (95% CI, 36.0-44.0 weeks; data not shown). Considering disease status at study entry, patients with relapsed disease had a median PFS of 45.0 weeks (95% CI, 43.6-50.4 weeks), while those with relapsed-refractory myeloma had a median PFS of 32.4 weeks (95% CI, 25.9-58.1 weeks; P = .48; data not shown).
Subgroup and multivariate analyses
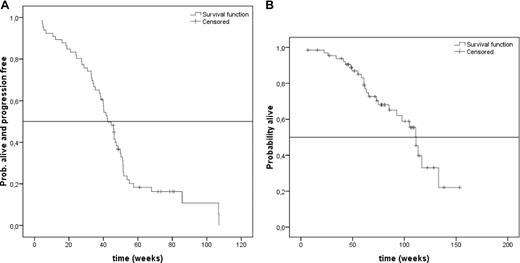
A total of 59 patients were assessable for levels of β2-microglobulin, with 17 (29%) patients displaying elevated concentrations (≥ 297.5 nM [3.5 mg/L]). Subjects with elevated β2-microglobulin were less likely to achieve at least PR (52%) compared with normal levels (88%; P = .002), and also had significantly shorter TTP (median, 31 weeks) compared with normal values (median, 48 weeks; P < .001; Figure 2A).
Time to progression in relation to prognostic factors. (A) Median TTP depending on normal or elevated (≥ 297.5 nM [3.5 mg/L]) levels of β2-microglobulin. The vertical axis represents the proportion of patients who are alive and progression-free. The horizontal axis represents time in weeks from first dose of study drug. The solid line represents patients with normal (less than 297.5 nM) β2-microglobulin (n = 42) and the dotted line represents patients with elevated (≥ 297.5 nM) β2-microglobulin (n = 17). (B) Median TTP in weeks depending on cytogenetic aberration 17p−. The vertical axis represents the proportion of patients who are alive and progression-free. The horizontal axis represents time in weeks from first dose of study drug. The solid line represents patients without 17p− (n = 32), and the dotted line represents patients with 17p− (n = 5).
Time to progression in relation to prognostic factors. (A) Median TTP depending on normal or elevated (≥ 297.5 nM [3.5 mg/L]) levels of β2-microglobulin. The vertical axis represents the proportion of patients who are alive and progression-free. The horizontal axis represents time in weeks from first dose of study drug. The solid line represents patients with normal (less than 297.5 nM) β2-microglobulin (n = 42) and the dotted line represents patients with elevated (≥ 297.5 nM) β2-microglobulin (n = 17). (B) Median TTP in weeks depending on cytogenetic aberration 17p−. The vertical axis represents the proportion of patients who are alive and progression-free. The horizontal axis represents time in weeks from first dose of study drug. The solid line represents patients without 17p− (n = 32), and the dotted line represents patients with 17p− (n = 5).
FISH data were available for 37 patients. 13q− was found in 15 (41%) patients, t(4;14) in 4 (11%) patients, and 17p− in 5 (14%) patients. Response was not significantly different for patients displaying 13q− (ORR, 67%) compared with those lacking this abnormality (ORR, 82%; P = .4). ORR for t(4;14)-positive patients (ORR, 50%) was also not significantly different compared with patients without t(4;14) (ORR, 73%; P = .176). Although the absolute number of patients was low, presence of 17p− was identified as an adverse prognostic factor with respect to response (20% vs 87%; P = .001) and median TTP (20 vs 45.5 weeks; P = .025; Figure 2B).
On multivariate analysis, the independent influence of several known prognostic factors on TTP was examined by a Cox regression: elevated β2-microglobulin, presence of 13q−, prior stem cell transplantation, prior exposure to bortezomib, and prior exposure to thalidomide, respectively. However, only β2-microglobulin concentrations of at least 297.5 nM were significantly associated with shortend TTP at a hazard ratio of 2.55 (95% CI, 1.29-5.02; P = .007; Table 5).
