Abstract
Acute myeloid leukemia (AML) is most common in the elderly, and most elderly are thought to be unfit for intensive treatment because of the risk of fatal toxicity. The Swedish Acute Leukemia Registry covers 98% of all patients with AML (nonacute promyelocytic leukemia) diagnosed in 1997 to 2005 (n = 2767), with a median follow-up of 5 years, and reports eligibility for intensive therapy, performance status (PS), complete remission rates, and survival. Outcomes were strongly age and PS dependent. Early death rates were always lower with intensive therapy than with palliation only. Long-term survivors were found among elderly given intensive treatment despite poor initial PS. Total survival of elderly AML patients was better in the geographic regions where most of them were given standard intensive therapy. This analysis provides unique real world data from a large, complete, and unselected AML population, both treated and untreated, and gives background to treatment decisions for the elderly. Standard intensive treatment improves early death rates and long-term survival compared with palliation. Most AML patients up to 80 years of age should be considered fit for intensive therapy, and new therapies must be compared with standard induction.
Introduction
Acute myeloid leukemia (AML) presents in all ages but is mainly a disease of the elderly with a median age of 69 years in the white US population of the Surveillance, Epidemiology, and End Results database.1 Age has a major impact on the management and outcome for patients with AML. Elderly patients have more comorbidity, more often poor performance status (PS) at diagnosis,2-4 and less tolerance to intensive therapy, and are therefore less frequently judged to be fit for remission induction.5,6 Furthermore, older patients have a higher incidence of poor prognostic factors, such as high-risk cytogenetics7 and secondary leukemia. Together, this results in the well-known dismal prognosis for elderly patients with AML.
Medical management is based on clinical studies, but most published information on AML concerns young patients. Studies on the elderly involve few and highly selected patient populations2 where, with few exceptions,8,9 no information on the selection process is available. The degree of selection in study populations compared with the real world increases with age.10 In addition, data from few databases1,11-13 and several single-institution observation studies are available.14-19 The lack of validated guidelines on the use of intensive treatment for elderly patients has led to individual and variable management of older AML patients but also created an opportunity to study new therapies for patients “unfit” for standard therapy.20-23
With the use of the large and almost complete and, therefore, unselected Swedish National Acute Leukemia Registry, we here show that age has a strong prognostic impact also within the group of elderly and that common assumptions with strong implications for management are untrue. Importantly, we show that standard intensive therapy decreases rather than increases early death rates and is a prerequisite for long-term survival in most patients up to 80 years of age.
Methods
Swedish population registries were introduced by law in the year 1686 for taxation and military purposes, with the first report on survival in 1746. The Swedish Cancer Registry is a nation-wide compulsory dual-report system developed in 1958, aided by the personal identification code system for all Swedish citizens established in 1947. First, all pathology specimens indicating malignancy are reported by the pathologist to the Regional Tumor Registry; and second, all patients with a newly diagnosed cancer are reported by the clinic; missing data are actively requested.
The Swedish Adult Acute Leukemia Registry was founded in 1997 by the Swedish Society of Hematology. It is supported by the Swedish Board for Health and Welfare and run in collaboration with the Regional Tumor Registry in each of the 6 Swedish healthcare regions, covering populations ranging from 0.9 to 1.9 million people, in total 9 million. Each region contains 1 or 2 university hospitals and 3 to 8 county hospitals treating leukemia, and patients are not referred for treatment outside the home region. No patients are seen at private hospitals. Reporting of data on all newly diagnosed patients with acute leukemia, de novo or secondary (blastic phase of chronic myeloid leukemia excluded), has thus been compulsory since 1997; therefore, almost all patients have 3 separate registrations (pathology, clinical report to national cancer registry, and report to leukemia registry), although sometimes given retrospectively. This analysis includes patients diagnosed until October 2005, when a new National Care Program for AML in Sweden was launched, and excludes acute promyelocytic leukemia (APL); because APL patients are treated differently and have a better outcome; APL will be reported separately.
Survival was always checked using the Swedish Population Registry, with an update in September 2007, and is therefore complete and accurate. The study was performed in agreement with the ethics committees of all participating institutions and the Swedish Society of Hematology.
The initial registration form for the Leukemia Registry includes patient identification, use of diagnostic procedures, and French-American-British type. Furthermore, the physician is requested to report whether the patient at diagnosis is eligible for intensive combination chemotherapy or not. This decision is based on clinical data and local routine, but not on karyotype, because cytogenetic reports are not available when treatment should be initiated. In previous reports, we have documented that there are major differences between the different geographic regions in the proportion of elderly patients judged to be fit for remission induction.6 Remission induction always consists of an anthracycline plus cytosine arabinoside (Ara-C), according to regional protocols and estimated patient status, in general TAD, ′3 plus 7′, or similar,9 with possible dose reductions for the elderly. Patients in remission subsequently receive consolidation with 1 to 3 courses of combination chemotherapy, usually including Ara-C at more than or equal to 1 g/m2 per dose. The potential impact of consolidation and transplant is out of scope for this analysis, and patients were not censored at transplant or for any reason in survival analyses. Chemotherapy used with a palliative intent, such as single-drug, low-dose Ara-C, hydroxyurea, or thioguanin, is not regarded as remission induction, despite the potential for myelosuppression and achievement of remission.
Statistical analyses were performed using Statistica software (Tulsa, OK).
