Abstract
Dendritic cell (DC) populations play unique and essential roles in the detection of pathogens, but information on how different DC types work together is limited. In this study, 2 major DC populations of human blood, myeloid (mDCs) and plasmacytoid (pDCs), were cultured alone or together in the presence of pathogens or their products. We show that pDCs do not respond to whole bacteria when cultured alone, but mature in the presence of mDCs. Using purified stimuli, we dissect this cross-talk and demonstrate that mDCs and pDCs activate each other in response to specific induction of only one of the cell types. When stimuli for one or both populations are limited, they synergize to reach optimal activation. The cross-talk is limited to enhanced antigen presentation by the nonresponsive population with no detectable changes in the quantity and range of cytokines produced. We propose that each population can be a follower or leader in immune responses against pathogen infections, depending on their ability to respond to infectious agents. In addition, our results indicate that pDCs play a secondary role to induce immunity against human bacterial infections, which has implications for more efficient targeting of DC populations with improved vaccines and therapeutics.
Introduction
Dendritic cells (DCs) are arrayed with diverse pathogen sensors (eg, Toll-like receptors (TLR)) and reside in tissues throughout the body, rendering them uniquely poised to detect invading pathogens.1,2 During the initiation and amplification of the immune response, DCs rally other cells of both the innate and adaptive immune systems for the elimination of infections.3,4 In the context of different infections, DC populations are also critical in determining the quality of the response through the efficient and rapid production of discrete subsets of cytokines, chemokines, and interferons (IFNs), which selectively direct the recruitment and activation of other immune effectors.3,4 Because DCs are key antigen-presenting cells (APCs), the instructive role of DC soluble factors shapes adaptive immunity in various ways, resulting in focused and optimized antigen-specific responses to different pathogen classes (eg, viruses vs bacteria).5,6 There are numerous distinct DC populations that vary in their tissue distribution, cytokine/chemokine secretion, and/or their interactions with infectious agents and other cells of the host.7-10 Of these, blood myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) represent 2 well-characterized populations that differ in their morphology, phenotype, TLR expression, and cytokine, chemokine, and type I IFN production.10-14 These differences imply that mDCs and pDCs have evolved to sense distinct classes of pathogens and selectively steer subsequent innate and adaptive immunity. Even though both DC types are considered effective APCs,11,15 the nonoverlapping distribution of TLRs and the pattern of cytokine production in human mDCs and pDCs suggest specialized and perhaps complementary functions in immune responses.13,16 Of particular note is the restricted expression of TLR9 by pDCs compared with that of TLR4 by mDCs in humans.5,10,13,16 Moreover, the efficient production of IFN-α by pDCs argues that they are critical for resistance to many viral infections.17 Thus, coordinated responses of the 2 cell types have the potential to impact host defense in unique ways.
There is limited information about how the different DC types coordinate their responses to discrete classes of pathogens.18,19 Recently, however, it was shown in the mouse that the cross-talk between mDCs and pDCs is important in antibacterial and antitumor immune responses.18,20 Although the molecular mechanisms for this cooperation remain to be elucidated, contact between pDCs and mDCs and CD40 engagement were found to be necessary. In this study, an in vitro model was developed to dissect human mDC and pDC responses to TLR agonists and live or fixed whole pathogens using highly purified populations of cells. The results demonstrate that human mDC/pDC cooperation is necessary to induce pDC maturation in response to whole bacteria. The cross-talk is dependent on cell contact and soluble factors, but not CD40 engagement, as seen for murine DCs. Activation of the nonresponding population was restricted to enhanced APC function, and no changes in cytokine, chemokine, and IFN-α production were observed. That pDCs are refractory to bacterial stimuli in the context of the whole microorganism provides a new and intriguing view of how and when pDCs and their pathogen sensors function in immunity.
Methods
Reagents
Antibodies used were as follows: CD3-, CD14-, CD19-, and CD56-phycoerythrin (PE)-Cy5 (Beckman Coulter, Fullerton, CA), or -fluorescein isothiocyanate (FITC; Pharmingen–Becton Dickinson, San Jose, CA), human histocompatibility leukocyte antigen (HLA)-DR-, CD11c-APC, CD16-PE, CD86-, CD80-, and CD40-FITC; tumor necrosis factor (TNF)–α neutralizing (Pharmingen–Becton Dickinson) and IFN-α/β receptor neutralizing (PBL Biomedical Laboratories, New Brunswick, NJ), both used at 10 μg/mL; and CD1c- and blood dendritic cell antigen (BDCA)–2-PE, BDCA-4microbead kits (Miltenyi Biotec, Bergisch Gladbach, Germany), and TLR2-PE (eBioscience, San Diego, CA). A CD40:Fc recombinant protein (Alexis-Axxora Life Sciences, San Diego, CA) was used for neutralization studies. Cytokines used were as follows: interleukin (IL)–8 (R&D Systems, Minneapolis, MN), IL-3 (R&D Systems), IFN-α (PBL), and TNF-α (Gentaur, Brussels, Belgium). TLR agonists used were as follows: R848, imidazoquinoline imiquimod (synthesized in-house), palmitoyl3Cys-Ser-Lys4 (Pam3CSK4; Alexis), lipopolysaccharide (LPS; Alexis), unmethylated CpG oligodeoxynucleotide (CpG) 2216 (type A), and CpG 2006 (type B; synthesized in-house). Fixed and live bacterial preparations were as follows: Staphylococcus aureus wood strain without protein A alexa fluor 488 conjugate (Molecular Probes-Invitrogen-Life Technologies, Carlsbad, CA) and S aureus wild-type strain; Escherichia coli alexa fluor 488 conjugate (Molecular Probes-Invitrogen-Life Technologies) and E coli K12 strain; Yersinia pestis pLCR mutant strain without type III secretion system; group B Streptococcus (GBS) COHI; and pHrodo E coli and S aureus bioparticles (Molecular Probes-Invitrogen-Life Technologies). Live bacteria were grown in the appropriate medium, and the bacterial particles were prepared using paraformaldehyde. Fixed and live bacteria were cultured with DCs at the bacterial cell/DC ratios of 10:1, 1:1, and 1:10. All mammalian cells were cultured in RPMI 1640 medium (Invitrogen-Life Technologies) supplemented with 10% fetal calf serum (FCS; HyClone, Logan, UT) and 1% penicillin-streptomycin-glutamine solution (PSG; Invitrogen-Life Technologies) or Dulbecco modified Eagle medium (DMEM) high glucose (Invitrogen-Life Technologies), 10% FCS, and 1% PSG. Where not indicated, the stimuli used were as follows: IL-8, 100 ng/mL; IFN-α, 5 μg/mL; TNF-α, 1 μg/mL; LPS, 1 μg/mL; R848, 5 μM; and CpG (type A or B), 10 μg/mL.