Multivariate analysis of the correlation of potential risk factors with time to progression (TTP)
| Variable . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Elevated (≥ 3.5 mg/L) β2 microglobulin | 2.55 | 1.29, 5.02 | .007 |
| Presence of 13q− | 1.35 | 0.89, 2.05 | .152 |
| Prior (autologous/allogeneic) SCT | 0.91 | 0.43, 1.93 | .816 |
| Prior bortezomib treatment | 0.71 | 0.38, 1.33 | .294 |
| Prior thalidomide treatment | 0.64 | 0.29, 1.41 | .276 |
| Variable . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Elevated (≥ 3.5 mg/L) β2 microglobulin | 2.55 | 1.29, 5.02 | .007 |
| Presence of 13q− | 1.35 | 0.89, 2.05 | .152 |
| Prior (autologous/allogeneic) SCT | 0.91 | 0.43, 1.93 | .816 |
| Prior bortezomib treatment | 0.71 | 0.38, 1.33 | .294 |
| Prior thalidomide treatment | 0.64 | 0.29, 1.41 | .276 |
Discussion
In this phase 1/2 study we evaluated dosing, safety, and efficacy of lenalidomide, adriamycin (doxorubicin), and dexamethasone when administered as a VAD-like regimen.
Side effects of RAD (mainly hematologic toxicity and infections) were moderate and manageable, and no treatment-related death was observed. As recruitment was restricted to patients with only mild preexisting neuropathy, we did not observe severe newly developing neurologic events. Severe infections occurred in roughly 10% of patients, whereas thromboembolism was rare with only one grade 3 case. This was probably due to mandatory aspirin (100 mg daily), whereas patients with newly implanted indwelling catheters received low-molecular-weight heparin. Overall tolerability of the protocol was underscored by an 89% achievement of the targeted doses in the phase 2 part of this trial.
Combined CR+VGPR rate was significantly higher in the phase 2 part compared with the phase 1 part. Considering fewer patients had received prior bortezomib on the latter dose levels (with otherwise comparable pretreatment), this suggests dose-dependent efficacy of the RAD regimen. In two phase 3 trials, lenalidomide plus dexamethasone (RD) induced CR+VGPR in 24% of patients,9,10 versus 74% with RAD-DL 5+G. Although the superiority of RAD needs to be demonstrated in a randomized trial, addition of adriamycin to RD seems to further improve depth of response. Similarly, the combination of lenalidomide with pegylated liposomal doxorubicin (PLD), dexamethasone, and vincristine17 for the treatment of relapsed MM induced a comparable ORR with fewer deep responses. The high response quality in our trial might be due to the continuous infusion of doxorubicin, which may have lead to a synergism in efficacy between the anthracycline and lenalidomide. By combining the latter with bortezomib for relapsed myeloma, a response rate of 58% including CR plus near CR (nCR) in 6% of patients was achieved.18
Treatment duration in the RAD study was relatively short (24 weeks) compared with the RD registration trials (approximately 48 weeks9,10 ). Median TTP, however, was in the same range: 10.4 months (RAD) and slightly more than 11 months (RD), respectively.9,10 Perhaps due to the small numbers in any of the categories (CR, VGPR, PR), no TTP differences were observed for responding patients within the RAD trial.
Other chemotherapy and “novel drug” combinations tested in the setting of relapsed-refractory myeloma were bortezomib, cyclophosphamide, and dexamethasone (VCD)19 ; bortezomib and PLD20 ; lenalidomide, cyclophosphamide, and dexamethasone (RCD)21 ; or the 2 “novel compounds” bortezomib and thalidomide along with melphalan and prednisone (VMPT).22 Treatment durations in 3 of those trials (RCD, VMPT, VCD) were longer than ours, with PFS ranging from less than 6 months (RCD) to approximately 12 months, respectively. We were also able to demonstrate activity of the RAD regimen in relapsed-refractory disease: ORR was identical for relapsed and relapsed-refractory patients, while PFS was slightly longer in relapsed patients without reaching the level of significance.
Results, of course, have to be interpreted carefully due to small sample sizes in this phase 1/2 trial.
Serum β2-microglobulin and cytogenetic abnormalities are 2 of the major prognostic factors in MM.23,24 In the present study, neither TTP nor response rate of patients with a 13q− or chromosomal translocation t(4;14) were significantly different from those without these abnormalities. This is in line with recent reports on combinations of bortezomib or lenalidomide, which seem to overcome the negative clinical impact of these variables.25,26 In contrast to t(4;14) and 13q−, 17p− and increased levels of β2-microglobulin appear to remain adverse prognostic factors. On multivariate analysis (not incorporating 17p− due to a low number of events), TTP was negatively affected merely by increased β2-microglobulin, whereas neither 13q− nor pretreatment modality had significant influence. Thus, in the RAD trial, detection of 17p− or elevated β2-microglobulin were both associated with poor response and a significantly shorter TTP. Even though sample size was relatively small, these results confirm observations from 1 study with RD in relapsed myeloma showing that RD overcame adverse genetic abnormalities except 17p−.27
Taken together, it seems that the novel drugs alone or in combination may change the profile of adverse prognostic factors. However, patients with 17p− appear to remain a challenge, representing a subgroup with a particularly bad prognosis even in the era of novel drugs. Very recently, a retrospective analysis demonstrated 17p− to retain prognostic significance even after allogeneic stem cell transplantation, a treatment modality that is claimed to be curative in myeloma.28 This underscores the great clinical need for the development of novel therapeutic strategies for patients with loss of the p53 tumor suppressor gene.