Results
The Swedish Acute Leukemia Registry contained 3371 adult patients over 16 years with acute leukemia, according to French-American-British criteria, diagnosed from January 1997 through September 2005, not including teenage patients treated at pediatric departments. There were 2866 cases with AML, including 99 with APL, 408 with acute lymphoblastic leukemia, and 97 with acute undifferentiated/unclassified leukemia. This corresponds to 98% of all patients with acute leukemia in the Swedish Cancer Registry. Missing patients had a similar age and sex distribution as patients in the Leukemia Registry.6 The current report is thus based on 2767 patients with non-APL AML. The incidence in relation to age is shown in Figure 1, with the highest incidence in ages 80 to 85 years and a subsequent decrease in the very old. The median age was 72 years (quartile values, 60-79 years; range, 16-97 years; mean, 68 years), 71 years for males, and 72 years for females.
Incidence of AML (non-APL) in 1997 to 2005 (new cases per 100 000 inhabitants, based on the Swedish population in 2005) according to age and sex.
Incidence of AML (non-APL) in 1997 to 2005 (new cases per 100 000 inhabitants, based on the Swedish population in 2005) according to age and sex.
Diagnosis
Diagnostic procedures included immune phenotyping in more than 90% of patients younger than 70 years, and in 80% of those between 70 and 80 years. Cytogenetics was performed in more than 90% of patients younger than 60 years, and in 84%, 65%, and 30%, respectively, in subsequent age decades. Results of karyotyping were not captured by the whole registry; however, in one region, 293 karyotypes were available, with 1% favorable, 69% intermediate, 25% high-risk karyotypes according to Grimwade et al,7 and 5% unknown in the population 60 to 79 years of age (G.J., B. Johansson, unpublished data, January 2008). In another region, karyotype was reported in 193 patients older than 60 years during the period 1982 to 1998, with 4% favorable, 69% intermediate, 20% high-risk karyotypes, and 7% unknown.9
In the current study, 24% had AML secondary to previous hematologic disease and 4% had therapy-related AML; the peak proportion was found in ages 70 to 74 years, where 32% of AMLs were secondary and 6% therapy-related. In subsequent analyses, therapy-related AML and AML secondary to previous hematologic disease are grouped together as secondary AML.
Performance status
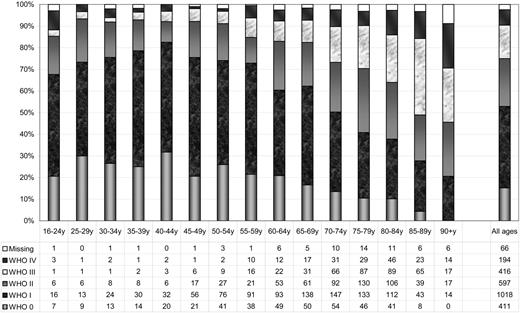
Data on World Health Organization (WHO)/Eastern Cooperative Oncology Group (ECOG) PS24 at the time of diagnosis were available in 2696 patients (97.4%). The distribution of PS according to age is shown in Figure 2, with the best PS in ages 40 to 44 years and declining with increasing age. In total, half of the patients had PS 0 or I at diagnosis.
Proportion of AML (non-APL) patients with WHO/ECOG performance status 0 to IV at diagnosis according to age. The numbers of patients are given at the bottom of the figure.
Proportion of AML (non-APL) patients with WHO/ECOG performance status 0 to IV at diagnosis according to age. The numbers of patients are given at the bottom of the figure.
Therapy
The primary intention with therapy (remission induction or palliation with or without chemotherapy) at time of diagnosis was recorded in all but 8 patients (99.7%). The proportions of patients eligible for remission induction (onwards referred to as intensive treatment) according to age and PS are shown in Table 1.
Patients reported fit for intensive chemotherapy
| Age, y . | All . | WHO 0 . | WHO I . | WHO II . | WHO III . | WHO IV . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | |
| < 50 | 336 | 342 | 98 | 86 | 86 | 100 | 171 | 174 | 98 | 51 | 52 | 98 | 13 | 14 | 93 | 9 | 10 | 90 |
| 50-54 | 155 | 160 | 97 | 42 | 42 | 100 | 73 | 76 | 96 | 26 | 28 | 93 | 9 | 9 | 100 | 2 | 2 | 100 |
| 55-59 | 165 | 181 | 91 | 38 | 38 | 100 | 87 | 94 | 93 | 20 | 21 | 95 | 13 | 16 | 81 | 6 | 11 | 55 |