Donors
Buffy coats from healthy HIV-, hepatitis B virus-, and hepatitis C virus–negative donors were obtained from the Blood Transfusion Section, Alta Val D'Elsa Hospital (Poggibonsi, Italy). Informed consent was obtained before all blood donations. The study protocol was approved by the Novartis Research Center ethical committee and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Cell purification
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats by Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation, following standard procedures. Highly pure (≥ 99%, data not shown) mDCs or mDC subsets were obtained, as previously described.21 Highly pure pDCs (≥ 99%, data not shown) were obtained by enriching pDCs from PBMCs using a BDCA-4 microbead kit (Miltenyi Biotec), according to the manufacturer's instruction. The enriched pDCs were stained using anti-lineage (CD3/CD14/CD19/CD56) and anti-CD11c antibodies, and pDCs were sorted as lineage and CD11c double-negative. Purified T cells were obtained by staining PBMCs with anti-CD14, anti-CD19, anti-CD56, and anti-HLA-DR antibodies, followed by sorting as CD14-, CD19-, CD56-, and HLA-DR–negative cells.
Primary cell cultures
PBMCs or blood mDCs and pDCs cultured alone or together were incubated in complete RPMI medium in conditions indicated in the text, using 96-well U-bottom plate and plating 106 PBMCs/well or 104 DCs per each cell type/well. The medium used for DC stimulation with live bacteria did not contain PSG. For measurement of intracellular TNF-α, brefeldin A (Sigma-Aldrich, St Louis, MO) was added after 4 hours of PBMC culture at a final concentration of 5 μg/mL.
Flow cytometric analysis
DC cultures were stained using anti-CD40 or anti-CD80 antibodies and analyzed in a FACS Canto (Pharmingen–Becton Dickinson). In mDC-pDC cocultures, mDCs and pDCs were identified as CD11c+ and CD11c−, respectively, identifying live cells by using a live/dead stain kit (Invitrogen) according to the manufacturer's instruction. PBMCs used for analysis of surface CD40 were stained with anti-lineage, anti-CD11c, anti–BDCA-2, and anti-CD40 antibodies. For measurement of intracellular TNF-α production, PBMCs were stained with anti-lineage plus anti-CD11c, or anti-lineage plus anti–BDCA-2 antibody mixtures, and fixed and permeabilized using the cytofix/cytoperm kit (Pharmingen–Becton Dickinson), according to manufacturer's instructions. Permeabilized cells were stained with anti–TNF-α antibodies.
Phagocytic assay
Freshly isolated mDCs and pDCs or stimulated pDCs were incubated with E coli or S aureus fluorescent bioparticles at a final concentration of 100 μg/mL or 10 μg/mL in the indicated conditions, with or without cytochalasin D (Calbiochem-Merck, Darmstadt, Germany) at a final concentration of 5 μg/mL. Stained cells were washed, resuspended in phosphate-buffered saline plus trypan blue 0.4% as quencher (Sigma-Aldrich), and analyzed in a FACS Canto (Pharmingen–Becton Dickinson). Alternatively, PBMCs or cocultures of freshly isolated mDC-pDC were incubated in the indicated conditions with E coli or S aureus pHrodo bioparticles, following the manufacturer's instructions. After incubation, the mDC-pDC cocultures were directly analyzed by flow cytometry (FACS Canto), whereas PBMCs were previously stained using BDCA-2 and CD11c antibodies.
Cytokine measurements
Culture supernatants were analyzed using a Bio-Plex multicytokine array system (Bio-Rad, Hercules, CA), according to manufacturer's instructions. Cytokines analyzed were as follows: IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, Eotaxin, fibroblast growth factor basic (FGF basic), granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), IFN-γ, IFN-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, platelet-derived growth factor BB (PDGF-BB), regulated on activation, normal T-cell expressed and secreted (RANTES), TNF-α and vascular endothelial growth factor (VEGF). IFN-α was measured using either Bio-Plex technology or human IFN-α enzyme-linked immunosorbent assay kit (PBL).
T-cell stimulation
T cells were incubated with 1 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes-Invitrogen-Life Technologies) at 2 × 107 cells/mL for 8 minutes at room temperature in the dark. CFSE was quenched for 1 minute at room temperature with 1 volume of FCS, and cells were washed twice. Loaded T cells were cultured in complete RPMI medium at 105 cells/well in 96-well U-bottom plates at different DC/T-cell ratios and analyzed after 5 to 6 days of culture. The overall number of stimulator cells was kept constant at 104 total DCs, and DC populations were mixed accordingly.
Transfectants
Human embryonic kidney cells were stably transfected with a luciferase reporter gene under the control of nuclear factor-κB promoter (HEK293-Luc) and stably cotransfected with plasmids coding for either TLR2-flag (HEK293-TLR2) or TLR4-hemagglutinin (HA) and MD2-CD14 (HEK293-TLR4) or TLR7 (HEK293-TLR7) or TLR9-HA (HEK293-TLR9).
Luciferase induction
HEK293 transfectants were plated in complete DMEM at 4 × 104 cells/well and stimulated for 6 hours, as indicated. The supernatant was removed, and the cells were lysed using 20 μL of passive lysis buffer (Promega, Madison, WI). Lysates were read using the SpectraMax luminometer (Molecular Devices, Concord, ON).