In conclusion, the RAD protocol provides a substantial proportion of high-quality response with substantial durability in patients with extensively pretreated myeloma and overcomes several well-known adverse prognostic factors. It is therefore tempting to speculate that RAD followed by a further means of consolidative treatment (both in primary as well as in later treatment lines) allows for the extension of TTP.
The online version of this article contains a data supplement.
Presented in abstract form at the 48th Annual Meeting of the American Society of Hematology, Orlando, FL, December 11, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This trial was supported by the Early Clinical Development Unit (ECDU) of the IZKF (Interdisziplinaeres Zentrum fuer Klinische Forschung) and CCCM (Comprehensive Cancer Center Mainfranken) of the University of Wuerzburg. H.E. and R.C.B. were supported by the German José Carreras Foundation (Clinical Unit for Myeloma-Specific Patient Care).
Authorship
Contribution: S.K. coordinated data flow, interpreted data, provided patient care, and wrote the manuscript; C.G. coordinated the database, interpreted data, and wrote the manuscript; P.L. provided patient care, performed FISH analysis, and provided input in data interpretation; M.S.T., O.S., and U.P. provided significant contributions in patient care; C.V. coordinated data flow; K.F. and A.G. significantly contributed to trial design and data interpretation and reviewed the manuscript; U.M. analyzed data, performed statistical analysis, and reviewed the manuscript; H.E. provided significant input on trial design and expert patient care and reviewed the manuscript; and R.C.B. wrote and implemented the protocol, contributed to patient and data flow, and wrote the manuscript.
Conflict-of-interest disclosure: S.K., P.L., M.S.T., U.P., H.E., and R.C.B. served as (compensated) consultants and lecturers for Celgene Germany. The remaining authors declare no competing financial interests.
A complete list of the investigators of the German Myeloma Study Group DSMM appears in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Stefan Knop, Department of Internal Medicine II, Division of Hematology, University Hospital of Wuerzburg, Klinikstr 6-8, 97070 Wuerzburg, Germany; e-mail: knop_s@klinik.uni-wuerzburg.de.
References
Author notes
*S.K. and C.G. contributed equally to this work.

![Figure 2. Time to progression in relation to prognostic factors. (A) Median TTP depending on normal or elevated (≥ 297.5 nM [3.5 mg/L]) levels of β2-microglobulin. The vertical axis represents the proportion of patients who are alive and progression-free. The horizontal axis represents time in weeks from first dose of study drug. The solid line represents patients with normal (less than 297.5 nM) β2-microglobulin (n = 42) and the dotted line represents patients with elevated (≥ 297.5 nM) β2-microglobulin (n = 17). (B) Median TTP in weeks depending on cytogenetic aberration 17p−. The vertical axis represents the proportion of patients who are alive and progression-free. The horizontal axis represents time in weeks from first dose of study drug. The solid line represents patients without 17p− (n = 32), and the dotted line represents patients with 17p− (n = 5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/18/10.1182_blood-2008-10-184135/6/m_zh80170933940002.jpeg?Expires=1763479712&Signature=eKUofUMNlf0CAGXgwebx--P77h~M1FhuGVH1TlEunwYhNP1Dm9eYsMEBOYVM4azKDausnTrUma8JP~BsJa~B0acLtKC1qJ1e3CQEMaw9runGATEZU7jp5wJ~CVXwQnQm-sZhHPvrXsLTQ5Ihi1EChvaamq7X12g50F~sr-k-gbYCOwCGgsliTWDnaU0narHB2EETsZK6~1j1XSVczvxvjIaJDA5JJnRNY59C-TKYZxaro5nZJce9NNDAQIs2NJ6MWyua6JzvxSYvvz~etybf1wQsUgaxidJWzlVSdrsLIUeSKnZ2fTqzYZ5-XInPfkamnCQfM4UEbBa96SajGc0ykQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)