| 60-64 | 223 | 242 | 92 | 50 | 50 | 100 | 95 | 98 | 97 | 46 | 53 | 87 | 20 | 23 | 87 | 7 | 12 | 58 |
| 65-69 | 246 | 308 | 80 | 48 | 51 | 94 | 126 | 140 | 90 | 45 | 62 | 73 | 13 | 31 | 42 | 9 | 17 | 53 |
| 70-74 | 281 | 419 | 67 | 48 | 56 | 86 | 127 | 159 | 80 | 60 | 95 | 63 | 29 | 66 | 44 | 9 | 31 | 29 |
| 75-79 | 202 | 448 | 45 | 28 | 46 | 61 | 79 | 137 | 58 | 58 | 133 | 44 | 26 | 89 | 29 | 6 | 29 | 21 |
| 80-84 | 96 | 411 | 23 | 12 | 41 | 29 | 34 | 114 | 30 | 24 | 106 | 23 | 20 | 90 | 22 | 5 | 46 | 11 |
| 85+ | 11 | 256 | 4 | 2 | 8 | 25 | 4 | 58 | 7 | 2 | 58 | 3 | 3 | 82 | 4 | 0 | 38 | 0 |
| All ages | 1715 | 2767 | 62 | 354 | 418 | 85 | 796 | 1050 | 76 | 332 | 608 | 55 | 146 | 420 | 35 | 53 | 196 | 27 |
| Age, y . | All . | WHO 0 . | WHO I . | WHO II . | WHO III . | WHO IV . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | Intensive . | Total . | %Int . | |
| < 50 | 336 | 342 | 98 | 86 | 86 | 100 | 171 | 174 | 98 | 51 | 52 | 98 | 13 | 14 | 93 | 9 | 10 | 90 |
| 50-54 | 155 | 160 | 97 | 42 | 42 | 100 | 73 | 76 | 96 | 26 | 28 | 93 | 9 | 9 | 100 | 2 | 2 | 100 |
| 55-59 | 165 | 181 | 91 | 38 | 38 | 100 | 87 | 94 | 93 | 20 | 21 | 95 | 13 | 16 | 81 | 6 | 11 | 55 |
| 60-64 | 223 | 242 | 92 | 50 | 50 | 100 | 95 | 98 | 97 | 46 | 53 | 87 | 20 | 23 | 87 | 7 | 12 | 58 |
| 65-69 | 246 | 308 | 80 | 48 | 51 | 94 | 126 | 140 | 90 | 45 | 62 | 73 | 13 | 31 | 42 | 9 | 17 | 53 |
| 70-74 | 281 | 419 | 67 | 48 | 56 | 86 | 127 | 159 | 80 | 60 | 95 | 63 | 29 | 66 | 44 | 9 | 31 | 29 |
| 75-79 | 202 | 448 | 45 | 28 | 46 | 61 | 79 | 137 | 58 | 58 | 133 | 44 | 26 | 89 | 29 | 6 | 29 | 21 |
| 80-84 | 96 | 411 | 23 | 12 | 41 | 29 | 34 | 114 | 30 | 24 | 106 | 23 | 20 | 90 | 22 | 5 | 46 | 11 |
| 85+ | 11 | 256 | 4 | 2 | 8 | 25 | 4 | 58 | 7 | 2 | 58 | 3 | 3 | 82 | 4 | 0 | 38 | 0 |
| All ages | 1715 | 2767 | 62 | 354 | 418 | 85 | 796 | 1050 | 76 | 332 | 608 | 55 | 146 | 420 | 35 | 53 | 196 | 27 |
Patients reported fit for intensive chemotherapy according to age and WHO/ECOG performance status (number of eligible patients/total number/percentage).
Complete remission and early death rates
Data on complete remission (CR) after intensive treatment were available in 98% of those eligible. CR rates and early death rates, that is, proportion of patients dead within 30 days from diagnosis, according to age, therapeutic decision, and PS, are shown in Table 2 and Figures 3 and 4. The CR rates among intensively treated patients were 65% in patients with de novo AML and 41% with secondary AML (all ages, χ2 = 59, P < .001).
Early death rates
| Age, y . | All . | Therapy . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive . | Palliative . | ||||||||
| ED . | Total . | %ED . | ED . | Total . | %ED . | ED . | Total . | %ED . | |
| < 50 | 15 | 342 | 4 | 14 | 336 | 4 | 1 | 6 | 17 |
| 50-54 | 14 | 160 | 9 | 12 | 155 | 8 | 2 | 5 | 40 |
| 55-59 | 25 | 181 | 14 | 17 | 165 | 10 | 8 | 16 | 50 |
| 60-64 | 27 | 242 | 11 | 20 | 223 | 9 | 7 | 19 | 37 |
| 65-69 | 43 | 308 | 14 | 20 | 246 | 8 | 23 | 61 | 38 |
| 70-74 | 83 | 419 | 20 | 35 | 281 | 12 | 47 | 137 | 34 |
| 75-79 | 98 | 448 | 22 | 30 | 202 | 15 | 67 | 244 | 27 |
| 80-84 | 125 | 411 | 30 | 25 | 96 | 26 | 100 | 312 | 32 |
| 85+ | 103 | 256 | 40 | 1 | 11 | 9 | 101 | 244 | 41 |
| All groups | 533 | 2767 | 19 | 174 | 1715 | 10 | 356 | 1044 | 34 |
| WHO/ECOG PS 0-II | |||||||||
| 16-55 | 21 | 491 | 4 | 3 | 12 | 25 | |||
| 56-65 | 22 | 344 | 6 | 6 | 22 | 27 | |||
| 66-75 | 35 | 435 | 8 | 27 | 131 | 21 | |||
| 76-89 | 29 | 211 | 14 | 67 | 397 | 17 | |||
| WHO/ECOG PS III-IV | |||||||||
| 16-55 | 10 | 38 | 26 | 2 | 4 | 50 | |||
| 56-65 | 12 | 43 | 28 | 12 | 19 | 63 | |||
| 66-75 | 21 | 62 | 34 | 50 | 92 | 54 | |||
| 76-89 | 20 | 56 | 36 | 142 | 271 | 52 | |||
| Age, y . | All . | Therapy . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive . | Palliative . | ||||||||
| ED . | Total . | %ED . | ED . | Total . | %ED . | ED . | Total . | %ED . | |
| < 50 | 15 | 342 | 4 | 14 | 336 | 4 | 1 | 6 | 17 |
| 50-54 | 14 | 160 | 9 | 12 | 155 | 8 | 2 | 5 | 40 |