Quantitative real-time polymerase chain reaction
Polymerase chain reaction (PCR) procedures and primers were used, as previously described.21
Results
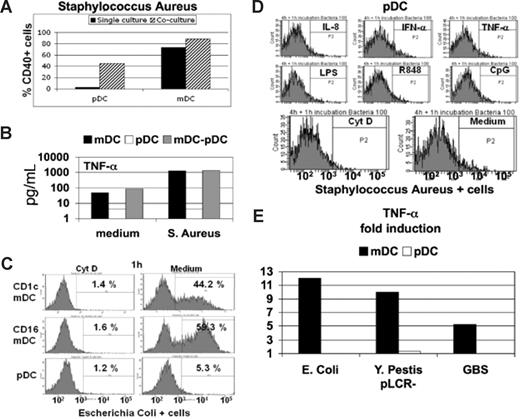
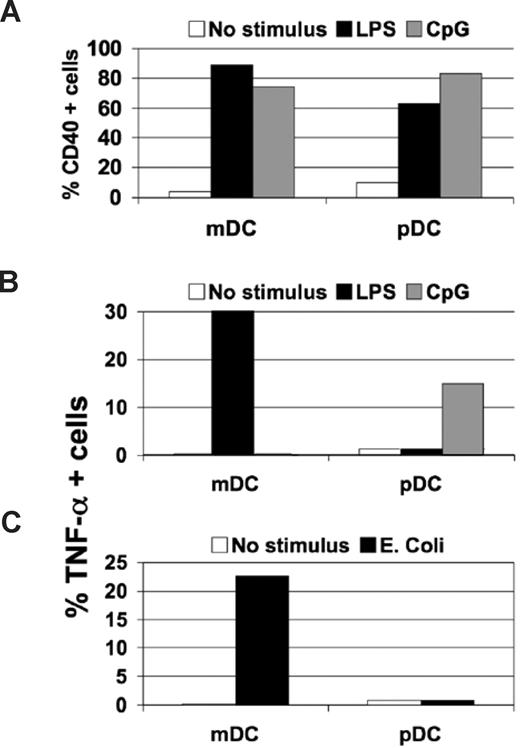
In pathogen infection, a wide range of molecular patterns is available for the activation of pDCs and mDCs. Therefore, we tested the maturation of these populations in the presence of whole pathogens. Initially, fixed or live bacteria were used as stimuli in mDC, pDC, or mDC/pDC cultures, using highly purified cells (Figure 1). No changes in maturation marker expression or cytokine/chemokine/IFN production by pDCs cultured alone were detected after overnight treatment with different fixed bacteria (Figure 1A,B, and data not shown). However, coculture of the unresponsive pDC population with responsive mDCs led to increases in pDC maturation marker expression (Figure 1A). This phenomenon is consistent among different donors, in spite of the huge donor-dependent variability of responses (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). This effect was limited to enhanced antigen-presenting capacity, as no changes in the cytokine signatures observed for mDCs cultured alone or with pDCs in the presence of different fixed bacteria were revealed (Figure 1B and data not shown). Based on this observation, we used fluorescently labeled, fixed bacteria, to compare the phagocytic capacity of the 2 cell types (Figure 1C,D). As expected, total mDCs or the CD1c+ or CD16+ mDC subsets21 readily phagocytosed bacterial particles, and this could be blocked by cytochalasin D, a disruptor of actin polymerization (Figure 1C). By contrast, pDCs exhibited no phagocytic activity even after stimulation with IFN-α, TNF-α, IL-8, IL-3, and various TLR agonists (Figure 1D and data not shown). This difference in phagocytic ability is maintained even when mDCs and pDCs are cultured together using either a coculture system of purified cells or PBMCs (Figure S1B,C). Because fixed bacteria may lack signals for pDC activation, mDCs and pDCs were cultured overnight with 3 different types of both Gram-positive and Gram-negative live bacteria. No pDC response in terms of cytokine production (Figure 1E) or maturation marker expression (data not shown) was observed, whereas mDCs responded vigorously. In addition, this central finding is strictly reproducible among the different donor responses (Figure S1D). These results demonstrate that pDCs are not equipped with pattern recognition receptors (PRRs) capable of sensing bacterial stimuli in the context of the whole organism. Thus, pDCs appear to have a limited role in human bacterial infections and most likely depend on cross-talk with mDCs to participate in antibacterial responses.
Plasmacytoid DCs are not responsive to whole bacteria. (A) pDCs and mDCs cultured in isolation (■) or together (▨) in presence of fixed S aureus bacterial particles, and the percentage of cells expressing CD40 was determined. A representative experiment of 3 is shown, and similar results were obtained using other bacteria (data not shown). (B) TNF-α production was measured in the culture supernatant of pDCs (□) and mDCs (■) cultured in isolation or together (▩) in the presence or absence of fixed S aureus. A representative experiment of 3 is shown. (C) Phagocytosis of fluorescently labeled E coli fixed bacterial particles by CD16 and CD1c mDC subsets or pDCs was measured by flow cytometry in the presence (left panels) or absence (right panels) of cytochalasin D (Cyt D). Similar results were obtained performing the experiments at the time points of incubation, as follows: 15 and 30 minutes; 1, 4, 6, and 8 hours; and overnight (data not shown). One representative experiment of 5 is shown. (D) Uptake of fluorescently labeled S aureus bacterial particles by pDCs pretreated for 4 hours with the indicated stimuli. Similar results were obtained after overnight pretreatment of pDCs. A representative experiment of 5 is shown. (E) Fold induction of TNF-α production measured in the supernatant of mDCs (■) or pDCs (□) cultured in presence of live growing E coli, Y pestis (pLCR-mutant strain), and GBS. One representative experiment of 3 is shown.
Plasmacytoid DCs are not responsive to whole bacteria. (A) pDCs and mDCs cultured in isolation (■) or together (▨) in presence of fixed S aureus bacterial particles, and the percentage of cells expressing CD40 was determined. A representative experiment of 3 is shown, and similar results were obtained using other bacteria (data not shown). (B) TNF-α production was measured in the culture supernatant of pDCs (□) and mDCs (■) cultured in isolation or together (▩) in the presence or absence of fixed S aureus. A representative experiment of 3 is shown. (C) Phagocytosis of fluorescently labeled E coli fixed bacterial particles by CD16 and CD1c mDC subsets or pDCs was measured by flow cytometry in the presence (left panels) or absence (right panels) of cytochalasin D (Cyt D). Similar results were obtained performing the experiments at the time points of incubation, as follows: 15 and 30 minutes; 1, 4, 6, and 8 hours; and overnight (data not shown). One representative experiment of 5 is shown. (D) Uptake of fluorescently labeled S aureus bacterial particles by pDCs pretreated for 4 hours with the indicated stimuli. Similar results were obtained after overnight pretreatment of pDCs. A representative experiment of 5 is shown. (E) Fold induction of TNF-α production measured in the supernatant of mDCs (■) or pDCs (□) cultured in presence of live growing E coli, Y pestis (pLCR-mutant strain), and GBS. One representative experiment of 3 is shown.