| 55-59 | 25 | 181 | 14 | 17 | 165 | 10 | 8 | 16 | 50 |
| 60-64 | 27 | 242 | 11 | 20 | 223 | 9 | 7 | 19 | 37 |
| 65-69 | 43 | 308 | 14 | 20 | 246 | 8 | 23 | 61 | 38 |
| 70-74 | 83 | 419 | 20 | 35 | 281 | 12 | 47 | 137 | 34 |
| 75-79 | 98 | 448 | 22 | 30 | 202 | 15 | 67 | 244 | 27 |
| 80-84 | 125 | 411 | 30 | 25 | 96 | 26 | 100 | 312 | 32 |
| 85+ | 103 | 256 | 40 | 1 | 11 | 9 | 101 | 244 | 41 |
| All groups | 533 | 2767 | 19 | 174 | 1715 | 10 | 356 | 1044 | 34 |
| WHO/ECOG PS 0-II | |||||||||
| 16-55 | 21 | 491 | 4 | 3 | 12 | 25 | |||
| 56-65 | 22 | 344 | 6 | 6 | 22 | 27 | |||
| 66-75 | 35 | 435 | 8 | 27 | 131 | 21 | |||
| 76-89 | 29 | 211 | 14 | 67 | 397 | 17 | |||
| WHO/ECOG PS III-IV | |||||||||
| 16-55 | 10 | 38 | 26 | 2 | 4 | 50 | |||
| 56-65 | 12 | 43 | 28 | 12 | 19 | 63 | |||
| 66-75 | 21 | 62 | 34 | 50 | 92 | 54 | |||
| 76-89 | 20 | 56 | 36 | 142 | 271 | 52 | |||
Early death (ED) rates (number of deaths within 30 days from diagnosis/total number/percentage) according to age and type of therapy, and according to WHO/ECOG performance status.
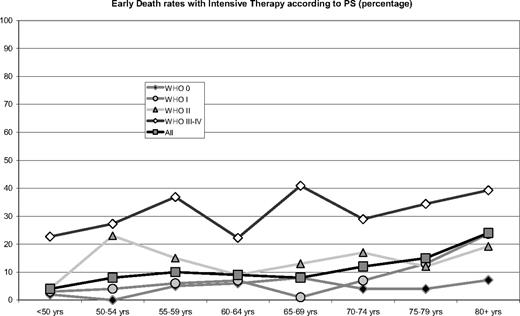
Early death rates (within 30 days from diagnosis) with intensive therapy according to age and performance status. The numbers of patients are given in Table 1.
Early death rates (within 30 days from diagnosis) with intensive therapy according to age and performance status. The numbers of patients are given in Table 1.
Complete remission rates with intensive treatment according to age and WHO/ECOG performance status. The numbers of patients are given in Table 1.
Complete remission rates with intensive treatment according to age and WHO/ECOG performance status. The numbers of patients are given in Table 1.
Early death rates were dependent on both age and PS. However, old patients with good PS had low early death rates, and patients with poor PS had increased early death rates in all ages (Figure 3, PS 0-II vs PS III-IV: all ages, χ2 = 115, P < .001; ages < 55 years, χ2 = 31, P < .001; ages 56-65 years, χ2 = 22, P < .001; ages 66-75 years, χ2 = 36, P < .001; ages 76-89 years, χ2 = 14, P = .002). The relative risk of early death according to age and PS was calculated with generalized linear models and was found to increase by 13% per 5-year age group when adjusting for PS and by 21% without such adjustment. The worse PS in older age groups (Figure 2) partly explained the increased early death rates with age, but when adjusting for PS, high age was still associated with an increased early death rate (P < .001).
There was no difference in early death rates between de novo and secondary AML cohorts with the same age (all ages, χ2 = 3.4, P = .07, data not shown). Early death rates were in all ages considerably lower in patients receiving intensive rather than palliative therapy (χ2 = 240, P < .001, Table 2), also when stratified for performance status (Table 2).
Overall survival
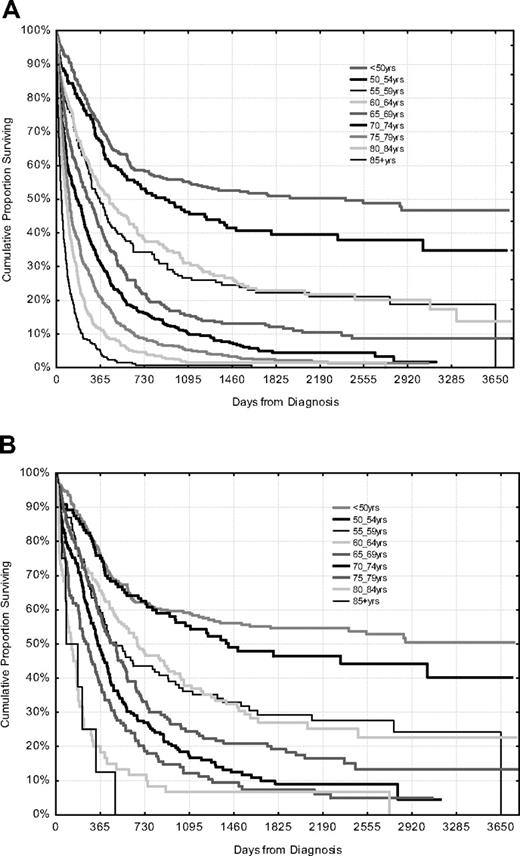
The median observation time of the 422 surviving patients was 1855 days, ie, over 5 years (quartiles, 1164-2600 days; mean, 1945 days); and because the median survival of all deceased patients was 126 days (quartiles, 36-347 days; mean, 271 days), the survival analysis is mature. Surviving patients had a median age of 53 years at diagnosis (quartiles, 42-62 years; maximum, 87 years). Overall survival according to age group is shown in Figure 5. Survival decreased with every 5-year age group, except for ages 55 to 64 years. In all ages the median survival was very much shorter than the expected residual life span of all Swedish people, which is 10.6 and 12.9 years for Swedish 75-year-old males and females, respectively, and slightly longer than in most other countries. The 5-year survival of the overall Swedish population 70 to 79 years of age is 80%.6
Overall survival according to age irrespective of management (top, n = 2767), and patients with de novo AML, fit for intensive treatment, and with WHO/ECOG performance status 0 to II (bottom, n = 1229).