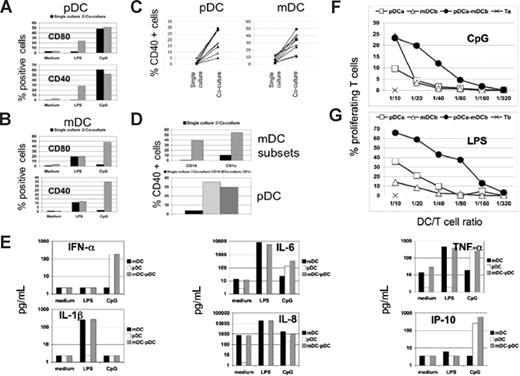
Given that the responsiveness to TLR4 and TLR9 engagement is restricted to human mDCs and pDCs, respectively, the use of purified and selective TLR agonists provided a powerful experimental model to dissect more in detail the features of DC cross-talk. We studied the effects of the TLR4 agonist lipopolysaccharide (LPS) or the TLR9 agonist unmethylated CpG oligodeoxynucleotide (CpG) on highly pure populations of mDCs or pDCs cultured in isolation or together (Figure 2). Investigation of maturation marker expression demonstrated that CpG stimulation of pDCs alone led to up-regulation of CD40 and CD80, whereas no effect was observed on mDCs under these conditions (Figure 2A,B). Conversely, LPS treatment led to a similar mDC response, but did not increase pDC maturation (Figure 2A,B). When mDCs and pDCs were treated with the selective TLR agonists in coculture, consistent and significant increases in CD40 and CD80 expression were observed on the previously nonresponsive population in all donors tested (Figure 2A-C). These effects were also seen using distinct mDC subsets expressing CD1c or CD16,21 indicating that this is a general phenomenon (Figure 2D). Given the clear cooperation between the 2 cell types in reciprocally up-regulating maturation marker expression, it was surprising that production of numerous cytokines, chemokines, and IFN-α remained unchanged after coculture in the presence of LPS or CpG (Figures 2E, S2A). Despite the clear lack of alterations in soluble factor production after coculture, the cooperation of mDC/pDC significantly increased their ability to drive the proliferation and IFN-γ production by alloreactive T cells compared with either cell type alone (Figures 2F,G, S2B,C, and data not shown). Thus, the mDC/pDC cross-talk appears to specifically enhance the antigen-presenting capacity without changing the overall cytokine/chemokine signature from that observed for the responsive cell type alone. The results reported in Figures 1,2 were generated using a mDC/pDC coculture ratio of 1:1, and all experiments shown throughout this study were performed with the same ratio. However, similar results were obtained using 1:10 or 10:1 ratios (data not shown) of mDCs to pDCs, demonstrating the robustness of the effect.
Cooperation between mDCs and pDCs enhances T-cell stimulation capacity without changes in cytokine production. (A,B) Costimulatory molecule expression was measured by flow cytometry on pDCs (A) and mDCs (B) after stimulation with CpG or LPS, as indicated. The cells were cultured alone (■) or together ( ), and the results are reported as the percentage of CD80+ and CD40+ cells in each population. One representative experiment of 50 is shown. (C) The effect of mDC/pDC cooperation on CD40 expression for 10 different donors was determined as in A and B. The percentage of CD40+ cells was determined for pDCs in the presence of LPS (left panel) and mDCs in the presence of CpG (right panel) from 10 different donors cultured alone (single culture) or together (coculture). (D) CD16+ or CD1c+ mDC subsets in the presence of CpG (top panel) and pDCs in the presence of LPS (bottom panel) were cultured in isolation (single culture) or together (coculture), and the percentage of cells expressing CD40 is shown. The indicated mDC subset was cultured in isolation (■) or with pDCs (
), and the results are reported as the percentage of CD80+ and CD40+ cells in each population. One representative experiment of 50 is shown. (C) The effect of mDC/pDC cooperation on CD40 expression for 10 different donors was determined as in A and B. The percentage of CD40+ cells was determined for pDCs in the presence of LPS (left panel) and mDCs in the presence of CpG (right panel) from 10 different donors cultured alone (single culture) or together (coculture). (D) CD16+ or CD1c+ mDC subsets in the presence of CpG (top panel) and pDCs in the presence of LPS (bottom panel) were cultured in isolation (single culture) or together (coculture), and the percentage of cells expressing CD40 is shown. The indicated mDC subset was cultured in isolation (■) or with pDCs ( ), whereas pDCs were cultured in isolation (■) either with CD16 mDC subset (gray striped bar) or CD1c mDC subset (▩). One representative experiment of 3 is shown. (E) Measurement of the indicated cytokines in the supernatants of mDCs (■) or pDCs (□) cultured in isolation or together (▩) in presence of LPS or CpG, as indicated. One representative experiment of 5 is shown. (F,G) T-cell stimulation by DC under the different culture conditions was measured as percentage of proliferating cells. CFSE-loaded T cells were cultured with the following: autologous pDCs (□), allogeneic mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratios, in presence of CpG (F); allogeneic pDCs (□), autologous mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratio in presence of LPS (G). One representative experiment of 3 is shown.
), whereas pDCs were cultured in isolation (■) either with CD16 mDC subset (gray striped bar) or CD1c mDC subset (▩). One representative experiment of 3 is shown. (E) Measurement of the indicated cytokines in the supernatants of mDCs (■) or pDCs (□) cultured in isolation or together (▩) in presence of LPS or CpG, as indicated. One representative experiment of 5 is shown. (F,G) T-cell stimulation by DC under the different culture conditions was measured as percentage of proliferating cells. CFSE-loaded T cells were cultured with the following: autologous pDCs (□), allogeneic mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratios, in presence of CpG (F); allogeneic pDCs (□), autologous mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratio in presence of LPS (G). One representative experiment of 3 is shown.
Cooperation between mDCs and pDCs enhances T-cell stimulation capacity without changes in cytokine production. (A,B) Costimulatory molecule expression was measured by flow cytometry on pDCs (A) and mDCs (B) after stimulation with CpG or LPS, as indicated. The cells were cultured alone (■) or together ( ), and the results are reported as the percentage of CD80+ and CD40+ cells in each population. One representative experiment of 50 is shown. (C) The effect of mDC/pDC cooperation on CD40 expression for 10 different donors was determined as in A and B. The percentage of CD40+ cells was determined for pDCs in the presence of LPS (left panel) and mDCs in the presence of CpG (right panel) from 10 different donors cultured alone (single culture) or together (coculture). (D) CD16+ or CD1c+ mDC subsets in the presence of CpG (top panel) and pDCs in the presence of LPS (bottom panel) were cultured in isolation (single culture) or together (coculture), and the percentage of cells expressing CD40 is shown. The indicated mDC subset was cultured in isolation (■) or with pDCs (
), and the results are reported as the percentage of CD80+ and CD40+ cells in each population. One representative experiment of 50 is shown. (C) The effect of mDC/pDC cooperation on CD40 expression for 10 different donors was determined as in A and B. The percentage of CD40+ cells was determined for pDCs in the presence of LPS (left panel) and mDCs in the presence of CpG (right panel) from 10 different donors cultured alone (single culture) or together (coculture). (D) CD16+ or CD1c+ mDC subsets in the presence of CpG (top panel) and pDCs in the presence of LPS (bottom panel) were cultured in isolation (single culture) or together (coculture), and the percentage of cells expressing CD40 is shown. The indicated mDC subset was cultured in isolation (■) or with pDCs ( ), whereas pDCs were cultured in isolation (■) either with CD16 mDC subset (gray striped bar) or CD1c mDC subset (▩). One representative experiment of 3 is shown. (E) Measurement of the indicated cytokines in the supernatants of mDCs (■) or pDCs (□) cultured in isolation or together (▩) in presence of LPS or CpG, as indicated. One representative experiment of 5 is shown. (F,G) T-cell stimulation by DC under the different culture conditions was measured as percentage of proliferating cells. CFSE-loaded T cells were cultured with the following: autologous pDCs (□), allogeneic mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratios, in presence of CpG (F); allogeneic pDCs (□), autologous mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratio in presence of LPS (G). One representative experiment of 3 is shown.
), whereas pDCs were cultured in isolation (■) either with CD16 mDC subset (gray striped bar) or CD1c mDC subset (▩). One representative experiment of 3 is shown. (E) Measurement of the indicated cytokines in the supernatants of mDCs (■) or pDCs (□) cultured in isolation or together (▩) in presence of LPS or CpG, as indicated. One representative experiment of 5 is shown. (F,G) T-cell stimulation by DC under the different culture conditions was measured as percentage of proliferating cells. CFSE-loaded T cells were cultured with the following: autologous pDCs (□), allogeneic mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratios, in presence of CpG (F); allogeneic pDCs (□), autologous mDCs (△), or a 1/1 mix of the 2 DC types (●) at the indicated total DC/T-cell ratio in presence of LPS (G). One representative experiment of 3 is shown.
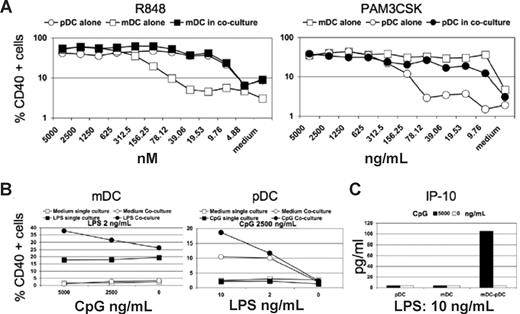
In situations when pathogens and their proinflammatory products are limiting, such as early or late in infection, the cooperation between mDCs and pDCs shown in Figure 2 may have profound effects on the initiation, amplification, or maintenance of effective immunity. To test this potential for synergistic mDC/pDC responses, the 2 cell types were cultured alone or together with different doses of TLR agonists that activate both DC populations (Figure 3). The imidazoquinoline resiquimod (R848) is a synthetic TLR7/8 agonist that activates both isolated pDCs and mDCs, although we observed that pDCs are significantly more responsive to R848. In coculture, the effects of R848 stimulation on mDC CD40 expression were greatly enhanced at low doses, but there was little effect on pDC responsiveness when mDCs were present (Figure 3A left panel). When using the bacterial lipopeptide palmitoyl3Cys-Ser-Lys4 (Pam3CSK4), a TLR2 agonist that triggers mDCs more efficiently than pDCs, we observed the opposite pattern (Figure 3A right panel). Activation of pDCs is increased at lower doses, whereas mDC responses are largely unchanged. We confirmed these observations, taking advantage again of the mDC and pDC selectivity for LPS (TLR4) and CpG (TLR9). In the presence of suboptimally CpG-activated pDCs and a low dose of LPS, mDCs gain responsiveness (Figure 3B left panel), whereas the converse experiment demonstrates that partially LPS-activated mDCs drive more efficient stimulation of pDCs by CpG (Figure 3B left panel). To highlight the consistency of the results in spite of the donor variability, we reported the synergy observed in the 3 donors tested, at the combination and concentrations of stimuli (LPS and CpG) for which the effect was most evident (Figure S3A). Together these findings demonstrate that mDC/pDC cross-talk augments antigen presentation function of each population in response to suboptimal pathogenic stimuli. Under conditions in which both cell types are capable of responding to the TLR agonists used, the cytokine/chemokine signatures from the cocultures are mixed, as expected (data not shown). However, unlike maturation markers and similar to the results in Figure 2, there were no dramatic changes in the cytokine/chemokine signatures observed with DC populations cultured alone versus together. The one exception was synergistic mDC-pDC cross-talk resulting in the production of IP-10 when a low dose of LPS was used in combination with a suboptimal dose of CpG (Figures 3C, S3B). This chemokine is central in T-cell recruitment,22 and its synergistic production is therefore consistent with a specific role of mDC/pDC cooperation in enhancing T-cell activation.