Overall survival according to age irrespective of management (top, n = 2767), and patients with de novo AML, fit for intensive treatment, and with WHO/ECOG performance status 0 to II (bottom, n = 1229).
Figure 5 also shows the overall survival according to age in patients selected for criteria usually applied in clinical studies, that is, de novo AML with PS 0 to II, and fit for intensive therapy. This selected group had more than a doubling of the median overall survival (60% increase in ages 56-65 years; Table 3), compared with the global patient population, also including those excluded by these criteria.
Median overall survival
| Age, y . | All . | Selected . | Proportion . | OS ratio . | ||
|---|---|---|---|---|---|---|
| n . | OS . | n . | OS . | Selected/all % . | Selected/all . | |
| 16-55 | 554 | 1119 | 434 | 2546 | 78 | 2.3 |
| 56-65 | 437 | 359 | 275 | 562 | 63 | 1.6 |
| 66-75 | 738 | 184 | 341 | 385 | 46 | 2.1 |
| 76-89 | 968 | 80 | 178 | 189 | 18 | 2.4 |
| Total | 2697 | 196 | 1228 | 500 | 46 | 2.3 |
| Age, y . | All . | Selected . | Proportion . | OS ratio . | ||
|---|---|---|---|---|---|---|
| n . | OS . | n . | OS . | Selected/all % . | Selected/all . | |
| 16-55 | 554 | 1119 | 434 | 2546 | 78 | 2.3 |
| 56-65 | 437 | 359 | 275 | 562 | 63 | 1.6 |
| 66-75 | 738 | 184 | 341 | 385 | 46 | 2.1 |
| 76-89 | 968 | 80 | 178 | 189 | 18 | 2.4 |
| Total | 2697 | 196 | 1228 | 500 | 46 | 2.3 |
Median overall survival in days according to age for all patients versus those selected for de novo AML, fit for intensive treatment, and PS 0-II.
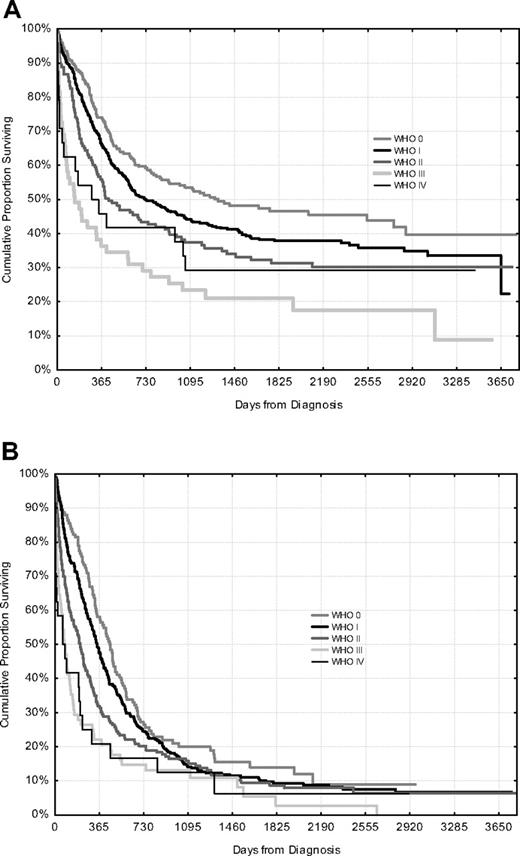
Overall survival with intensive treatment for patients younger than and older than 65 years according to PS is shown in Figure 6. Despite a high early death rate in PS III or IV, long-term survivors were found.
Overall survival according to WHO/ECOG performance status, only patients fit for intensive treatment. Patients younger than 65 years (top, n = 864) and 65 to 79 years (bottom, n = 711).
Overall survival according to WHO/ECOG performance status, only patients fit for intensive treatment. Patients younger than 65 years (top, n = 864) and 65 to 79 years (bottom, n = 711).
Consequences of the decision to treat
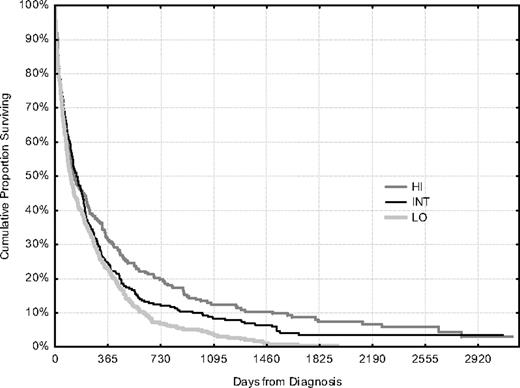
We know since 10 years, that is, the first report from the registry, that there is a variation in the proportion of elderly patients given intensive therapy between the 6 Swedish healthcare regions.6 This variation is greatest among patients 70 to 79 years of age, and we therefore analyzed this cohort according to the regions, grouped according to their individual therapeutic strategy in low (41% given intensive treatment), intermediate (58%), and high (75%) (Table 4); there were no other differences with prognostic implications, such as the distribution of PS or secondary leukemia, or survival expectancy of the general population. Early death rates and CR rates were as good in the high as in the low region and resulted in an improved CR rate and survival of the global AML cohort of that age in regions where a greater proportion of the patients received intensive treatment (Table 4; Figure 7). The statistics were very strong as regards the clinically important number of long-term survivors (P < .001). However, the log rank test provided a borderline P value (χ2 = 5.4, P = .066). The log rank test ranks survival without considering the clinical relevance of the survival time. In this elderly cohort with high initial death rates, similar in all patient groups (Figure 7), the clinically important value of the subsequently decreased death rates in some groups gives little impact in the statistics. Landmark analysis is a way to evaluate subsequent survival from a defined time point and is useful when the survival difference emerges later. We therefore performed landmark analyses, which resulted in significant survival differences also according to the log-rank test (30 days, χ2 = 7.4, P = .024; 90 days, χ2 = 9.8, P = .007).
AML, non-APL patients 70-79 years old (N = 864)
| Region . | Low . | Intermediate . | High . | Comparison: χ2 (P) . | |
|---|---|---|---|---|---|
| Low versus high . | |||||
| All patients | |||||
| Intensive/total (%) | 141/348 (41%) | 153/264 (58%) | 189/252 (75%) | 70 (P ≪ .001) | |
| With intensive therapy | |||||
| PS (0/I/II/III/IV) number | 25/65/25/15/6 | 24/59/41/22/4 | 27/82/52/18/5 | 0.57 (P = .45)* | |
| PS (0/I/II/III/IV) % | 18/48/18/11/4 | 16/39/27/15/3 | 15/45/28/10/3 | ||
| Early death rates | 77/349 (22%) | 50/265 (19%) | 54/253 (21%) | 0.04 (P = .83) | |
| With intensive treatment | 20/141 (16%) | 14/153 (9%) | 31/189 (16%) | 0.30 (P = .58) | |
| With palliation only | 56/207 (27%) | 36/111 (32%) | 22/63 (35%) | 1.46 (P = .23) | |
| CR rates | |||||
| Intensive treatment | 58/141 (41%) | 80/153 (52%) | 93/189 (49%) | 2.12 (P = .15) | |
| Intensive and palliative | 68/349 (19%) | 83/265 (31%) | 93/253 (37%) | 22.3 (P < .001) | |
| Patients alive | |||||
| At 2 y | 24 | 31 | 50 | 22.7 (P < .001) | |
| At 5 y | 1 | 5 | 13 | 15 (P = .001) | |
| Region . | Low . | Intermediate . | High . | Comparison: χ2 (P) . | |
|---|---|---|---|---|---|
| Low versus high . | |||||
| All patients | |||||
| Intensive/total (%) | 141/348 (41%) | 153/264 (58%) | 189/252 (75%) | 70 (P ≪ .001) | |
| With intensive therapy | |||||
| PS (0/I/II/III/IV) number | 25/65/25/15/6 | 24/59/41/22/4 | 27/82/52/18/5 | 0.57 (P = .45)* | |
| PS (0/I/II/III/IV) % | 18/48/18/11/4 | 16/39/27/15/3 | 15/45/28/10/3 | ||
| Early death rates | 77/349 (22%) | 50/265 (19%) | 54/253 (21%) | 0.04 (P = .83) | |
| With intensive treatment | 20/141 (16%) | 14/153 (9%) | 31/189 (16%) | 0.30 (P = .58) | |
| With palliation only | 56/207 (27%) | 36/111 (32%) | 22/63 (35%) | 1.46 (P = .23) | |
| CR rates | |||||
| Intensive treatment | 58/141 (41%) | 80/153 (52%) | 93/189 (49%) | 2.12 (P = .15) | |
| Intensive and palliative | 68/349 (19%) | 83/265 (31%) | 93/253 (37%) | 22.3 (P < .001) | |
| Patients alive | |||||
| At 2 y | 24 | 31 | 50 | 22.7 (P < .001) | |
| At 5 y | 1 | 5 | 13 | 15 (P = .001) | |
Features according to geographical region grouped and ordered according to proportion of patients given intensive therapy.
Value is for PS 0-II versus III-IV.
Overall survival of all patients, treated and untreated, 70 to 79 years of age according to geographic region, with different proportions of patients given intensive therapy (Table 4).
Overall survival of all patients, treated and untreated, 70 to 79 years of age according to geographic region, with different proportions of patients given intensive therapy (Table 4).
A summary of important features of AML in the elderly (APL excluded) is given in Table 5.