Simultaneous stimulation of both DC populations with limited amounts of TLR agonists leads to synergistic activation. (A) Percentage of CD40+ pDCs (○) and mDCs (□) was cultured in isolation or together (● and ■, respectively). Cultures were treated with the indicated concentrations of the TLR7/8 agonists R848 (left panel) or Pam3CSK4 (right panel), a TLR2 agonist. A representative experiment of 3 is shown. (B) Percentages of CD40+ mDCs (left panel) and pDCs (right panel) cultured in isolation (squares) or together (circles) in presence of the indicated concentration of LPS and CpG (filled symbols). A representative experiment of 3 is shown. (C) Measurement of IP-10 secretion by pDCs and mDCs cultured in isolation or together at the indicated concentration of LPS. CpG was added (■) or not (□) to the cultures, and the effects on IP-10 production are shown. The data are from a representative experiment of 3 performed.
Simultaneous stimulation of both DC populations with limited amounts of TLR agonists leads to synergistic activation. (A) Percentage of CD40+ pDCs (○) and mDCs (□) was cultured in isolation or together (● and ■, respectively). Cultures were treated with the indicated concentrations of the TLR7/8 agonists R848 (left panel) or Pam3CSK4 (right panel), a TLR2 agonist. A representative experiment of 3 is shown. (B) Percentages of CD40+ mDCs (left panel) and pDCs (right panel) cultured in isolation (squares) or together (circles) in presence of the indicated concentration of LPS and CpG (filled symbols). A representative experiment of 3 is shown. (C) Measurement of IP-10 secretion by pDCs and mDCs cultured in isolation or together at the indicated concentration of LPS. CpG was added (■) or not (□) to the cultures, and the effects on IP-10 production are shown. The data are from a representative experiment of 3 performed.
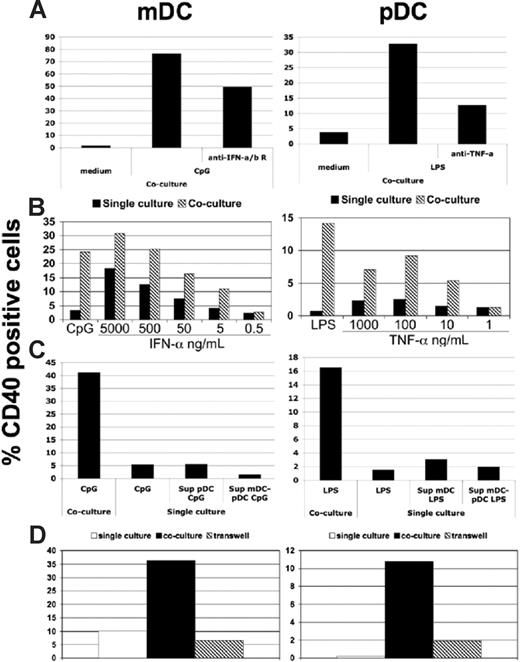
The general mechanisms governing the likely complex cooperation between mDC/pDC were next examined (Figure 4). Because TNF-α and IFN-α have been shown to impact pDC and mDC maturation,3,14,23 and both soluble factors are produced in response to the selective TLR agonists used in this study, their effects on CD40 up-regulation in cocultures were examined. Addition of neutralizing antibodies against type I IFN receptors and TNF-α to CpG- or LPS-treated mDC/pDC cocultures, respectively, partially or strongly suppressed CD40 up-regulation (Figures 4A, S4A). Moreover, treatment of these same cocultures with high doses of recombinant IFN-α or TNF-α could to some extent recapitulate the activities of CpG and LPS, showing that the coculture conditions provide additional factors (soluble or cell bound) for DC maturation (Figure 4B). Importantly, treatment of mDCs with supernatant from CpG-stimulated pDCs or mDC/pDC cultures or treatment of pDCs with supernatant from LPS-stimulated mDCs or mDC/pDC cultures had little to no effect (Figure 4C). This indicated that soluble factors produced by the stimulated population were necessary, but not sufficient for the cross-talk, and suggested that cell contact is essential. The requirement for mDC/pDC contact was confirmed by separating of the 2 cell types in transwells, which completely abolished cross-maturation (Figures 4D, S4B). While CD40-CD40L interaction has been described to have a role in mouse mDC-pDC cross-talk,18 we used a soluble CD40:Fc chimeric molecule to block this interaction without any effect on the reciprocal maturation between mDCs and pDCs (data not shown). Thus, human mDC and pDC cross-talk is distinct from that described in the mouse and dependent on a combination of soluble factors and cell surface receptors.
DC cross-talk depends on TNF-α, IFN-α, and cell contact. (A) The percentage of CD40+ pDCs and mDCs was determined after mDC/pDC coculture, as in Figure 1. The effects of LPS treatment with or without neutralizing anti-TNF-α mAbs (right panel) or CpG stimulation in the presence or absence of neutralizing anti-type I interferon receptor mAbs (left panel) were determined for pDCs and mDCs, respectively. (B) CD40 expression by pDCs treated with TNF-α (right panel) or mDCs treated with IFN-α (left panel) is compared for mDCs and pDCs cultured alone (■) or in coculture ( ). (C) pDCs were treated with supernatants from mDCs cultured alone or with pDCs after LPS stimulation, and CD40 expression was determined (right panel). Similarly, mDCs were treated with supernatants from pDCs alone or in coculture that was treated with CpG (left panel). (D) The percentage of CD40+ pDCs (right panel) and mDCs (left panel) after culture in isolation without stimulation (□) and stimulated together either in the same well (■) or separated by a semipermeable membrane (
). (C) pDCs were treated with supernatants from mDCs cultured alone or with pDCs after LPS stimulation, and CD40 expression was determined (right panel). Similarly, mDCs were treated with supernatants from pDCs alone or in coculture that was treated with CpG (left panel). (D) The percentage of CD40+ pDCs (right panel) and mDCs (left panel) after culture in isolation without stimulation (□) and stimulated together either in the same well (■) or separated by a semipermeable membrane ( ). As before, LPS (right panel) or CpG (left panel) was used as mDC- or pDC-selective stimuli. All experiments in this figure are representative of 3 performed.
). As before, LPS (right panel) or CpG (left panel) was used as mDC- or pDC-selective stimuli. All experiments in this figure are representative of 3 performed.