Summary of important features of AML in elderly (APL excluded)
| AML in the elderly . |
|---|
| Epidemiology |
| The median age of adult AML patients is 72 y, and the mean age is 68 y; similar for males and females. |
| AML has a peak incidence at 80-84 y. |
| Males over 70 years have a higher incidence than females of the same age. |
| At least 70% of patients up to age 80 have a performance status of 0-II. |
| One-fourth of the AML patients have a previous hematological disease. |
| The proportion of secondary AML is largest in ages 70-74, when one-third have a previous hematological disease. |
| Clinical |
| Most patients up to age 80 benefit from standard intensive treatment. |
| Standard intensive treatment decreases rather than increases early death rate. |
| Complete remission is achieved with intensive treatment in at least half of the patients up to age 75, and in patients with good performance status up to age 80. |
| Complete remission is less frequently achieved in secondary AML as compared to de novo AML, but early death rates are similar. |
| Performance status is more predictive for early death rate than age. |
| Long-term survival may be achieved also in patients with initial poor performance status. |
| Selection criteria commonly used for inclusion into clinical studies have a major impact on reported outcome. |
| From previous studies |
| Patients in remission from AML require less supportive care and hospitalization and have a better quality of life than patients during palliation. |
| Interpretation |
| Remission, even if of short duration, is a reasonable aim also in elderly patients with AML. |
| Most patients up to age 80 should be considered for standard intensive treatment. |
| New treatments should be compared to standard intensive treatment, also in elderly. |
| AML in the elderly . |
|---|
| Epidemiology |
| The median age of adult AML patients is 72 y, and the mean age is 68 y; similar for males and females. |
| AML has a peak incidence at 80-84 y. |
| Males over 70 years have a higher incidence than females of the same age. |
| At least 70% of patients up to age 80 have a performance status of 0-II. |
| One-fourth of the AML patients have a previous hematological disease. |
| The proportion of secondary AML is largest in ages 70-74, when one-third have a previous hematological disease. |
| Clinical |
| Most patients up to age 80 benefit from standard intensive treatment. |
| Standard intensive treatment decreases rather than increases early death rate. |
| Complete remission is achieved with intensive treatment in at least half of the patients up to age 75, and in patients with good performance status up to age 80. |
| Complete remission is less frequently achieved in secondary AML as compared to de novo AML, but early death rates are similar. |
| Performance status is more predictive for early death rate than age. |
| Long-term survival may be achieved also in patients with initial poor performance status. |
| Selection criteria commonly used for inclusion into clinical studies have a major impact on reported outcome. |
| From previous studies |
| Patients in remission from AML require less supportive care and hospitalization and have a better quality of life than patients during palliation. |
| Interpretation |
| Remission, even if of short duration, is a reasonable aim also in elderly patients with AML. |
| Most patients up to age 80 should be considered for standard intensive treatment. |
| New treatments should be compared to standard intensive treatment, also in elderly. |
Discussion
The poor prognosis of AML in the elderly is not sufficiently explained by their more frequent adverse prognostic factors,7,9,25 including poor PS (Figure 2). Remission induction requires prolonged hospitalization and intensive supportive care, which is costly and cumbersome, and high early death rates of 17% to 54% among elderly have been reported.3 With more comorbidity in older people, reluctance toward intensive therapy is common. However, low-dose therapy or dose reduction of intensive therapy may lead to lower CR rates with delayed recovery of blood counts, which may be worse than the toxicity of standard intensive treatment.9,26 Patients in remission from acute leukemia require less supportive care and hospitalization and have a better quality of life than patients during palliation. Remission, even if short-lived, is therefore a reasonable aim also in elderly patients.
The consequences of intensive compared with palliative therapy (with or without chemotherapy) are important to assess but difficult to address in clinical trials, and observation studies are unreliable because of large and uncontrolled patient selections. A small randomized study was published in 1989, in which 31 AML patients older than 65 years receiving combination chemotherapy had a median survival of 21 weeks compared with 11 weeks for 29 patients on a “wait-and-see” strategy,27 and there was no difference in time spent in hospital. Another small study performed in the 1980s on 87 patients older than 65 years failed to show benefit from intensive therapy resulting from increased early death rates.28 The recent large United Kingdom AML14 study aimed to randomize between intensive and nonintensive treatment, but only 8 of 1400 patients were randomized.5 Finally, a case-control study starting with 457 AML patients in a single institution compared 34 patients over 60 years given nonintensive therapy to 102 patients receiving induction therapy14 ; intensive treatment resulted in 63% CR and a reduced early death rate and improved survival compared with nonintensive therapy. Two population-based or rather hospital-based registry studies in patients older than 56 years13 and 60 years12 compared intensive vs nonintensive treatment, with no apparent benefit from intensive therapy.
The Swedish Adult Acute Leukemia Registry is a true population-based registry, and the size, completeness, and procedures for data collection make this, by far, the most valid source for information on elderly AML currently available, despite that some items may have been captured retrospectively and are not monitored and validated as closely as in clinical studies. However, long-term follow-up and survival are completely accurate.
Many of the current analyses evaluated outcome for patients eligible for intensive therapy because this item was reported on the initial form with almost no missing data and reflects therapeutic strategy more than actually given therapy, that is, an intention-to-treat analysis. There were occasional reports on discrepancies between eligibility and given therapy; however, similar results were always achieved from corresponding analyses according to given therapy.
Until recently, our registry did not include karyotype, and the impact of cytogenetic risk group could therefore not be assessed, although it is probable that most of our older patients had intermediate karyotypes and very few favorable (APL was excluded). It is well known that adverse cytogenetics confer reduced response rates and durations as well as impaired survival,29 and that adults older than 55 years have more complex and less favorable abnormalities than younger adults.7,25,30 However, few of the large cytogenetic studies have an appropriate proportion of patients over 70 years. There is also the conflict between waiting for cytogenetic results for treatment decision and the aim to start induction as soon as possible.31 In the current improved web-based Swedish registry, cytogenetics will be recorded, permitting future analyses on the impact of karyotype.
Intensive treatment was always given using established treatment protocols that have not changed for decades. Many randomized trials, for example, from Medical Research Council,32 European Organisation for the Research and Treatment of Cancer,33 Cancer and Leukemia Group B Study,34 Southwest Oncology Group,35 ECOG,31 and Leukemia Group of Middle Sweden (LGMS),8 have failed to show improvement with modifications of the standard therapy. Indeed, no newer treatment options, such as gemtuzumab,36 clofarabine,21 tipifarnib,22 or fludarabine combinations,37 have provided better or even the same level of response and survival as we show here in an unselected total population given standard intensive treatment. Newer treatments are often studied in patients judged to be unfit for standard treatment, but this seems not validated comparing ages and PS distributions in those studies to ours.18,20
One important piece of information in this report is the finding of improved early death rates with intensive treatment compared with palliation only. This was seen in all age groups and all PS levels. In the study by SWOG,2 9 of 11 patients older than 75 years with a PS of III died within 30 days. In our study of unselected patients, 20 of 56 patients 76 to 89 years of age and PS III or IV given intensive treatment suffered an early death (36%), compared with 142 of 271 such patients receiving palliation only (52%) (Table 2, χ2 = 5.2, P = .023). Furthermore, the early death rates with impaired PS were increased in all ages (Figure 3), whereas there were long-term survivors also with poor initial PS. It is therefore not justified to deny intensive treatment to this patient group based on presumed early death rates only.
We and others used 30 days as cutoff for early deaths because most responding patients will have returning blood counts and transfusion independence at that time point. Kantarjian et al17 suggests analysis of death rates within 8 weeks to involve consideration of deaths resulting from lack of response. In our study, the early death rates increased by a mean factor of 1.4 using the 8-week cutoff (data not shown); however, this was similar in all subcategories and did therefore not change the results of any comparison.
PS evaluations are widely used in clinical studies. The WHO/ECOG system24 is simpler than the original Karnofsky score, but in AML there is a problem of potential variation resulting from major changes in PS that can occur during the diagnostic workup, for example, when a patient with initial severe anemia and sepsis is transfused and prepared for leukemia therapy. The PS classification does not differentiate between functional impairment resulting from leukemia, which is reversible with intensive treatment, compared with irreversible comorbidity unrelated to leukemia. Therefore, comorbidity scoring38 may have a greater potential for providing basis for treatment decisions than PS39 but has not been recorded in the Swedish registry previously.
It is obvious from our tables and figures that age and PS are independently influencing outcomes. It is also clear that the correlations to outcome are not overall linear, but breakpoints can be identified at different levels for different populations and outcomes. No additional information would be achieved from multivariate analysis, which was therefore not performed. Linear models, however, were used to show that age and PS had independent influence on early death rates.
Our study, in contrast to most other reports, gives data on all AML patients irrespective of management, but it could still be argued that our results are skewed because of selection and the possible existence of additional pretreatment prognostic factors. However, we have used our knowledge that the proportion of elderly patients given intensive therapy is different in the 6 Swedish healthcare regions,6 all having all the required facilities for the management of AML, with no out-of-region referrals that would lead to selection bias, and no difference in the health status of the populations, that is, similar survival expectancy and immediate access to advanced health care. The difference between the regions was greatest in the age group 70 to 79 years, and we therefore again6 chose this cohort for the analysis of early death rates, CR rates, and overall survival in relation to the proportion of patients given intensive therapy (Table 4). If a narrow selection of patients is applied, choosing only those with the best potential for a positive outcome, you would expect a higher CR rate and a lower early death rate among the treated, and the same survival for the global AML cohort compared with when intensive treatment is given to a larger proportion of elderly, according to the well-known Will Rogers phenomenon.40 However, here we found the same CR rates and early death rates in all regions, but clinically significant improvements of overall survival where more patients were given intensive treatment (Figure 7), supporting a benefit of intensive treatment for most elderly patients.
We thus claim that standard intensive treatment for elderly patients with AML up to 80 years of age reduces early death rates and improves the chance for a survival that is far from satisfactory but clinically significant and longer than with palliation only. New treatments need to be compared directly with standard intensive treatment and not simply given to patients stated to be unfit for standard treatment.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We appreciate the work of data manager Cecilia Arnesson, Regional Tumor Registry Lund, and statistical support from Professor John Carstensen, Linköping. The authors are all board members of the Swedish Acute Myeloid Leukemia Group. We thank all Swedish hematologists who have carefully reported all their patients to the Registry.
The Registry was supported by the Swedish National Board of Health and Welfare. G.J. is Professor of Hematology at the Lund Strategic Research Center for Stem Cell Biology and Cell Therapy.
Authorship
Contribution: G.J. is chairman of the Acute Myeloid Leukemia Registry Group and who was mainly responsible for the analyses and wrote the manuscript; M.H. is chairman; G.J., P.A., Å.D., S.L., L.M., D.S., U.T., and A.W. are board members of the Swedish National AML group; and all authors have participated in data reporting and have been involved in the evaluation of data and manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of all participating centers of the Swedish Acute Myeloid Leukemia Registry Group appears in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Gunnar Juliusson, Stem Cell Center BMC B10, Lund University, SE 22185 Lund, Sweden; e-mail: Gunnar.Juliusson@med.lu.se.