DC cross-talk depends on TNF-α, IFN-α, and cell contact. (A) The percentage of CD40+ pDCs and mDCs was determined after mDC/pDC coculture, as in Figure 1. The effects of LPS treatment with or without neutralizing anti-TNF-α mAbs (right panel) or CpG stimulation in the presence or absence of neutralizing anti-type I interferon receptor mAbs (left panel) were determined for pDCs and mDCs, respectively. (B) CD40 expression by pDCs treated with TNF-α (right panel) or mDCs treated with IFN-α (left panel) is compared for mDCs and pDCs cultured alone (■) or in coculture ( ). (C) pDCs were treated with supernatants from mDCs cultured alone or with pDCs after LPS stimulation, and CD40 expression was determined (right panel). Similarly, mDCs were treated with supernatants from pDCs alone or in coculture that was treated with CpG (left panel). (D) The percentage of CD40+ pDCs (right panel) and mDCs (left panel) after culture in isolation without stimulation (□) and stimulated together either in the same well (■) or separated by a semipermeable membrane (
). (C) pDCs were treated with supernatants from mDCs cultured alone or with pDCs after LPS stimulation, and CD40 expression was determined (right panel). Similarly, mDCs were treated with supernatants from pDCs alone or in coculture that was treated with CpG (left panel). (D) The percentage of CD40+ pDCs (right panel) and mDCs (left panel) after culture in isolation without stimulation (□) and stimulated together either in the same well (■) or separated by a semipermeable membrane ( ). As before, LPS (right panel) or CpG (left panel) was used as mDC- or pDC-selective stimuli. All experiments in this figure are representative of 3 performed.
). As before, LPS (right panel) or CpG (left panel) was used as mDC- or pDC-selective stimuli. All experiments in this figure are representative of 3 performed.
Although, in an infectious setting, numerous TLR and non-TLR sensors are available to each DC type for pathogen detection, the cross-maturation of pDCs is identical in the cocultures with mDCs stimulated with whole bacteria or with LPS. This observation indicates that our model is useful to understand the response of pDCs exposed to bacteria.
In all experiments with isolated mDCs and pDCs, the rules for cooperation were remarkably consistent; however, we wanted to test the model when other cell types were present. Using total human PBMCs as a more physiologic setting, we stimulated these mixed populations with the pDC-selective TLR9 agonist, CpG, or the mDC-selective one, LPS, and measured activation marker expression and TNF-α production by each population using flow cytometry. Similar to our previous results, CpG and LPS treatment resulted in the increased maturation marker expression by both subsets (Figures 5A, S5), yet TNF-α production was found only in mDCs after LPS stimulation and pDCs after CpG treatment (Figure 5B). The same pattern of pDC activation marker expression in the absence of TNF-α production was observed when PBMCs were incubated with E coli (Figure 5C). This indicates that the mDC/pDC cross-talk and the pDC specialization described in this study are not influenced by the presence of other immune cells and most likely occur in vivo.
The main features of mDC-pDC cooperation are found also in PBMC. (A) Percentage of CD40+ mDCs and pDCs in unstimulated PBMCs (□) was compared after treatment with LPS (■) or CpG (▩). (B) TNF-α–positive mDCs and pDCs in PBMCs were determined using intracellular cytokine staining of unstimulated PBMCs (□) or PBMCs treated with LPS (■) or CpG (▩). (C) Percentage of intracellular TNF-α+ mDCs and pDCs in unstimulated PBMCs (□) or PBMCs exposed to E coli fixed bacterial particles (■). All data are from a representative experiment of 3 performed.
The main features of mDC-pDC cooperation are found also in PBMC. (A) Percentage of CD40+ mDCs and pDCs in unstimulated PBMCs (□) was compared after treatment with LPS (■) or CpG (▩). (B) TNF-α–positive mDCs and pDCs in PBMCs were determined using intracellular cytokine staining of unstimulated PBMCs (□) or PBMCs treated with LPS (■) or CpG (▩). (C) Percentage of intracellular TNF-α+ mDCs and pDCs in unstimulated PBMCs (□) or PBMCs exposed to E coli fixed bacterial particles (■). All data are from a representative experiment of 3 performed.
Discussion
A hallmark of host defense is the communication, coordination, complementarity, and cooperation (the 4 Cs) of numerous and distinct cell types and soluble factors that work in concert to focus the immune response most effectively on specific invading pathogens. In this study, we investigated the 4 Cs of human mDC and pDC interactions, uncovering unique and surprising features of mDC/pDC cross-talk. At first glance, the limited scope of mDC/pDC cooperation in response to selective TLR agonists and whole bacteria appears counterintuitive. In most studies of immune cell cooperation, strong and broad synergies have been observed, resulting in a general amplification of all functions and the overall immune response. In this study, only APC function appeared to be enhanced, because the quality and quantity of cytokines, chemokines, and IFNs produced by the responsive cell type alone were not different from those seen in coculture. With the view that DCs are not only key initiators and amplifiers of immunity, but also navigators, the limited effects of mDC/pDC cross-talk observed in this study make sense. DC populations possess different capacities for detecting distinct classes of pathogens early and in steering subsequent responses that are more effective for a given agent. Given the lack of gross changes in the soluble factors released by the responding cell types alone and in coculture, we propose that mDCs and pDCs may play primary and secondary roles depending on the infectious context. Because soluble factors such as type I interferons, IL-1β, IL-6, IL-8, or IL-12 have profound effects on the quality and effectiveness of antigen-specific responses,24 robust cytokine production from a DC population not specialized for a given pathogen during the innate immune response could be counterproductive. In essence, it would obscure the nature of the infectious agent (eg, viral vs bacterial) and confound the instructive role of the innate immune response.1,11 The noninstructive or sterile antigen presentation elicited by mDC/pDC cross-talk serves to enhance the immune response quantitatively, but not qualitatively. Thus, mDCs and pDCs can be leaders or followers during an immune response, depending on the type of pathogen encountered. It is tempting to speculate that their mutually exclusive capacities for IFN-α production (pDC viruses) or phagocytosis (mDC bacteria) are key determinants for pathogen specificity of immune responses.
The complementary response of mDCs and pDCs to TLR engagement and the sterile antigen presentation highlight fundamental differences between human and mouse DC cross-talk.18,20 As shown in this study, human mDC-pDC cooperation is bidirectional, whereas in mice it has been shown to function only from pDCs to mDCs. Cooperation in both species is contact dependent, but murine DC cross-talk is dependent on CD40-CD40L interactions and IL-15 secretion, whereas TNF-α and IFN-α partially drive the human system. Moreover, TLR9 is expressed by both murine populations, but only human pDCs and not mDCs express this pathogen sensor.25-27 Thus, the molecular players and the functional outcomes of human DC cooperation differ from those in mice, and it will be important to dissect these species-specific mechanisms in future studies.
Another important effect of the cooperation described in this study is the overall increased sensitivity of the immune system to less potent or limited stimuli. Not only is the more sensitive DC type able to shift the dose-response behavior of the other population, but amplification is also achieved due to the ability of fewer responsive DCs to induce activation of less responsive cells. When stimuli are insufficient for one DC type, the combination of the 2 populations can still lead to activation. The strong induction of the T-cell chemoattractant IP-10 in this setting most likely contributes to heightened T-cell priming. Thus, whereas the concept of sterile antigen presentation described above ensures specificity, simultaneous activation of both populations most likely enhances the general sensitivity of the DC system for pathogen detection as well.
When using live pathogens, we focused our attention on bacteria and the lack of pDC responses because both DC populations undergo activation when infected with different viruses.28-31 Like virtually all cell types, DCs productively infected by viruses produce type I interferons17 ; moreover, they sense the viruses through TLR7/8 (mDCs and pDCs),32,33 TLR3 (mDCs),14,16,34 TLR9 (pDCs), and other mechanisms. The major difference between pDCs and other virally infected cells is the speed and amount in the IFN-α secreted17 that render them the leaders in innate antiviral responses.19 In addition, productive virus infection of pDCs is not necessary to induce high amounts of IFN-α, because TLR engagement is sufficient to induce this response.17,35 In contrast, mDCs do not produce IFN-α upon TLR engagement, but require infection for this type of response. Thus, in terms of innate antiviral effector function through IFN-α, mDCs can be considered secondary to pDCs. Nevertheless, mDCs can directly participate in antiviral T-cell activation. Conversely, our novel findings clearly show that pDC responses are dependent on mDCs in the presence of bacteria and are restricted to T-cell activation. This indicates that, during bacterial infections, pDCs are not engaged in innate responses, but participate as followers of mDCs by presenting antigen to T cells.
The complete unresponsiveness of pDCs to live and fixed bacteria raises important questions not only about their role in bacterial infections, but how they sense pathogens and how they should be targeted with vaccines and therapeutics. Our results suggest that pDCs do not express surface PRRs for bacteria or their products, and their lack of phagocytic activity shields their intracellular pathogen sensors from bacterial stimuli. Of particular interest are TLR2 and TLR9 because both have long been considered bacterial sensors with TLR2-detecting lipids and lipopeptides, and TLR9 is historically viewed as a receptor for bacterial DNA.6,36 However, TLR9 has been proposed as a sensor of viral nucleic acids as well,17 and TLR2 has been implicated in viral infections,37-39 consistent with our results. We show that pDCs are responsive to the TLR2 agonist Pam3CSK4, yet do not respond to either Gram-negative or Gram-positive bacteria. These bacteria all activate HEK293T cells transfected to express TLR2, but not untransfected cells (Figure S6). Human pDCs have been described previously to lack TLR210,11,16 and to be unresponsive to the TLR agonists such as peptidoglycan and lipotechoic acid. We do not detect significant TLR2 expression in pDCs by flow cytometry or PCR (Figure S7). Together these results argue that Pam3CSK4 sensing by pDCs is TLR2 independent, and it is likely that whatever detection mechanism is involved is intracellular because of the lack of pDC responses to whole bacteria. For TLR9, its role as a sensor for CpG motifs in bacterial DNA36 is called into question because TLR9-dependent activation of pDCs in response to whole bacteria was not apparent. Overall, the issue of using purified and/or synthetic pathogen stimuli to dissect TLR selectivity and innate immune cell function has implications for clinical application. Many licensed bacterial vaccines are produced using fixed cells, and based on the results in this work, it is anticipated that they would represent less than ideal activators of pDCs directly. It is unclear whether direct targeting of pDCs for bacterial vaccines or antibacterial therapeutics is the best approach to increasing efficacy, and therefore, the precise dissection of mDC/pDC cooperation and pDC specialization has clinical utility.
In conclusion, our study argues that the complementary activity of mDC and pDC populations alerts the immune system to fight bacterial or viral infection, respectively. Currently, mDC phagocytic capacity and pDC IFN-α production appear to be critical and exclusive functions that steer antigen-specific responses countering bacterial versus viral insult. The sequestering of pDC PRRs to avoid recognition of bacterial stimuli when encountering an intact organism suggests that human pDCs serve a secondary role in immune response against bacterial infections, and presents unique and unexpected challenges in the prophylactic and therapeutic clinical targeting of both pDCs and mDCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ugo D'Oro for providing TLR transfectants; Renata Grifantini for providing Y pestis; Immaculada Magarit Y Ros for providing S aureus; Domenico Maione for providing GBS; all of the people above, Ennio De Gregorio, and Rino Rappuoli for helpful discussions; Erwin Swennen, Giacomo Romagnoli, and Salvatore Nocadello for providing E coli; Stefania Crotta for providing E coli and for influenza experiments; and Angela Spagnuolo and Maria Rita Fontana for Neisseria gonorrhoeae experiments.
This work was supported in part by a grant from European Commission 6th Framework Program (Contract LSHB-CT-2004-512074 DC-THERA NETWORK OF EXCELLENCE).
Authorship
Contribution: D.P. designed and performed research, analyzed and interpreted data, and wrote the manuscript; C.S., S.T., and S.N. performed flow cytometry cell sorting; E.F., A.M., and A.N. performed experiments with bacteria; S.A. and S.V. performed experiments with transfectants; E.B. performed PCR experiments; and A.W. and N.M.V. analyzed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Andreas Wack, Novartis Vaccines and Diagnostics Research Center, via Fiorentina 1, 53100 Siena, Italy; e-mail: andreas.wack@novartis.com; or Dr Nicholas M. Valiante, Novartis Vaccines and Diagnostics Research Center, 45 Sidney St, Cambridge, MA 02139; e-mail: nicholas.valiante@novartis.com.
References
Author notes
*A.W. and N.M.V. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal